Abstract
Objectives
The objective of this study was to investigate survival of ovarian cancer patients with BRCA1 and BRCA2 mutations compared to those without mutations in a population-based sample of incident epithelial ovarian cancer cases.
Methods
Follow-up for vital status was performed on a population-based sample of 232 women with incident epithelial ovarian cancer recruited between December 13, 2000 and September 30, 2003 in the Tampa Bay area. Survival analysis using Cox regression was performed on (1) all 232 cases and (2) the 209 invasive epithelial ovarian cancer cases. Results of the two analyses were similar, thus data involving the 209 invasive epithelial cancer cases are presented, as this was judged to be more clinically relevant.
Results
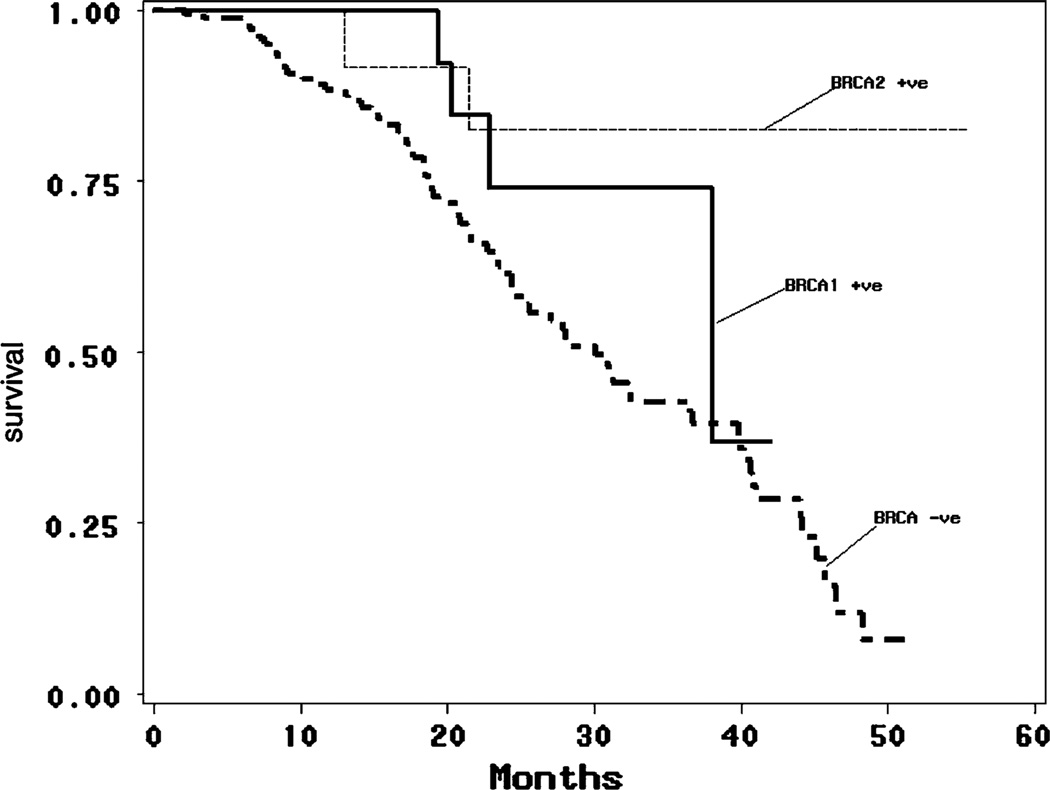
In the multivariate analysis, BRCA status and stage were statistically significant, and were adjusted for in the survival analysis model. The Kaplan–Meier method estimated expected survival at 4 years of 83% of BRCA2 carriers compared to 37% of BRCA1 carriers and 12% of non-carriers. There was a statistically significant difference between BRCA2 carriers and non-carriers (p = 0.013). No statistically significant survival differences were seen for BRCA1 carriers when compared with either BRCA2 carriers or non-carriers.
Conclusion
These data suggest that BRCA2 mutation carriers with ovarian cancer may have better survival than BRCA1 carriers and non-carriers. The etiology of this possible survival advantage is currently unknown. Larger studies are needed to confirm these results and to clarify their etiology and clinical significance.
Keywords: hereditary ovarian cancer carcinoma, BRCA1, BRCA2, Survival
Introduction
Approximately 5–14% of invasive ovarian cancers are believed to be due to hereditary susceptibility [1–3], of which mutations in the BRCA1 and BRCA2 genes account for the majority [4, 5]. The lifetime risk of developing ovarian cancer in BRCA1 and BRCA2 carriers is 28–44% [1, 2, 6, 7]. Estimates of the frequency of BRCA1 and BRCA2 germline mutations in ovarian cancer patients have ranged between 2–12% and 2–6% for BRCA1 and BRCA2 mutations, respectively [2, 3, 8–22].
