Abstract
Background
Data on the association between TNF-alpha and TNF-beta gene polymorphisms and migraine are conflicting.
Methods
We performed a systematic review and meta-analysis of studies published until January 2011. We used data from published papers and as provided after contact with the authors. We calculated study specific odds ratios (OR) and 95% confidence intervals (CI) assuming additive, dominant, and recessive genetic models as well as pooled effect estimates.
Results
Among the ten studies identified the best evidence is available for the TNF-alpha -308G>A and TNF-beta 252A>G polymorphisms indicating no overall association with migraine. Subgroup analyses suggested that the A allele of the TNF-alpha -308G>A variant more than doubles the risk for migraine among populations with a heterogeneous ethnic background, which was driven by associations for MO (additive model: pooled OR=2.87; 95% CI 1.86–4.43). Further, the risk for MA was increased among Asian populations (additive model: pooled OR=1.71; 95% CI 1.07–2.71). Both observed effects were stronger among females than males.
Conclusions
Our results indicate no overall association between TNF-alpha and TNF-beta gene variants. However, associations differed among specific populations. Our findings need to be treated with caution and further targeted research is warranted to evaluate population-specific effects including population stratification.
Keywords: migraine, tumor necrosis factor, lymphotoxin, polymorphism, meta-analysis
Introduction
Migraine is a common neurological disorder affecting 10–20% of the population and women 3–4 times more often than men (1). Clinically migraine presents with recurrent headache attacks, associated symptoms of vegetative disturbance, and hypersensitivity of various functional systems of the nervous system (1, 2). Further, about one-third of migraineurs experience transient neurological symptoms mostly involving the visual system prior to or during a migraine attack, which are known as migraine aura (2).
Migraine pathophysiology is incompletely understood. Current concepts view migraine as an inherited brain disorder intermittently leading to neuronal dysfunctions of various parts of the nervous system (1, 3). A “neurogenic inflammation” appears to be a key mechanism in activating the trigeminal system and causing the headache (4).
Tumor necrosis factor-alpha (TNF-alpha) is an important inflammatory cytokine and modulator of immune responses, which also appears to play a role in migraine. For example, studies have reported changes in serum (5–7) and urine concentrations (8) of TNF-alpha as well as altered serum concentrations of the soluble TNF-alpha receptor (9) among migraineurs. A link between TNF-alpha and migraine is further plausible, since TNF-alpha can stimulate transcription of calcitonin gene-related peptide (CGRP), which plays a pivotal role in migraine pathophysiology (10).
The genes coding for TNF-alpha and the closely related TNF-beta (lymphotoxin-alpha, LT-alpha) are located in the class III gene cluster of the major histocompatibility complex on chromosome 6 (11). Variants in these genes have been shown to modulate cytokine levels of TNF-alpha and TNF-beta (12, 13); hence, various polymorphisms in the TNF-alpha and TNF-beta genes have been investigated among migraineurs. The most frequently studied variants are the -308G>A polymorphism in the promoter region of the TNF-alpha gene (rs1800629) and the 252A>G polymorphism in intron 1 of the TNF-beta gene (rs909253). However, results are conflicting with some studies suggesting associations with migraine for the TNF-alpha -308G>A (14–18) and the TNF-beta 252A>G polymorphism (19, 20), while others do not (14, 17, 19–23).
We sought to summarize the current evidence on the association between polymorphisms in the TNF-alpha/TNF-beta gene cluster and migraine including migraine with aura (MA) and migraine without aura (MO) by systematically reviewing the literature and performing a meta-analysis.
Methods
Selection of studies
We followed the guidelines for systematic reviews of genetic association studies (24). Two investigators (M.S., P.M.R.) independently searched MEDLINE, EMBASE, and Science Citation Index from inception to January 2011 combining text words and MESH terms, where appropriate, for tumor necrosis factor and lymphotoxin (“tumor necrosis factor” or “tumor necrosis factor-alpha” or “tumor necrosis factor-beta” or lymphotoxin or “lymphotoxin-alpha” or “lymphotoxin-beta” or TNF or LTA) with terms for genetic variations (“gene” or “polymorphism” or “genetic variation”) and terms for headache and migraine (“headache” or “headache disorders” or “migraine” or “migraine disorders”). The search terms were combined with the “explode” feature where applicable. We considered all publications without language restrictions. In addition, we manually searched the reference list of all primary articles and review articles.
A priori, we defined the following criteria for inclusion:
Studies must have a cross-sectional, case-control or cohort design.
Authors must investigate patients with migraine, diagnosed according to the criteria of the International Headache Society (IHS) (25, 26) or according to modified IHS criteria and healthy control subjects.
Authors must investigate genetic variants located in the TNF-alpha and/or TNF-beta gene.
Information on genotype frequencies for the polymorphisms investigated among migraineurs and non-migraineneurs must be presented in the publication or be obtainable from the authors upon request.
In publications with overlapping cases and/or controls the largest study with usable genetic data was included.
In a first step, two investigators (M.S., P.M.R.) by consensus identified all studies not meeting any of the pre-specified criteria by screening the title and abstracts. These studies were excluded. In a second step, the same investigators evaluated the full-paper publications of the remaining studies. Studies were excluded if they did not meet all criteria.
Data extraction and contact with authors
Two investigators (M.S., P.M.R.) independently extracted data from the published studies and entered them in a customized database. Disagreements were resolved by consensus. The extracted data included authors’ names and title of study, year of publication, country of origin, setting (clinic vs. population), study design, genotyping method, migraine status (migraine, MA, MO), age range and gender of study individuals, study size, allele and genotype frequencies, and additional potentially relevant information. We sought to collect genetic information for all migraineurs and migraineurs with and without aura separately as well as for the whole study population and females and males separately. If not presented in the paper, allele and genotype frequencies were calculated where possible. For studies, which did not allow extraction of all relevant information including genotype and allele frequencies, we contacted the authors to obtain the missing information. We wrote e-mails to the corresponding authors explaining our project and asking them to provide the additional data. Authors not responding within two weeks were sent up to four reminder e-mails.
Statistical analysis
We first used logistic regression to calculate odds ratios (ORs) and 95% confidence intervals (CIs) for the association between genetic polymorphisms and migraine assuming additive, dominant, and recessive genetic models for each study. The additive model assumes that the risk for migraine among carriers of the heterozygous genotype is half way between carriers of the homozygous genotypes. While the dominant model assumes that carriers of the heterozygous and homozygous variant genotypes have the same risk of developing migraine compared with carriers of the homozygous wild-type genotype, a recessive model assumes that carrying the homozygous variant genotype is necessary to alter the risk for migraine compared to carriers of the heterozygous and homozygous wild-type genotype. We also determined Hardy-Weinberg Equilibrium (HWE) for each study. We investigated (overall) migraine, MA, and MO. We further performed analyses stratified by gender and country of origin (countries with European populations vs. countries with Asian populations vs. countries with populations of heterogeneous ethnic background [Turkey and Iran]), where applicable.