Ovarian cancer has the highest mortality rate among gynecologic cancers, with more than two thirds of patients presenting with late-stage metastatic disease at initial diagnosis and a 5-year survival rate of only 20– 30% [23–27]. Conversely, at early stages, the long-term survival rate approaches 90% [28].
It has been hypothesized that ovarian cancer patients with BRCA mutations may have improved survival compared to those without mutations [29], possibly due to earlier age of onset in BRCA1 carriers and better response to platinum-based chemotherapy [30–32] with longer periods of remission [29]. Most [29, 33–37], but not all [38, 39], of the studies conducted to date suggest a survival advantage, however all studies where stage information is available are inclusive of a disproportionate number of late-stage cases. Furthermore, these studies have been based on retrospective cases and none have been population-based. We investigated survival in a population-based sample of 232 incident epithelial ovarian cancer cases, including 32 BRCA mutation carriers.
Materials and methods
Subjects
Patients in the current investigation included subjects ascertained through The Tampa Bay Ovarian Cancer Study (TBOCS), a population-based study of newly diagnosed patients with incident epithelial ovarian cancer in the Tampa Bay area. Further details about study design, population, and data collection methods have been published previously [3]. Briefly, study participants were from a heavily populated two-county region of west central Florida, with a population in excess of two million. The study was approved by the institutional review board of the University of South Florida.
Cases were women aged 18–80 with histologically confirmed epithelial ovarian cancer diagnosed between December 13, 2000 and September 30, 2003. Clinical stage was determined according to the International Federation of Gynecologists and Obstetricians criteria [40] and histological subtype was evaluated according to the World Health Organization classification [41].
Of the 362 cases of epithelial ovarian cancer identified through the state registry data, 232 agreed to participate (i.e., case participation rate of 64%). The distribution of TBOCS cases by race and ethnicity was similar to the distribution of cancer cases in the catchment area. The distribution of histologic subtypes and stage was also similar to that seen in the general population. Of the 232 women enrolled in the TBOCS study, 32 had mutations in BRCA1 or BRCA2: 20 in BRCA1 and 12 in BRCA2. Of the 23 women with borderline ovarian tumors, none had BRCA1 or BRCA2 mutations.
Methods
The date of initial surgery was used as the starting date of observation. Survival time was calculated as the months from that date to the date of death or the date last contact for which the patient was known to be alive. In the latter case, survival time was censored as of that date. Demographic information, including date of birth and social security numbers of study participants were used to search the National Death Index (NDI) to obtain vital status on study subjects. Additionally, in an effort to compile the most current information available, local hospital cancer registries and medical offices were contacted. Date of last contact was recorded as the last date found in the patient’s medical chart. Date of death was recorded from the medical record, including day, month, and year of death. When searching the NDI for vital status, only year of death was recorded; thus, for the purposes of analysis, when more accurate medical record information was not available, date of death was defined as July 1 of the year of death. This occurred four times.
Statistical analysis
Descriptive statistics were generated for continuous and categorical variables. The chi-square test was used to compare frequencies. The Cox regression analysis was used to assess the association of various factors to survival using both univariate and multivariate analyses. The Kaplan–Meier method was used to estimate overall survival over time, and the log-rank test was used to compare the survival curves. Asymptotic p-values were calculated, and statistical significance was defined as a two-sided p-value less than 0.05. SAS® statistical software (version 9.1) was used.
BRCA1 and BRCA2 analysis
Lymphocyte DNA was extracted from whole blood by standard procedures. All samples were analyzed for BRCA1 and BRCA2 through full gene sequencing and rearrangement testing per published protocol [42] through Myriad Genetics Laboratories Inc. This analysis identifies gene mutations in all of the known protein coding sequences and additional adjacent areas in the genes and detects approximately 90–95% of mutations in BRCA1 and BRCA2. The samples were also analyzed for the presence of five BRCA1 genomic rearrangements using recombination-specific PCR using primers specific for the normal gene as well as for the rearrangement, including: a 3.8-kb deletion of exon 13 and a 510-bp deletion of exon 22 described in individuals of Dutch ancestry [43], a 6-kb duplication of exon 13 described in individuals of European (particularly British) ancestry [44, 45], a 7.1-kb deletion of exons 8 and 9 described in individuals of European ancestry [46], and a 26-kb deletion of exons 14–20 [47].
Results
As described previously [3], of the 232 TBOCS cases, 209 were diagnosed with invasive cancer and 23 were diagnosed with borderline tumors. The average age at diagnosis was 53 years among BRCA1 carriers (n = 20 women), 58 years among BRCA2 carriers (n = 12 women), and 57 years among women with invasive sporadic tumors (n = 177). The distribution of histologic subtypes and stage of the TBOCS patients is shown in Table 1. The Wilcoxon rank sum test was performed to evaluate the association between stage and BRCA status, however the results were insignificant (p = 0.29).