We weighted the log of the ORs by the inverse of their variance to obtain pooled estimates. We ran random-effects models, which include assumptions about the variability between studies. We performed the DerSimonian and Laird Q test for heterogeneity and also calculated the I2 statistic for each analysis (27). We used Galbraith plots to visually examine the impact of individual studies on the overall homogeneity test statistic. We further used meta-regression to investigate whether gender, country of study origin, or clinical phenotype (migraine with aura, migraine without aura) significantly impact the pooled results. We evaluated potential small study effects such as publication bias statistically with the methods described by Begg and Mazumdar (28) and Egger (29).
All analyses were performed using SAS version 9.1 (SAS Institute Inc, Cary, NC) and STATA 10.1 (Stata, College Station, Texas, USA).
Since we only utilized data from previously published studies, we did not obtain approval of an ethics committee or written informed consent.
Results
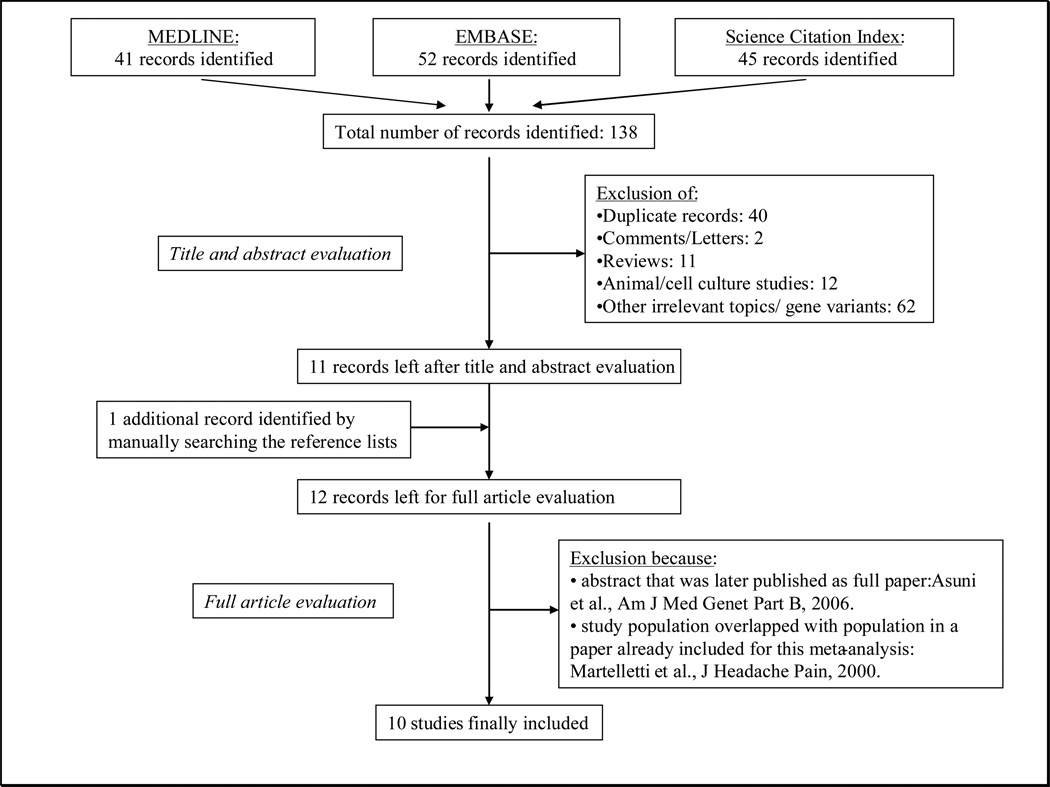
Figure 1 summarizes the process of identifying eligible studies. After title and abstract evaluation we were left with 11 records (14–23, 30). We identified one additional publication by manually searching the reference lists of the records (31). Of the twelve records, we excluded two: one abstract (30), because detailed results were later published in full (19) and one paper (31), because the study population overlapped with that of a record already included for our analysis (20). We were finally left with ten studies (14–23).
Figure 1.
Process of identifying studies
Contact with authors
In eight papers genotype and allele frequencies were only reported for all participants and/or for overall migraine, but not stratified by gender and/or aura (16–23). After contacting the authors we could obtain complete data for five studies (18–22); however, not for two studies (16, 23). We also included genotype and allele frequencies from our previous study (17).
Study characteristics
Table 1a summarizes the characteristics of the studies included according to the polymorphisms investigated. Ten studies have investigated the TNF-alpha -308G>A (rs1800629) (14–23), six the TNF-beta 252A>G (rs909253) (14, 17, 19–22), and two the TNF-alpha -238G>A (rs361525) and TNF-alpha -376G>A (rs1800750) polymorphisms (17, 22). Additional polymorphisms in the TNF-alpha and TNF-beta genes have been investigated in single studies (Table 1b). All studies used standard polymerase chain reaction genotyping methods.
Table 1.
Characteristics of the included studies according to the polymorphisms investigated
| a) Polymorphisms that were investigated in at least 2 studies | ||||||||
|---|---|---|---|---|---|---|---|---|
| TNF-alpha -308G>A (rs1800629) polymorphism | ||||||||
| Study size with genotype information | ||||||||
| Author | Country | Setting | Participants | Controls | Migraine | MA | MO | Comment |
| Trabace 2002 (20) | Italy | clinic | all | 101 | 79 | 32 | 47 |
Other gene variants investigated:
TNF-beta 252A>G (rs909253). |
| females | 45 | 62 | 27 | 35 | ||||
| males | 56 | 17 | 5 | 12 | ||||
| Rainero 2004 (16) | Italy | clinic | all | 306 | 299 | 38 | 261 | |
| females | 231 | 215 | NA | NA | ||||
| males | 75 | 84 | NA | NA | ||||
| Herken 2005 (23) | Turkey | clinic | all | 60 | 60 | 40 | 20 | Cases and controls are predominantly male. |
| females | 8 | 11 | NA | NA | ||||
| males | 52 | 49 | NA | NA | ||||
| Mazaheri 2006 (15) | Iran | clinic | all | 183 | 221 | ---- | 221 | |
| females | 123 | 196 | ---- | 196 | ||||
| males | 60 | 25 | ---- | 25 | ||||
| Lee 2007 (21) | Korea | clinic | all/females | 382 | 439 | 65 | 327 |
Other gene variants investigated: total of 15 SNPs in the TNF-alpha/TNF-beta region. |
| Asuni 2009 (19) | Italy (Sardinia) | clinic | all | 278 | 299 | ---- | 299 |
Other gene variants investigated:
TNF-beta 252A>G (rs909253). |
| females | 144 | 261 | ---- | 261 | ||||
| males | 134 | 38 | ---- | 38 | ||||
| Schürks 2009 (17) | US | population | all/females | 20,425 | 4577 | 1275 | 1951 |
Other gene variants investigated:
TNF-beta 252A>G (rs909253), rs1041981; TNF-alpha -238G>A (rs361525), -376G>A (rs1800750), 244G>A (rs673). |
| Ghosh 2010 (14) | India | clinic | all | 216 | 216 | 84 | 132 |
Other gene variants investigated:
TNF-beta 252A>G (rs909253). |
| females | 152 | 152 | 63 | 89 | ||||
| males | 64 | 64 | 21 | 43 | ||||
| Yilmaz 2010 (18) | Turkey | clinic | all | 96 | 67 | ---- | 67 |
Other gene variants investigated:
IL-1RA VNTR, IL- 1beta 3953C>T (rs1143634), IL-1alpha 4845G>T (rs17561). |
| females | 83 | 57 | ---- | 57 | ||||
| males | 13 | 10 | ---- | 10 | ||||
| Pappa 2010 (22) | Northern Greece | clinic | all | 178 | 103 | ---- | 103 |
Other gene variants investigated:
TNF-alpha - 238G>A (rs361525), TNF-alpha -376A>G (rs1800750), TNF-alpha -857C>T (rs1799724), TNF- alpha -1031T>C (rs1799964), TNF-beta 252A>G (rs909253). |
| females | 60 | 57 | ---- | 57 | ||||
| males | 118 | 46 | ---- | 46 | ||||
| Total number of subjects | 22,227 | 6360 | 1534 | 3428 | ||||
| TNF-beta 252A>G (rs909253) polymorphism | ||||||||
| Study size with genotype information | ||||||||
| Author | Country | Setting | Participants | Controls | Migraine | MA | MO | Comment |
| Trabace 2002 (20) | Italy | clinic | all | 101 | 77 | 30 | 47 |
Other gene variants investigated:
TNF-alpha -308G>A (rs1800629). |
| females | 43 | 60 | 25 | 35 | ||||
| males | 58 | 17 | 5 | 12 | ||||
| Lee 2007 (21) | Korea | clinic | all/females | 382 | 439 | 65 | 327 |
Other gene variants investigated: total of 15 SNPs in the TNF-alpha/TNF-beta region. |
| Asuni 2009 (19) | Italy (Sardinia) | clinic | all | 278 | 299 | ---- | 299 |
Other gene variants investigated:
TNF-alpha -308G>A (rs1800629). |
| females | 144 | 261 | ---- | 261 | ||||
| males | 134 | 38 | ---- | 38 | ||||
| Schürks 2009 (17) | US | population | all/females | 19,269 | 4332 | 1213 | 1824 |
Other gene variants investigated:
TNF-beta rs1041981; TNF-alpha -238G>A (rs361525), - 308G>A (rs1800629), -376G>A (rs1800750), 244G>A (rs673). |
| Ghosh 2010 (14) | India | clinic | all | 216 | 216 | 84 | 132 |
Other gene variants investigated:
TNF-beta 252A>G (rs909253). |
| females | 152 | 152 | 63 | 89 | ||||
| males | 64 | 64 | 21 | 43 | ||||
| Pappa 2010 (22) | Northern Greece | clinic | all | 178 | 103 | ---- | 103 |
Other gene variants investigated:
TNF-alpha -238G>A (rs361525), TNF-alpha -376A>G (rs1800750), TNF- alpha -857C>T (rs1799724), TNF-alpha -1031T>C (rs1799964), TNF-beta 252A>G (rs909253). |
| females | 60 | 57 | ---- | 57 | ||||
| males | 118 | 46 | ---- | 46 | ||||
| Total number of subjects | 20,424 | 5466 | 1392 | 2732 | ||||
| TNF-alpha -238G>A (rs361525) and TNF-alpha -376G>A (rs1800750) polymorphisms | ||||||||
| Study size with genotype information | ||||||||
| Author | Country | Setting | Participants | Controls | Migraine | MA | MO | Comment |
| Schürks 2009 (17) | US | population | all/females | 20,425 | 4577 | 1275 | 1951 |
Other gene variants investigated:
TNF-beta rs1041981; TNF-alpha -238G>A (rs361525), -376G>A (rs1800750), 244G>A (rs673) |
| Pappa 2010 (22) | Northern Greece | clinic | all | 178 | 103 | ---- | 103 |
Other gene variants investigated:
TNF-alpha -308G>A (rs1800629), TNF-alpha -857C>T (rs1799724), TNF alpha -1031T>C (rs1799964), TNF-beta 252A>G (rs909253). |
| females | 60 | 57 | ---- | 57 | ||||
| males | 118 | 46 | ---- | 46 | ||||
| Total number of subjects | 20,603 | 4680 | 1275 | 2054 | ||||
| b) Polymorphisms that were investigated in single studies | ||||||||
|---|---|---|---|---|---|---|---|---|
| Author | Country | Setting | Gene | Polymorphism | Association | |||
| Lee 2007 (21) | Korea | clinic | TNF-alpha/ TNF-beta | rs13192469; rs2516390; rs928815; rs2844483; rs2009658; rs915654; rs2071590; rs2239704; rs3889157; rs1800630; rs3093664; rs769177 |
No | |||
| TNF-beta | rs2844482 | Yes | ||||||
| Schürks 2009 (17) | US | population | TNF-alpha | rs673 | Yes | |||
| TNF-beta | rs1041981 | No | ||||||
| Pappa 2010 (22) | Northern Greece | clinic | TNF-alpha | rs1799724; rs1799964 | No | |||
TNF: tumor necrosis factor; IL-1: Interleukin 1; MA: migraine with aura; MO: migraine without aura; NA: not available.
Eight studies investigated mixed female and male populations (14–16, 18–20, 22, 23), while two study populations consisted only of females (17, 21). Migraineurs in six studies consisted of migraineurs with aura and without aura (14, 16, 17, 20, 21, 23), while four studies only investigated MO (15, 18, 19, 22). One study had a cohort design (17), the remainder were case-control studies (14–16, 18–23). One study was performed among children/adolescents (22), the other studies among adults (14–21, 23). Five studies were performed in European populations (16, 17, 19, 20, 22), two in Turkish populations (18, 23), two in Asian populations (14, 21), and one in a Iranian population (15).
The allele and genotype frequencies for the investigated polymorphisms among migraineurs and controls as well as the p-value for the Hardy-Weinberg Equilibrium (HWE) are listed in Table 2.
Table 2.