Table 1.
Tumor histology: subtype and stage among BRCA mutation carriers and non-carriers
| Tumor type | Total sample (N = 232) |
Invasive cancers (n = 209) |
Borderline tumors (n = 23) |
BRCA1+ (n = 20) |
BRCA2+ (n = 12) |
Total BRCA+ (N = 32) |
Total non-carriers (N = 200) |
|---|---|---|---|---|---|---|---|
| Serous | 135 (58.2%) | 121 (57.9%) | 14 (60.9%) | 14 (70%) | 6 (50%) | 20 (63%) | 115 (57.5%) |
| Endometrioid | 30 (12.9%) | 29 (13.9%) | 1 (4.3%) | 1 (5%) | 2 (16.8%) | 3 (9%) | 27 (13.5%) |
| Transitional | 3 (1.3%) | 3 (14.4%) | 0 (0%) | 1 (5%) | 0 (0%) | 1 (3%) | 2 (0.1%) |
| Mucinous | 19 (8.2%) | 13 (6.2%) | 6 (26.1%) | 0 (0%) | 0 (0%) | 0 (0%) | 19 (9.5%) |
| Mixed | 20 (8.6%) | 18 (8.6%) | 2 (8.7%) | 4 (20%) | 1 (8.3%) | 5 (16%) | 15 (7.5%) |
| Peritoneal | 6 (2.6%) | 6 (2.9%) | 0 (0%) | 0 (0%) | 1 (8.3%) | 1 (3%) | 5 (2.5%) |
| Brenner | 3 (1.3%) | 3 (1.4%) | 0 (0%) | 0 (0%) | 1 (8.3%) | 1 (3%) | 1 (1%) |
| Clear cell | 9 (3.9%) | 9 (4.3%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 9 (4.5%) |
| Unknown | 7 (3.0%) | 7 (3.3%) | 0 (0%) | 0 (0%) | 1 (8.3%) | 1 (3%) | 6 (3%) |
| Stage | |||||||
| I | 47 (20.3%) | 28 (13.4%) | 19 (82.6%) | 1 (5%) | 4 (33.4%) | 5 (15.6%) | 42 (21.0%) |
| II | 20 (8.6%) | 20 (9.6%) | 0 (0%) | 3 (15%) | 1 (8.3%) | 4 (12.5%) | 16 (8.0%) |
| III | 136 (58.6%) | 132 (63.15%) | 4 (17.4%) | 13 (65%) | 6 (50%) | 19 (59.4%) | 117 (58.5%) |
| IV | 28 (12.1%) | 28 (13.4%) | 0 (0%) | 3 (15%) | 1 (8.3%) | 4 (12.5%) | 24 (12.0%) |
| Unknown | 1 (0.4%) | 1 (0.45%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0.5%) |
Survival analysis using Cox regression was initially performed on all 232 TBOCS cases (data not shown) and subsequently performed including only the 209 invasive epithelial ovarian cancer cases. Results of these two analyses were similar, thus results involving the 209 invasive epithelial cancer cases are shown, as this was judged to be more clinically relevant. Total observation time for all subjects was 4,083 months, with a median of 18.42 months. Variables examined in the univariate Cox regression analysis were age at diagnosis, BRCA status, grade, stage (early vs. late), and histologic subtype (serous vs. non-serous). Statistically significant associations with survival were found for age at diagnosis (p = 0.040), BRCA status (p = 0.009), and stage (p = 0.009). Variables identified as statistically significant in the univariate model were included in the multivariate analysis. In the multivariate analysis, the association of age at diagnosis with survival lost statistical significance, however BRCA status and stage remained statistically significant (Table 2).
Table 2.