Allele and genotype frequencies as well as Hardy-Weinberg Equilibrium according to the investigated polymorphisms
| TNF-alpha -308G>A (rs1800629) polymorphism | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Allele frequencies, n (%) | Genotype frequencies, n (%) | |||||||||
| Author | Participants | Disease status | Study size | HWE | G | A | GG | GA | AA | |
| Trabace 2002 (20) | all | controls | 101 | 0.052 | 189 (93.6) | 13 (6.4) | 90 (89.1) | 9 (8.9) | 2 (2.0) | |
| migraine | 79 | 1.000 | 146 (92.4) | 12 (7.6) | 67 (84.8) | 12 (15.2) | 0 (0.0) | |||
| MA | 32 | 1.000 | 57 (89.1) | 7 (10.9) | 25 (78.1) | 7 (21.9) | 0 (0.0) | |||
| MO | 47 | 1.000 | 89 (94.7) | 5 (5.3) | 42 (89.4) | 5 (10.6) | 0 (0.0) | |||
| females | controls | 45 | 0.116 | 85 (94.4) | 5 (5.6) | 41 (91.1) | 3 (6.7) | 1 (2.2) | ||
| migraine | 62 | 1.000 | 114 (91.9) | 10 (8.1) | 52 (83.9) | 10 (16.1) | 0 (0.0) | |||
| MA | 27 | 1.000 | 48 (88.9) | 6 (11.1) | 21 (77.8) | 6 (22.2) | 0 (0.0) | |||
| MO | 35 | 1.000 | 66 (94.3) | 4 (5.7) | 31 (88.6) | 4 (11.4) | 0 (0.0) | |||
| males | controls | 56 | 0.234 | 104 (92.9) | 8 (7.1) | 49 (87.5) | 6 (10.7) | 1 (1.8) | ||
| migraine | 17 | 1.000 | 32 (94.1) | 2 (5.9) | 15 (88.2) | 2 (11.8) | 0 (0.0) | |||
| MA | 5 | 1.000 | 9 (90.0) | 1 (10.0) | 4 (80.0) | 1 (20.0) | 0 (0.0) | |||
| MO | 12 | 1.000 | 23 (95.8) | 1 (4.2) | 11 (91.7) | 1 (8.3) | 0 (0.0) | |||
| Rainero 2004 (16) | all | controls | 306 | 0.693 | 502 (82.0) | 110 (18.0) | 207 (67.6) | 88 (28.8) | 11 (3.6) | |
| migraine | 299 | 1.000 | 554 (92.6) | 44 (7.4) | 256 (85.6) | 42 (14.0) | 1 (0.3) | |||
| MA | 38 | 1.000 | 66 (86.8) | 10 (13.2) | 28 (73.7) | 10 (26.3) | 0 (0.0) | |||
| MO | 261 | 1.000 | 488 (93.5) | 34 (6.5) | 228 (87.4) | 32 (12.3) | 1 (0.4) | |||
| females | controls | 231 | 1.000 | 382 (82.7) | 80 (17.3) | 158 (68.4) | 66 (28.6) | 7 (3.0) | ||
| migraine | 215 | 0.611 | 401 (93.3) | 29 (6.7) | 186 (86.5) | 29 (13.5) | 0 (0.0) | |||
| males | controls | 75 | 0.475 | 120 (80.0) | 30 (20.0) | 49 (65.3) | 22 (29.3) | 4 (5.3) | ||
| migraine | 84 | 0.504 | 153 (91.1) | 15 (8.9) | 70 (83.3) | 13 (15.5) | 1 (1.2) | |||
| Herken 2005 (23) | all | controls | 62 | 1.000 | 115 (92.7) | 9 (7.3) | 53 (85.5) | 9 (14.5) | 0 (0.0) | |
| migraine | 60 | 0.170 | 113 (94.2) | 7 (5.8) | 54 (90.0) | 5 (8.3) | 1 (1.7) | |||
| MA | 40 | 1.000 | 76 (95.0) | 4 (5.0) | 36 (90.0) | 4 (10.0) | 0 (0.0) | |||
| MO | 20 | 0.079 | 37 (92.5) | 3 (7.5) | 18 (90.0) | 1 (5.0) | 1 (5.0) | |||
| Mazaheri 2006 (15) | all | controls | 183 | 0.0004 | 274 (74.9) | 92 (25.1) | 94 (51.4) | 86 (47) | 3 (1.6) | |
| MO | 221 | <0.0001 | 265 (60) | 177 (40) | 51 (23.1) | 163 (73.8) | 7 (3.2) | |||
| females | controls | 123 | 0.001 | 191 (77.6) | 55 (22.4) | 68 (55.3) | 55 (44.7) | 0 (0.0) | ||
| MO | 196 | <0.0001 | 233 (59.4) | 159 (40.6) | 44 (22.4) | 145 (74) | 7 (3.6) | |||
| males | controls | 60 | 0.141 | 83 (69.2) | 37 (30.8) | 26 (43.3) | 31 (51.7) | 3 (5.0) | ||
| MO | 25 | 0.007 | 32 (64.0) | 18 (36.0) | 7 (28.0) | 18 (72.0) | 0 (0.0) | |||
| Lee 2007 (21) | all/females | controls | 382 | 0.158 | 717 (93.8) | 47 (6.2) | 338 (88.5) | 41 (10.7) | 3 (0.8) | |
| migraine | 439 | 0.716 | 815 (92.8) | 63 (7.2) | 377 (85.9) | 61 (13.9) | 1 (0.2) | |||
| MA | 65 | 1.000 | 119 (91.5) | 11 (8.5) | 54 (83.1) | 11 (16.9) | 0 (0.0) | |||
| MO | 327 | 1.000 | 608 (93.0) | 46 (7.0) | 282 (86.2) | 44 (13.5) | 1 (0.3) | |||
| Asuni 2009 (19) | all | controls | 278 | 0.560 | 526 (94.6) | 30 (5.4) | 249 (89.6) | 28 (10.1) | 1 (0.4) | |
| MO | 299 | 0.476 | 570 (95.3) | 28 (4.7) | 272 (91.0) | 26 (8.7) | 1 (0.3) | |||
| females | controls | 144 | 1.000 | 273 (94.8) | 15 (5.2) | 129 (89.6) | 15 (10.4) | 0 (0.0) | ||
| MO | 261 | 0.452 | 497 (95.2) | 25 (4.8) | 237 (90.8) | 23 (8.8) | 1 (0.4) | |||
| males | controls | 134 | 0.333 | 253 (94.4) | 15 (5.6) | 120 (89.6) | 13 (9.7) | 1 (0.7) | ||
| MO | 38 | 1.000 | 73 (96.1) | 3 (3.9) | 35 (92.1) | 3 (7.9) | 0 (0.0) | |||
| Schürks 2009 (17) | all/females | controls | 20,425 | 0.559 | 33,771 (82.7) | 7079 (17.3) | 13947 (68.3) | 5877 (28.8) | 601 (2.9) | |
| migraine | 4577 | 0.094 | 7531 (82.3) | 1623 (17.7) | 3081 (67.3) | 1369 (29.9) | 127 (2.8) | |||
| MA | 1275 | 0.420 | 2060 (80.8) | 490 (19.2) | 827 (64.9) | 406 (31.8) | 42 (3.3) | |||
| MO | 1951 | 0.868 | 3240 (83.0) | 662 (17.0) | 1346 (69.0) | 548 (28.1) | 57 (2.9) | |||
| Ghosh 2010 (14) | all | controls | 216 | 0.557 | 406 (94.0) | 26 (6.0) | 191 (88.4) | 24 (11.1) | 1 (0.5) | |
| migraine | 216 | 0.235 | 391 (90.5) | 41 (9.5) | 175 (81.0) | 41 (19.0) | 0 (0.0) | |||
| MA | 84 | 0.595 | 149 (88.7) | 19 (11.3) | 65 (77.4) | 19 (22.6) | 0 (0.0) | |||
| MO | 132 | 0.604 | 242 (91.7) | 22 (8.3) | 110 (83.3) | 22 (16.7) | 0 (0.0) | |||
| females | controls | 152 | 0.446 | 285 (93.8) | 19 (6.3) | 134 (88.2) | 17 (11.2) | 1 (0.7) | ||
| migraine | 152 | 0.363 | 273 (89.8) | 31 (10.2) | 121 (79.6) | 31 (20.4) | 0 (0.0) | |||
| MA | 63 | 0.594 | 109 (86.5) | 17 (13.5) | 46 (73.0) | 17 (27.0) | 0 (0.0) | |||
| MO | 89 | 1.000 | 164 (92.1) | 14 (7.9) | 75 (84.3) | 14 (15.7) | 0 (0.0) | |||
| males | controls | 64 | 1.000 | 121 (94.5) | 7 (5.5) | 57 (89.1) | 7 (10.9) | 0 (0.0) | ||
| migraine | 64 | 1.000 | 118 (92.2) | 10 (7.8) | 54 (84.4) | 10 (15.6) | 0 (0.0) | |||
| MA | 21 | 1.000 | 40 (95.2) | 2 (4.8) | 19 (90.5) | 2 (9.5) | 0 (0.0) | |||
| MO | 43 | 1.000 | 78 (90.7) | 8 (9.3) | 35 (81.4) | 8 (18.6) | 0 (0.0) | |||
| Yilmaz 2010 (18) | all | controls | 96 | 0.590 | 174 (90.6) | 18 (9.4) | 79 (82.3) | 16 (16.7) | 1 (1.0) | |
| MO | 67 | 0.233 | 97 (72.4) | 37 (27.6) | 37 (55.2) | 23 (34.3) | 7 (10.4) | |||
| females | controls | 83 | 0.455 | 152 (91.6) | 14 (8.4) | 70 (84.3) | 12 (14.5) | 1 (1.2) | ||
| MO | 57 | 0.308 | 83 (72.8) | 31 (27.2) | 32 (56.1) | 19 (33.3) | 6 (10.5) | |||
| males | controls | 13 | 1.000 | 22 (84.6) | 4 (15.4) | 9 (69.2) | 4 (30.8) | 0 (0.0) | ||
| MO | 10 | 1.000 | 14 (70.0) | 6 (30.0) | 5 (50.0) | 4 (40.0) | 1 (10.0) | |||
| Pappa 2010 (22) | all | controls | 178 | 0.680 | 321 (90.2) | 35 (9.8) | 145 (81.5) | 31 (17.4) | 2 (1.1) | |
| MO | 103 | 1.000 | 192 (93.2) | 14 (6.8) | 89 (86.4) | 14 (13.6) | 0 (0) | |||
| females | controls | 60 | 0.516 | 107 (89.2) | 13 (10.8) | 48 (80.0) | 11 (18.3) | 1 (1.7) | ||
| MO | 57 | 1.000 | 105 (92.1) | 9 (7.9) | 48 (84.2) | 9 (15.8) | 0 (0.0) | |||
| males | controls | 118 | 1.000 | 214 (90.7) | 22 (9.3) | 97 (82.2) | 20 (16.9) | 1 (0.8) | ||
| MO | 46 | 1.000 | 87 (94.6) | 5 (5.4) | 41 (89.1) | 5 (10.9) | 0 (0.0) | |||
| TNF-beta 252A>G (rs909253) polymorphism | ||||||||||
| Allele frequencies, n (%) | Genotype frequencies, n (%) | |||||||||
| Author | Participants | Disease status | Study size | HWE | A | G | AA | AG | GG | |
| Trabace 2002 (20) | all | controls | 101 | 0.404 | 124 (61.4) | 78 (38.6) | 40 (39.6) | 44 (43.6) | 17 (16.8) | |
| migraine | 77 | 0.546 | 115 (74.7) | 39 (25.3) | 44 (57.1) | 27 (35.1) | 6 (7.8) | |||
| MA | 30 | 0.106 | 41 (68.3) | 19 (31.7) | 16 (53.3) | 9 (30.0) | 5 (16.7) | |||
| MO | 47 | 0.656 | 74 (78.72) | 20 (21.28) | 28 (59.57) | 18 (38.30) | 1 (2.13) | |||
| females | controls | 43 | 0.391 | 67 (77.9) | 19 (22.1) | 27 (62.8) | 13 (30.2) | 3 (7.0) | ||
| migraine | 60 | 0.345 | 87 (72.5) | 33 (27.5) | 33 (55.0) | 21 (35.0) | 6 (10.0) | |||
| MA | 25 | 0.230 | 31 (62.0) | 19 (38.0) | 11 (44.0) | 9 (36.0) | 5 (20.0) | |||
| MO | 35 | 1.000 | 56 (80.0) | 14 (20.0) | 22 (62.9) | 12 (34.3) | 1 (2.9) | |||
| males | controls | 58 | 0.328 | 84 (72.4) | 32 (27.6) | 32 (55.2) | 20 (34.5) | 6 (10.3) | ||
| migraine | 17 | 1.000 | 28 (82.4) | 6 (17.6) | 11 (64.7) | 6 (35.3) | 0 (0.0) | |||
| MA | 5 | --- | 10 (100.0) | 0 (0.0) | 5 (100.0) | 0 (0.0) | 0 (0.0) | |||
| MO | 12 | 0.536 | 18 (75.0) | 6 (25.0) | 6 (50.0) | 6 (50.0) | 0 (0.0) | |||
| Lee 2007 (21) | all/females | controls | 382 | 0.915 | 439 (57.5) | 325 (42.5) | 125 (32.7) | 189 (49.5) | 68 (17.8) | |
| migraine | 439 | 0.070 | 467 (53.2) | 411 (46.8) | 134 (30.5) | 199 (45.3) | 106 (24.1) | |||
| MA | 65 | 0.803 | 69 (53.1) | 61 (46.9) | 19 (29.2) | 31 (47.7) | 15 (23.1) | |||
| MO | 327 | 0.118 | 347 (53.1) | 307 (46.9) | 99 (30.3) | 149 (45.6) | 79 (24.2) | |||
| Asuni 2009 (19) | all | controls | 278 | 0.597 | 482 (86.7) | 74 (13.3) | 210 (75.5) | 62 (22.3) | 6 (2.2) | |
| MO | 299 | 1.000 | 488 (81.6) | 110 (18.4) | 199 (66.6) | 90 (30.1) | 10 (3.3) | |||
| females | controls | 144 | 1.000 | 244 (84.7) | 44 (15.3) | 103 (71.5) | 38 (26.4) | 3 (2.1) | ||
| MO | 261 | 1.000 | 429 (82.2) | 93 (17.8) | 176 (67.4) | 77 (29.5) | 8 (3.1) | |||
| males | controls | 134 | 0.207 | 238 (88.8) | 30 (11.2) | 107 (79.9) | 24 (17.9) | 3 (2.2) | ||
| MO | 38 | 1.000 | 59 (77.6) | 17 (22.4) | 23 (60.5) | 13 (34.2) | 2 (5.3) | |||
| Schürks 2009 (17) | all/females | controls | 19,269 | 0.557 | 25,570 (66.4) | 12,968 (33.6) | 8501 (44.1) | 8568 (44.5) | 2200 (11.4) | |