Multivariate Cox regression analysis
| Variable | p-value | Hazard ratio | Comments |
|---|---|---|---|
| Age at diagnosis | 0.207 | 1.014 | |
| BRCA1/2 | 0.025 | 0.380 | Reference = BRCA negative |
| BRCA1 only | 0.330 | 0.600 | Reference = BRCA negative |
| BRCA2 only | 0.036 | 0.221 | Reference = BRCA negative |
| Stage (early) | 0.021 | 0.374 | Reference = late stage |
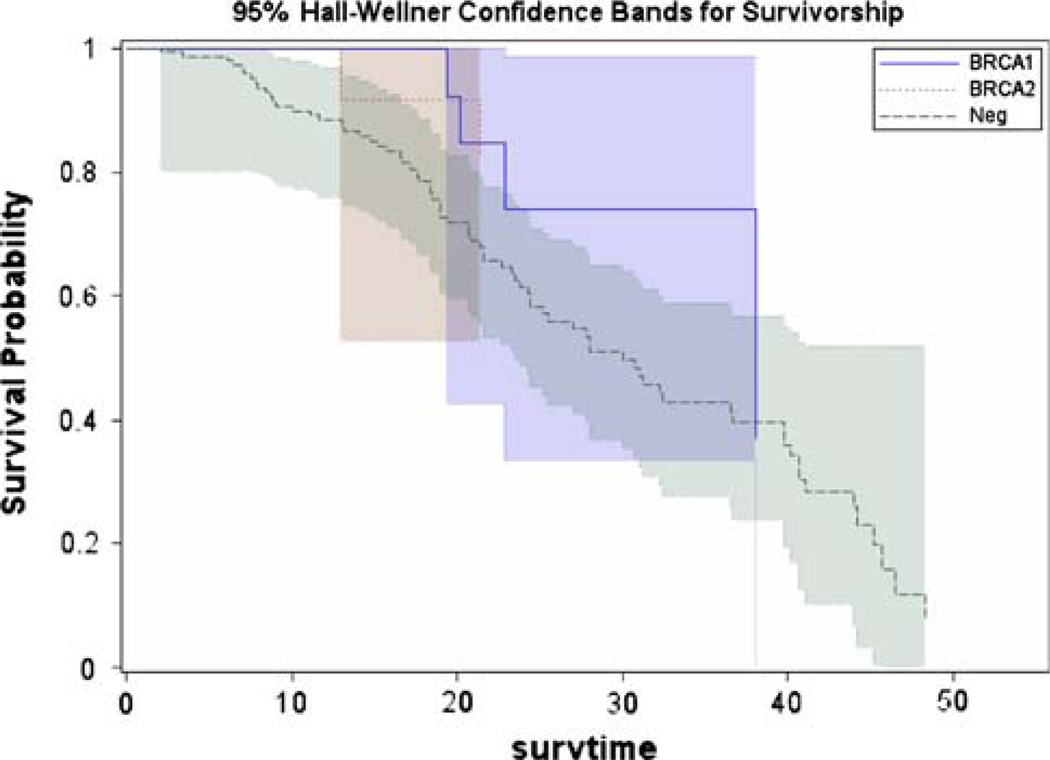
The Kaplan–Meier method was used to estimate the survival probabilities over time. Results showed estimated 4-year survival of 83% of BRCA2 carriers compared to 37% of BRCA1 carriers and 12% of non-carriers (Table 3 and Fig. 1). There was a statistically significant difference between BRCA2 carriers and non-carriers (p = 0.013), however no statistically significant survival differences were seen for BRCA1 carriers when compared with both BRCA2 carriers (p = 0.355) and non-carriers (p = 0.174). The Kaplan–Meier curve was also plotted using 95% Hall-Wellner confidence bands for survivorship (Fig. 2).
Table 3.
Survival by BRCA1 carriers vs. BRCA2 carriers vs. non-carriers (%)
| Years | BRCA1 carriers (No. at risk) | BRCA2 carriers (No. at risk) | BRCA non-carriers (No. at risk) | |
|---|---|---|---|---|
| 1 | 100 (14) | 100 (12) | 88 (111) | Log-rank p-value = 0.019 |
| 2 | 74 (7) | 83 (8) | 61 (57) | |
| 3 | 74 (2) | 83 (4) | 43 (28) | |
| 4 | 37 (1) | 83 (1) | 12 (3) |
Fig. 1.
Kaplan–Meier estimates of survival by BRCA1 carriers vs. BRCA2 carriers vs. non-carriers
Fig. 2.
Ninety-five percent Hall-Wellner confidence bands for survivorship
Discussion
Our study represents the first report of a population-based sample of incident epithelial ovarian cancer cases stratified by BRCA status, suggesting greater generalizability of our results. Furthermore, all previously published studies reporting a survival advantage in BRCA carriers [29, 33–36, 48] have been based predominantly on advanced stage cases, whereas our study is the first with a stage distribution similar to that seen in the general population, thus enhancing relevance to all women with BRCA-associated ovarian cancer. Moreover, the two previously published studies that reported no survival difference between BRCA carriers and non-carriers [38, 39] examined cases without stratifying for stage, which is currently the strongest prognostic variable in invasive epithelial ovarian cancer.
Among the few previous studies reporting a survival advantage were those based on specific population groups, including three studies in Ashkenazi Jewish women [29, 34, 35], and one study in Japanese women [33], limiting generalizability of the results to the US population. Two of the previous studies reporting a survival advantage investigated BRCA1 mutations only [33, 37], and found a survival advantage in BRCA1 carriers compared to sporadic cases, contrary to the findings of the current study. The study by Buller et al. [36] was based on familial cases, and was performed prior to the discovery of the BRCA1 and BRCA2 genes. Thus, the proportion of cases with BRCA mutations was unknown.