| migraine | 4332 | 0.564 | 5738 (66.2) | 2926 (33.8) | 1891 (43.7) | 1956 (45.2) | 485 (11.2) | |||
| MA | 1213 | 0.315 | 1597 (65.8) | 829 (34.2) | 517 (42.6) | 563 (46.4) | 133 (11.0) | |||
| MO | 1824 | 0.528 | 2420 (66.3) | 1228 (33.7) | 809 (44.4) | 802 (44.0) | 213 (11.7) | |||
| Ghosh 2010 (14) | all | controls | 216 | 0.858 | 328 (75.9) | 104 (24.1) | 125 (57.9) | 78 (36.1) | 13 (6.0) | |
| migraine | 216 | 0.354 | 328 (75.9) | 104 (24.1) | 127 (58.8) | 74 (34.3) | 15 (6.9) | |||
| MA | 84 | 0.162 | 125 (74.4) | 43 (25.6) | 49 (58.3) | 27 (32.1) | 8 (9.5) | |||
| MO | 132 | 1.000 | 203 (76.9) | 61 (23.1) | 78 (59.1) | 47 (35.6) | 7 (5.3) | |||
| females | controls | 152 | 0.467 | 241 (79.3) | 63 (20.7) | 97 (63.8) | 47 (30.9) | 8 (5.3) | ||
| migraine | 152 | 0.359 | 234 (77.0) | 70 (23.0) | 92 (60.5) | 50 (32.9) | 10 (6.6) | |||
| MA | 63 | 0.092 | 96 (76.2) | 30 (23.8) | 39 (61.9) | 18 (28.6) | 6 (9.5) | |||
| MO | 89 | 1.000 | 138 (77.5) | 40 (22.5) | 53 (59.6) | 32 (36.0) | 4 (4.5) | |||
| males | controls | 64 | 0.563 | 87 (68.0) | 41 (32.0) | 28 (43.8) | 31 (48.4) | 5 (7.8) | ||
| migraine | 64 | 0.753 | 94 (73.4) | 34 (26.6) | 35 (54.7) | 24 (37.5) | 5 (7.8) | |||
| MA | 21 | 1.000 | 29 (69.0) | 13 (31.0) | 10 (47.6) | 9 (42.9) | 2 (9.5) | |||
| MO | 43 | 0.681 | 65 (75.6) | 21 (24.4) | 25 (58.1) | 15 (34.9) | 3 (7.0) | |||
| Pappa 2010 (22) | all | controls | 178 | 0.503 | 279 (78.4) | 77 (21.6) | 111 (62.4) | 57 (32.0) | 10 (5.6) | |
| MO | 103 | 0.747 | 167 (81.1) | 39 (18.9) | 68 (66.0) | 31 (30.1) | 4 (3.9) | |||
| females | controls | 60 | 0.709 | 95 (79.2) | 25 (20.8) | 38 (63.3) | 19 (31.7) | 3 (5.0) | ||
| MO | 57 | 1.000 | 94 (82.5) | 20 (17.5) | 38 (66.6) | 18 (31.6) | 1 (1.8) | |||
| males | controls | 118 | 0.590 | 184 (78.0) | 52 (22.0) | 73 (61.9) | 38 (32.2) | 7 (5.9) | ||
| MO | 46 | 0.366 | 73 (79.3) | 19 (20.7) | 30 (65.2) | 13 (28.3) | 3 (6.5) | |||
| TNF-alpha -238G>A (rs361525) polymorphism | ||||||||||
| - | Allele frequencies, n (%) | Genotype frequencies, n (%) | ||||||||
| Author | Participants | Disease status | Study size | HWE | G | A | GG | GA | AA | |
| Schürks 2009 (17) | all/females | controls | 20,425 | 0.006 | 38,780 (94.9) | 2070 (5.1) | 18,427 (90.2) | 1926 (9.4) | 72 (0.4) | |
| migraine | 4577 | 0.431 | 8705 (95.1) | 449 (4.9) | 4136 (90.4) | 433 (9.5) | 8 (0.2) | |||
| MA | 1275 | 0.763 | 2422 (95.0) | 128 (5.0) | 1149 (90.1) | 124 (9.7) | 2 (0.2) | |||
| MO | 1951 | 0.784 | 3730 (95.6) | 172 (4.4) | 1783 (91.4) | 164 (8.4) | 4 (0.2) | |||
| Pappa 2010 (22) | all | controls | 178 | 1.000 | 348 (97.8) | 8 (2.2) | 170 (95.5) | 8 (4.5) | 0 (0.0) | |
| MO | 103 | 1.000 | 199 (96.6) | 7 (3.4) | 96 (93.2) | 7 (6.8) | 0 (0.0) | |||
| females | controls | 60 | 1.000 | 119 (99.2) | 1 (0.8) | 59 (98.3) | 1 (1.7) | 0 (0.0) | ||
| MO | 57 | 1.000 | 111 (97.4) | 3 (2.6) | 54 (94.7) | 3 (5.3) | 0 (0.0) | |||
| males | controls | 118 | 1.000 | 229 (97.0) | 7 (3.0) | 111 (94.1) | 7 (5.9) | 0 (0.0) | ||
| MO | 46 | 1.000 | 88 (95.7) | 4 (4.3) | 42 (91.3) | 4 (8.7) | 0 (0.0) | |||
| TNF-alpha -376G>A (rs1800750) polymorphism | ||||||||||
| Allele frequencies, n (%) | Genotype frequencies, n (%) | |||||||||
| Author | Gender | Disease status | Study size | HWE | G | A | GG | GA | AA | |
| Schürks 2009 (17) | all/females | controls | 20,425 | 0.002 | 40,283 (98.6) | 567 (1.4) | 19,869 (97.3) | 545 (2.7) | 11 (0.1) | |
| migraine | 4578 | 0.307 | 9012 (98.4) | 144 (1.6) | 4436 (96.9) | 140 (3.1) | 2 (0.04) | |||
| MA | 1276 | 0.374 | 2504 (98.1) | 48 (1.9) | 1229 (96.3) | 46 (3.6) | 1 (0.1) | |||
| MO | 1951 | 1.000 | 3851 (98.7) | 51 (1.3) | 1900 (97.4) | 51 (2.6) | 0 (0.0) | |||
| Pappa 2010 (22) | all | controls | 178 | --- | 356 (100.0) | 0 (0.0) | 178 (100.0) | 0 (0.0) | 0 (0.0) | |
| MO | 103 | 1.000 | 205 (99.5) | 1 (0.5) | 102 (99.0) | 1 (1.0) | 0 (0.0) | |||
| females | controls | 60 | --- | 120 (100) | 0 (0.0) | 60 (100) | 0 (0.0) | 0 (0.0) | ||
| MO | 57 | 1.000 | 113 (99.1) | 1 (0.9) | 56 (98.2) | 1 (1.8) | 0 (0.0) | |||
| males | controls | 118 | --- | 236 (100) | 0 (0.0) | 118 (100) | 0 (0.0) | 0 (0.0) | ||
| MO | 46 | --- | 92 (100) | 0 (0.0) | 46 (100) | 0 (0.0) | 0 (0.0) | |||
TNF: tumor necrosis factor; HWE: p value from exact test for the Hardy-Weinberg Equilibrium; MA: migraine with aura; MO: migraine without aura.
Table 3 summarizes for each of the studies the ORs (95% CI) for the association between the polymorphisms and migraine.
Table 3.
Odds ratios (95% confidence intervals) for the association between the investigated polymorphisms and migraine
| TNF-alpha -308G>A (rs1800629) polymorphism | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| additive | dominant | recessive | |||||||
| Author | Participants | Disease status | Study size | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value |
| Trabace 2002 (20) | all | controls | 101 | Referent | Referent | Referent | |||
| migraine | 79 | 1.176 (0.541–2.559) | 0.682 | 1.465 (0.610–3.523) | 0.393 | --- | --- | ||
| MA | 32 | 1.665 (0.671–4.131) | 0.272 | 2.291 (0.805–6.521) | 0.120 | --- | --- | ||
| MO | 47 | 0.841 (0.314–2.253) | 0.730 | 0.974 (0.318–2.982) | 0.963 | --- | --- | ||
| females | controls | 45 | Referent | Referent | Referent | ||||
| migraine | 62 | 1.457 (0.494–4.294) | 0.495 | 1.970 (0.576–6.738) | 0.280 | --- | --- | ||
| MA | 27 | 1.982 (0.599–6.554) | 0.262 | 2.928 (0.744–11.524) | 0.124 | --- | --- | ||
| MO | 35 | 1.026 (0.294–3.573) | 0.968 | 1.323 (0.306–5.708) | 0.708 | --- | --- | ||
| males | controls | 56 | Referent | Referent | Referent | ||||
| migraine | 17 | 0.833 (0.185–3.754) | 0.812 | 0.933 (0.175–4.980) | 0.936 | --- | --- | ||
| MA | 5 | 1.368 (0.183–10.201) | 0.760 | 1.750 (0.170–17.987) | 0.638 | --- | --- | ||
| MO | 12 | 0.607 (0.081–4.543) | 0.627 | 0.636 (0.071–5.714) | 0.687 | --- | --- | ||
| Rainero 2004 (16) | all | controls | 306 | Referent | Referent | Referent | |||
| migraine | 299 | 0.365 (0.250–0.531) | <0.0001 | 0.351 (0.235–0.525) | <0.0001 | 0.090 (0.012–0.701) | 0.022 | ||
| MA | 38 | 0.694 (0.346–1.389) | 0.302 | 0.747 (0.349–1.598) | 0.452 | --- | --- | ||
| MO | 261 | 0.323 (0.214–0.487) | <0.0001 | 0.303 (0.196–0.468) | <0.0001 | 0.103 (0.013–0.804) | 0.030 | ||
| females | controls | 231 | Referent | Referent | Referent | ||||
| migraine | 215 | 0.339 (0.215–0.537) | <0.0001 | 0.337 (0.209–0.545) | <0.0001 | --- | --- | ||
| males | controls | 75 | Referent | Referent | Referent | ||||
| migraine | 84 | 0.415 (0.215–0.803) | 0.009 | 0.377 (0.179–0.794) | 0.010 | 0.214 (0.023–1.957) | 0.172 | ||
| Herken 2005 (23) | all | controls | 62 | Referent | Referent | Referent | |||
| migraine | 60 | 0.802 (0.297–2.165) | 0.664 | 0.654 (0.218–1.966) | 0.450 | --- | --- | ||
| MA | 40 | 0.654 (0.187–2.287) | 0.507 | 0.654 (0.187–2.287) | 0.507 | --- | --- | ||
| MO | 20 | 1.033 (0.283–3.766) | 0.961 | 0.655 (0.129–3.316) | 0.609 | --- | --- | ||
| Mazaheri 2006 (15) | all | controls | 183 | Referent | Referent | Referent | |||
| MO | 221 | 3.166 (2.113–4.745) | <0.0001 | 3.520 (2.298–5.393) | <0.0001 | 1.962 (0.500–7.697) | 0.334 | ||
| females | controls | 123 | Referent | Referent | Referent | ||||
| MO | 196 | 4.278 (2.655–6.895) | <0.0001 | 4.271 (2.620–6.963) | <0.0001 | --- | --- | ||
| males | controls | 60 | Referent | Referent | Referent | ||||
| MO | 25 | 1.415 (0.599–3.346) | 0.429 | 1.966 (0.715–5.406) | 0.190 | --- | --- | ||
| Lee 2007 (21) | all/females | controls | 382 | Referent | Referent | Referent | |||
| migraine | 439 | 1.178 (0.798–1.740) | 0.410 | 1.263 (0.836–1.910) | 0.268 | 0.289 (0.030–2.787) | 0.283 | ||
| MA | 65 | 1.390 (0.711–2.716) | 0.335 | 1.565 (0.761–3.216) | 0.223 | --- | --- | ||
| MO | 327 | 1.151 (0.759–1.745) | 0.509 | 1.226 (0.786–1.912) | 0.369 | 0.388 (0.040–3.747) | 0.413 | ||
| Asuni 2009 (19) | all | controls | 278 | Referent | Referent | Referent | |||
| MO | 299 | 0.864 (0.512–1.458) | 0.583 | 0.852 (0.491–1.479) | 0.570 | 0.929 (0.058–14.932) | 0.959 | ||
| females | controls | 144 | Referent | Referent | Referent | ||||
| MO | 261 | 0.916 (0.475–1.766) | 0.792 | 0.871 (0.441–1.719) | 0.690 | --- | --- | ||
| males | controls | 134 | Referent | Referent | Referent | ||||
| MO | 38 | 0.707 (0.205–2.436) | 0.583 | 0.735 (0.200–2.703) | 0.643 | --- | --- | ||
| Schürks 2009 (17) | all/females | controls | 20,425 | Referent | Referent | Referent | |||
| migraine | 4577 | 1.029 (0.969–1.092) | 0.356 | 1.046 (0.976–1.120) | 0.201 | 0.941 (0.775–1.143) | 0.542 | ||
| MA | 1275 | 1.136 (1.025–1.258) | 0.015 | 1.166 (1.036–1.313) | 0.011 | 1.124 (0.817–1.544) | 0.473 | ||
| MO | 1951 | 0.975 (0.893–1.064) | 0.568 | 0.968 (0.875–1.070) | 0.524 | 0.993 (0.754–1.308) | 0.959 | ||
| Ghosh 2010 (14) | all | controls | 216 | Referent | Referent | Referent | |||
| migraine | 216 | 1.683 (0.995–2.846) | 0.052 | 1.790 (1.045–3.065) | 0.034 | --- | --- | ||
| MA | 84 | 2.054 (1.084–3.893) | 0.027 | 2.234 (1.155–4.321) | 0.017 | --- | --- | ||
| MO | 132 | 1.436 (0.788–2.617) | 0.238 | 1.528 (0.823–2.838) | 0.180 | --- | --- | ||
| females | controls | 152 | Referent | Referent | Referent | ||||
| migraine | 152 | 1.750 (0.950–3.222) | 0.073 | 1.907 (1.015–3.583) | 0.045 | --- | --- | ||
| MA | 63 | 2.436 (1.191–4.983) | 0.015 | 2.751 (1.309–5.781) | 0.008 | --- | --- | ||
| MO | 89 | 1.284 (0.624–2.639) | 0.497 | 1.390 (0.654–2.952) | 0.392 | --- | --- | ||