All studies to date investigating both the BRCA1 and BRCA2 genes and reporting a survival advantage have been performed in the Ashkenazi Jewish population [29, 34, 35], and have included testing of the three Jewish founder mutations only. Boyd et al. [35] reported that BRCA1-linked cases survived significantly longer than sporadic cases (p = 0.008), and although BRCA2-linked cases displayed a trend toward longer survival, this difference did not achieve statistical significance (p = 0.09). Ben David et al. [34] reported no significant survival differences between women with BRCA1 and BRCA2 mutations. Cass et al. [29] did not specifically report survival differences between BRCA1 and BRCA2 carriers, however they examined disease-free intervals between BRCA2 and BRCA1 carriers, and found that BRCA2 carriers had a longer disease-free interval (57 months vs. 40 months), which may imply improved survival in BRCA2 carriers. Findings in the current study differed in that BRCA2 carriers, but not BRCA1 carriers, had improved survival compared to non-carriers. Possible reasons for this difference include the population-based ascertainment in this study, with a stage distribution that was different from previously published studies. Specifically, our study was representative of the stage distribution of ovarian cancer cases in the general population, and not heavily weighted on advanced stage cases, as was the case in all previous studies reported to date.
As suggested by Boyd et al. [35], the improved survival seen in BRCA2 carriers with ovarian cancer may be due to intrinsic factors within the tumor leading to more indolent clinical behavior. Alternatively, more favorable response to chemotherapy in BRCA2 carriers may be a factor in their longer survival times. Cass et al. reported a better response to platinum-based chemotherapy in BRCA mutation carriers compared to patients with sporadic ovarian cancer and suggested that in vitro chemoresistance was a better predictor of tumor response in BRCA mutation carriers than non-carriers [29]. The etiology of the increased chemosensitivity of BRCA-associated ovarian cancers may be related to the inability of BRCA-deficient cells to repair DNA damage [30–32]. Another hypothesis to explain better survival in BRCA mutation carriers is the younger age at diagnosis for BRCA1 carriers. However, in the current study, this variable was not found to be statistically significant (p = 0.207). This is not a surprising finding as the improved survival was seen in BRCA2 carriers, who are diagnosed with ovarian cancer at similar ages as the general population, compared with BRCA1 carriers, who are diagnosed with ovarian cancer approximately 10 years earlier than women in the general population [2].
The current study represents the first report based on population-based case ascertainment of incident epithelial ovarian cancer cases. This study design avoids two common sources of bias. Firstly, rapid ascertainment and inclusion of incident cases ensures no preferential inclusion of cases that survive longer. Secondly, the cases (including both BRCA carriers and non-carriers) in the current study were ascertained from seven gynecologic oncologists who are known to share similar practices for ovarian cancer treatment. Such treatment consists of primary cytoreductive surgery followed by chemotherapy. Thus, it does not seem likely that the form of treatment contributed to differences in length of survival between BRCA carriers and non-carriers.
Limitations of the study included a small sample size, with limited statistical power to detect survival differences between carriers and non-carriers. Study results need to be interpreted with caution due to the limited median follow-up time of 18 months and the relatively small sample size of BRCA mutation carriers. Thus, confirmation of study findings through a larger study with longer survival times is necessary to verify our observations. Another limitation was the inability to find the exact cause of death for some deceased patients. For example, for patients determined to be deceased based on information from the NDI, no information about cause of death was available. Thus, in these cases, we were unable to determine whether these patients died of ovarian cancer, or if they died of other causes.
The findings in the current study of improved survival in BRCA2 carriers require confirmation in a larger study, ideally with a similar study design based on incident population-based cases. Future studies investigating whether there is an improved response to platinum-based chemotherapy in BRCA carriers than non-carriers is indicated [29]. To that end, we are in the process of extracting treatment response data in the current study population of BRCA carriers and non-carriers.
Clinical implications of findings, if confirmed, include the addition of BRCA mutation status as a prognostic factor in patients diagnosed with ovarian cancer. Additionally, future treatment implications for BRCA mutation carriers with ovarian cancer might include recommendations regarding platinum-based chemotherapy, even at early stages of disease.
In summary, BRCA2 mutation carriers with ovarian cancer appear to have better survival than BRCA1 carriers and non-carriers. The etiology of this survival advantage is currently unknown. Larger studies are needed to confirm our results and to clarify the clinical significance and etiology of our findings.
Acknowledgments
The Tampa Bay Ovarian Cancer Study was supported by grant # DAMD 17-98-1-8659 from the Department of Defense. The ongoing Ovarian Cancer and Mismatch Repair Deficiency Study is supported by grant # R01CA111914-01 from the National Cancer Institute. We are grateful for the work of Cheryl Miree, M.S., Katie Shuler, Elizabeth Allen, A.R.N.P, Mary Reilly, R.N., and Dominique Garvin, A.R.N.P. for data abstraction. We would especially like to thank all of the women who participated in the Tampa Bay Ovarian Cancer Study.
Contributor Information
Tuya Pal, Division of Cancer Prevention and Control, H. Lee Moffitt Cancer Center and Research Institute, 12902 Magnolia Drive, MRC-CANCONT, Tampa, FL 33612, USA; Department of Interdisciplinary Oncology, College of Medicine, The University of South Florida, Tampa, FL, USA; Department of Pediatrics, College of Medicine, All Children’s Hospital, The University of South Florida, St Petersburg, FL, USA.
Jenny Permuth-Wey, Email: permutjm@moffitt.usf.edu, Division of Cancer Prevention and Control, H. Lee Moffitt Cancer Center and Research Institute, 12902 Magnolia Drive, MRC-CANCONT, Tampa, FL 33612, USA; Department of Interdisciplinary Oncology, College of Medicine, The University of South Florida, Tampa, FL, USA.
Rachna Kapoor, Biostatistics Core, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA.
Alan Cantor, Biostatistics Core, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA; Department of Interdisciplinary Oncology, College of Medicine, The University of South Florida, Tampa, FL, USA.
Rebecca Sutphen, Division of Cancer Prevention and Control, H. Lee Moffitt Cancer Center and Research Institute, 12902 Magnolia Drive, MRC-CANCONT, Tampa, FL 33612, USA; Department of Interdisciplinary Oncology, College of Medicine, The University of South Florida, Tampa, FL, USA; Department of Pediatrics, College of Medicine, All Children’s Hospital, The University of South Florida, St Petersburg, FL, USA.
References
- 1.Whittemore AS. Characteristics relating to ovarian cancer risk: implications for prevention and detection. Gynecol Oncol. 1994;55:S15–S19. doi: 10.1006/gyno.1994.1334. [DOI] [PubMed] [Google Scholar]
- 2.Risch HA, McLaughlin JR, Cole DE, et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am J Hum Genet. 2001;68:700–710. doi: 10.1086/318787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pal T, Permuth-Wey J, Betts JA, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104:2807–2816. doi: 10.1002/cncr.21536. [DOI] [PubMed] [Google Scholar]
- 4.Narod S, Ford D, Devilee P, et al. Genetic heterogeneity of breast–ovarian cancer revisited. Breast Cancer Linkage Consortium. Am J Hum Genet. 1995;57:957–958. [PMC free article] [PubMed] [Google Scholar]
- 5.Frank TS, Manley SA, Olopade OI, et al. Sequence analysis of BRCA1 and BRCA2: correlation of mutations with family history and ovarian cancer risk. J Clin Oncol. 1998;16:2417–2425. doi: 10.1200/JCO.1998.16.7.2417. [DOI] [PubMed] [Google Scholar]
- 6.Ford D, Easton DF, Bishop D. Tea: risks of cancer in BRCA1 mutation carriers. Lancet. 1994;343:692–695. doi: 10.1016/s0140-6736(94)91578-4. [DOI] [PubMed] [Google Scholar]
- 7.Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336:1401–1408. doi: 10.1056/NEJM199705153362001. [DOI] [PubMed] [Google Scholar]
- 8.Berchuck A, Heron KA, Carney ME, et al. Frequency of germline and somatic BRCA1 mutations in ovarian cancer. Clin Cancer Res. 1998;4:2433–2437. [PubMed] [Google Scholar]
- 9.Bjorge T, Lie AK, Hovig E, et al. BRCA1 mutations in ovarian cancer and borderline tumours in Norway: a nested case–control study. Br J Cancer. 2004;91:1829–1834. doi: 10.1038/sj.bjc.6602199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khoo US, Ngan HY, Cheung AN, et al. Mutational analysis of BRCA1 and BRCA2 genes in Chinese ovarian cancer identifies 6 novel germline mutations. Hum Mutat. 2000;16:88–89. doi: 10.1002/1098-1004(200007)16:1<88::AID-HUMU16>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 11.Malander S, Ridderheim M, Masback A, et al. One in 10 ovarian cancer patients carry germ line BRCA1 or BRCA2 mutations: results of a prospective study in Southern Sweden. Eur J Cancer. 2004;40:422–428. doi: 10.1016/j.ejca.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 12.Matsushima M, Kobayashi K, Emi M, et al. Mutation analysis of the BRCA1 gene in 76 Japanese ovarian cancer patients: four germline mutations, but no evidence of somatic mutation. Hum Mol Genet. 1995;4:1953–1956. doi: 10.1093/hmg/4.10.1953. [DOI] [PubMed] [Google Scholar]
- 13.Menkiszak J, Gronwald J, Gorski B, et al. Hereditary ovarian cancer in Poland. Int J Cancer. 2003;106:942–945. doi: 10.1002/ijc.11338. [DOI] [PubMed] [Google Scholar]
- 14.Modan B, Hartge P, Hirsh-Yechezkel G, et al. Parity, oral contraceptives, and the risk of ovarian cancer among carriers and noncarriers of a BRCA1 or BRCA2 mutation. N Engl J Med. 2001;345:235–240. doi: 10.1056/NEJM200107263450401. [DOI] [PubMed] [Google Scholar]
- 15.Rafnar T, Benediktsdottir KR, Eldon BJ, et al. BRCA2, but not BRCA1, mutations account for familial ovarian cancer in Iceland: a population-based study. Eur J Cancer. 2004;40:2788–2793. doi: 10.1016/j.ejca.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Rubin SC, Blackwood MA, Bandera C, et al. BRCA1, BRCA2 and hereditary nonpolyposis colorectal cancer gene mutations in an unselected ovarian cancer population: relationship to family history and implications for genetic testing. Am J Obstet Gynecol. 1998;178:670–677. doi: 10.1016/s0002-9378(98)70476-4. [DOI] [PubMed] [Google Scholar]
- 17.Sekine M, Nagata H, Tsuji S, et al. Mutational analysis of BRCA1 and BRCA2 and clinicopathologic analysis of ovarian cancer in 82 ovarian cancer families: two common founder mutations of BRCA1 in Japanese population. Clin Cancer Res. 2001;7:3144–3150. [PubMed] [Google Scholar]
- 18.Stratton JF, Gayther SA, Russell P, et al. Contribution of BRCA1 mutations to ovarian cancer. N Engl J Med. 1997;336:1125–1130. doi: 10.1056/NEJM199704173361602. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi H, Behbakht K, McGovern PE, et al. Mutation analysis of the BRCA1 gene in ovarian cancers. Cancer Res. 1995;55:2998–3002. [PubMed] [Google Scholar]
- 20.Van Der Looij M, Szabo C, Besznyak I, et al. Prevalence of founder BRCA1 and BRCA2 mutations among breast and ovarian cancer patients in Hungary. Int J Cancer. 2000;86:737–740. doi: 10.1002/(sici)1097-0215(20000601)86:5<737::aid-ijc21>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 21.Anton-Culver H, Cohen PF, Gildea ME, et al. Characteristics of BRCA1 mutations in a population-based case series of breast and ovarian cancer. Eur J Cancer. 2000;36:1200–1208. doi: 10.1016/s0959-8049(00)00110-6. [DOI] [PubMed] [Google Scholar]
- 22.Majdak EJ, De Bock GH, Brozek I, et al. Prevalence and clinical correlations of BRCA1/BRCA2 unclassified variant carriers among unselected primary ovarian cancer cases—preliminary report. Eur J Cancer. 2005;41:143–150. doi: 10.1016/j.ejca.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 23.American Cancer Society Inc. Cancer facts & figures 2005. Atlanta: American Cancer Society Inc; 2005. [Google Scholar]
- 24.Mok SC, Chao J, Skates S, et al. Prostasin, a potential serum marker for ovarian cancer: identification through microarray technology. J Natl Cancer Inst. 2001;93:1458–1464. doi: 10.1093/jnci/93.19.1458. [DOI] [PubMed] [Google Scholar]
- 25.Schink JC. Managing the care of patients with advanced ovarian cancer. Semin Oncol. 1999;26:59–61. [PubMed] [Google Scholar]
- 26.Schwartz PE, Taylor KJ. Is early detection of ovarian cancer possible? Ann Med. 1995;27:519–528. doi: 10.3109/07853899509002463. [DOI] [PubMed] [Google Scholar]
- 27.Taylor KJ, Schwartz PE. Screening for early ovarian cancer. Radiology. 1994;192:1–10. doi: 10.1148/radiology.192.1.8208918. [DOI] [PubMed] [Google Scholar]
- 28.Greenlee RT, Hill-Harmon MB, Murray T, et al. Cancer statistics, 2001. CA Cancer J Clin. 2001;51:15–36. doi: 10.3322/canjclin.51.1.15. [DOI] [PubMed] [Google Scholar]
- 29.Cass I, Baldwin RL, Varkey T, et al. Improved survival in women with BRCA-associated ovarian carcinoma. Cancer. 2003;97:2187–2195. doi: 10.1002/cncr.11310. [DOI] [PubMed] [Google Scholar]
- 30.Cortez D, Wang Y, Qin J, et al. Requirement of ATM-dependent phosphorylation of brca1 in the DNA damage response to double-strand breaks. Science. 1999;286:1162–1166. doi: 10.1126/science.286.5442.1162. [DOI] [PubMed] [Google Scholar]
- 31.Husain A, He G, Venkatraman ES, et al. BRCA1 up-regulation is associated with repair-mediated resistance to cis-diamminedichloroplatinum(II) Cancer Res. 1998;58:1120–1123. [PubMed] [Google Scholar]
- 32.Yang H, Jeffrey PD, Miller J, et al. BRCA2 function in DNA binding and recombination from a BRCA2–DSS1–ssDNA structure. Science. 2002;297:1837–1848. doi: 10.1126/science.297.5588.1837. [DOI] [PubMed] [Google Scholar]
- 33.Aida H, Takakuwa K, Nagata H, et al. Clinical features of ovarian cancer in Japanese women with germ-line mutations of BRCA1. Clin Cancer Res. 1998;4:235–240. [PubMed] [Google Scholar]
- 34.Ben David Y, Chetrit A, Hirsh-Yechezkel G, et al. Effect of BRCA mutations on the length of survival in epithelial ovarian tumors. J Clin Oncol. 2002;20:463–466. doi: 10.1200/JCO.2002.20.2.463. [DOI] [PubMed] [Google Scholar]
- 35.Boyd J, Sonoda Y, Federici MG, et al. Clinicopathologic features of BRCA-linked and sporadic ovarian cancer. JAMA. 2000;283:2260–2265. doi: 10.1001/jama.283.17.2260. [DOI] [PubMed] [Google Scholar]
- 36.Buller RE, Anderson B, Connor JP, et al. Familial ovarian cancer. Gynecol Oncol. 1993;51:160–166. doi: 10.1006/gyno.1993.1265. [DOI] [PubMed] [Google Scholar]
- 37.Rubin SC, Benjamin I, Behbakht K, et al. Clinical and pathological features of ovarian cancer in women with germline mutations of BRCA1. N Engl J Med. 1996;335:1413–1416. doi: 10.1056/NEJM199611073351901. [DOI] [PubMed] [Google Scholar]
- 38.Johannsson OT, Ranstam J, Borg A, et al. Survival of BRCA1 breast and ovarian cancer patients: a population-based study from southern Sweden. J Clin Oncol. 1998;16:397–404. doi: 10.1200/JCO.1998.16.2.397. [DOI] [PubMed] [Google Scholar]
- 39.Pharoah PD, Easton DF, Stockton DL, et al. Survival in familial, BRCA1-associated, and BRCA2-associated epithelial ovarian cancer. United Kingdom Coordinating Committee for Cancer Research (UKCCCR) Familial Ovarian Cancer Study Group. Cancer Res. 1999;59:868–871. [PubMed] [Google Scholar]
- 40.Ozols RF, Rubin SC, Thomas G, et al. Epithelial ovarian cancer. In: Hoskins WJ, Perez CA, Young RC, editors. Principles and principles of gynecologic oncology. Philadelphia: Lippincott-Raven Publishers; 1997. p. 958. [Google Scholar]
- 41.World Health Organization. WHO handbook for reporting results of cancer treatment. Geneva, Switzerland: WHO; 1979. [Google Scholar]
- 42.Frank TS, Deffenbaugh AM, Reid JE, et al. Clinical characteristics of individuals with germline mutations in BRCA1 and BRCA2: analysis of 10,000 individuals. J Clin Oncol. 2002;20:1480–1490. doi: 10.1200/JCO.2002.20.6.1480. [DOI] [PubMed] [Google Scholar]
- 43.Petrij-Bosch A, Peelen T, van Vliet M, et al. BRCA1 genomic deletions are major founder mutations in Dutch breast cancer patients. Nat Genet. 1997;17:341–345. doi: 10.1038/ng1197-341. [DOI] [PubMed] [Google Scholar]
- 44.The exon 13 duplication in the BRCA1 gene is a founder mutation present in geographically diverse populations. The BRCA1 Exon 13 Duplication Screening Group. Am J Hum Genet. 2000;67:207–212. [PMC free article] [PubMed] [Google Scholar]
- 45.Unger MA, Nathanson KL, Calzone K, et al. Screening for genomic rearrangements in families with breast and ovarian cancer identifies BRCA1 mutations previously missed by conformation-sensitive gel electrophoresis or sequencing. Am J Hum Genet. 2000;67:841–850. doi: 10.1086/303076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rohlfs EM, Puget N, Graham ML, et al. An Alu-mediated 7.1 kb deletion of BRCA1 exons 8 and 9 in breast and ovarian cancer families that results in alternative splicing of exon 10. Genes Chromosomes Cancer. 2000;28:300–307. doi: 10.1002/1098-2264(200007)28:3<300::aid-gcc8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 47.Ward BC, Hendrikson CA, Gaglio T, et al. Identification of a novel 26 kb deletion in BRCA1 haplotype pair analysis [abstract] Am J Hum Genet. 2002;71:249. [Google Scholar]
- 48.Rubin SC, Benjamin SC, Behbakht K, et al. Clinical and pathological features of ovarian cancer in women with germ-line mutations of BRCA-1. N Engl J Med. 1997;335:1413–1416. doi: 10.1056/NEJM199611073351901. [DOI] [PubMed] [Google Scholar]