| males | controls | 64 | Referent | Referent | Referent | ||||
| migraine | 64 | 1.508 (0.536–4.245) | 0.437 | 1.508 (0.536–4.245) | 0.437 | --- | --- | ||
| MA | 21 | 0.857 (0.164–4.486) | 0.855 | 0.857 (0.164–4.486) | 0.855 | --- | --- | ||
| MO | 43 | 1.861 (0.621–5.581) | 0.268 | 1.861 (0.621–5.581) | 0.268 | --- | --- | ||
| Yilmaz 2010 (18) | all | controls | 96 | Referent | Referent | Referent | |||
| MO | 67 | 3.321 (1.785–6.178) | 0.000 | 3.768 (1.849–7.676) | 0.0003 | 11.074 (1.330–92.207) | 0.026 | ||
| females | controls | 83 | Referent | Referent | Referent | ||||
| MO | 57 | 3.521 (1.772–6.996) | 0.000 | 4.206 (1.909–9.268) | 0.0004 | 9.644 (1.129–82.419) | 0.038 | ||
| males | controls | 13 | Referent | Referent | Referent | ||||
| MO | 10 | 2.473 (0.543–11.264) | 0.242 | 2.250 (0.407–12.439) | 0.353 | --- | --- | ||
| Pappa 2010 (22) | all | controls | 178 | Referent | Referent | Referent | |||
| MO | 103 | 0.667 (0.348–1.276) | 0.221 | 0.691 (0.351–1.362) | 0.286 | --- | --- | ||
| females | controls | 60 | Referent | Referent | Referent | ||||
| MO | 57 | 0.704 (0.287–1.725) | 0.443 | 0.750 (0.289–1.944) | 0.554 | --- | --- | ||
| males | controls | 118 | Referent | Referent | Referent | ||||
| MO | 46 | 0.555 (0.202–1.525) | 0.254 | 0.563 (0.199–1.596) | 0.280 | --- | --- | ||
| TNF-beta 252A>G (rs909253) polymorphism | |||||||||
| additive | dominant | recessive | |||||||
| Author | Participants | Disease status | Study size | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value |
| Trabace 2002 (20) | all | controls | 101 | Referent | Referent | Referent | |||
| migraine | 77 | 0.564 (0.359–0.885) | 0.013 | 0.492 (0.269–0.898) | 0.021 | 0.418 (0.156–1.116) | 0.082 | ||
| MA | 30 | 0.763 (0.427–1.362) | 0.360 | 0.574 (0.253–1.304) | 0.185 | 0.988 (0.331–2.947) | 0.983 | ||
| MO | 47 | 0.438 (0.246–0.780) | 0.005 | 0.445 (0.220–0.901) | 0.025 | 0.107 (0.014–0.833) | 0.033 | ||
| females | controls | 43 | Referent | Referent | Referent | ||||
| migraine | 60 | 1.296 (0.701–2.397) | 0.408 | 1.381 (0.620–3.075) | 0.430 | 1.481 (0.349–6.284) | 0.594 | ||
| MA | 25 | 1.925 (0.937–3.954) | 0.074 | 2.148 (0.788–5.855) | 0.135 | 3.332 (0.722–15.367) | 0.123 | ||
| MO | 35 | 0.886 (0.413–1.898) | 0.755 | 0.997 (0.396–2.510) | 0.995 | 0.392 (0.039–3.946) | 0.427 | ||
| males | controls | 58 | Referent | Referent | Referent | ||||
| migraine | 17 | 0.584 (0.226–1.507) | 0.266 | 0.671 (0.219–2.060) | 0.486 | --- | --- | ||
| MA | 5 | --- | --- | --- | --- | --- | --- | ||
| MO | 12 | 0.882 (0.330–2.353) | 0.801 | 1.231 (0.355–4.271) | 0.744 | --- | --- | ||
| Lee 2007 (21) | all/females | controls | 382 | Referent | Referent | Referent | |||
| migraine | 439 | 1.180 (0.975–1.430) | 0.090 | 1.107 (0.824–1.487) | 0.499 | 1.470 (1.045–2.068) | 0.027 | ||
| MA | 65 | 1.195 (0.822–1.737) | 0.351 | 1.178 (0.662–2.094) | 0.578 | 1.386 (0.735–2.611) | 0.313 | ||
| MO | 327 | 1.188 (0.966–1.462) | 0.103 | 1.120 (0.815–1.540) | 0.486 | 1.471 (1.022–2.118) | 0.038 | ||
| Asuni 2009 (19) | all | controls | 278 | Referent | Referent | Referent | |||
| MO | 299 | 1.461 (1.061–2.013) | 0.020 | 1.552 (1.078–2.233) | 0.018 | 1.569 (0.562–4.374) | 0.390 | ||
| females | controls | 144 | Referent | Referent | Referent | ||||
| MO | 261 | 1.205 (0.812–1.787) | 0.355 | 1.213 (0.778–1.893) | 0.395 | 1.486 (0.388–5.691) | 0.563 | ||
| males | controls | 134 | Referent | Referent | Referent | ||||
| MO | 38 | 2.155 (1.128–4.116) | 0.020 | 2.585 (1.190–5.612) | 0.016 | 2.426 (0.390–15.075) | 0.342 | ||
| Schürks 2009 (17) | all/females | controls | 19,269 | Referent | Referent | Referent | |||
| migraine | 4332 | 1.005 (0.957–1.056) | 0.828 | 1.019 (0.954–1.089) | 0.577 | 0.978 (0.881–1.086) | 0.680 | ||
| MA | 1213 | 1.024 (0.939–1.116) | 0.597 | 1.063 (0.945–1.195) | 0.309 | 0.955 (0.794–1.150) | 0.630 | ||
| MO | 1824 | 1.001 (0.931–1.075) | 0.988 | 0.990 (0.899–1.091) | 0.846 | 1.026 (0.883–1.192) | 0.736 | ||
| Ghosh 2010 (14) | all | controls | 216 | Referent | Referent | Referent | |||
| migraine | 216 | 1.000 (0.736–1.358) | 1.000 | 0.963 (0.657–1.411) | 0.845 | 1.165 (0.541–2.511) | 0.697 | ||
| MA | 84 | 1.080 (0.724–1.612) | 0.705 | 0.981 (0.589–1.636) | 0.942 | 1.644 (0.656–4.123) | 0.289 | ||
| MO | 132 | 0.948 (0.661–1.359) | 0.772 | 0.951 (0.613–1.476) | 0.823 | 0.874 (0.340–2.251) | 0.781 | ||
| females | controls | 152 | Referent | Referent | Referent | ||||
| migraine | 152 | 1.135 (0.781–1.648) | 0.507 | 1.150 (0.723–1.829) | 0.555 | 1.268 (0.486–3.304) | 0.628 | ||
| MA | 63 | 1.175 (0.734–1.880) | 0.502 | 1.085 (0.592–1.991) | 0.791 | 1.895 (0.630–5.705) | 0.256 | ||
| MO | 89 | 1.106 (0.710–1.722) | 0.655 | 1.198 (0.700–2.050) | 0.510 | 0.847 (0.248–2.897) | 0.791 | ||
| males | controls | 64 | Referent | Referent | Referent | ||||
| migraine | 64 | 0.759 (0.436–1.320) | 0.329 | 0.644 (0.321–1.294) | 0.217 | 1.000 (0.275–3.637) | 1.000 | ||
| MA | 21 | 0.947 (0.431–2.079) | 0.892 | 0.856 (0.318–2.299) | 0.757 | 1.242 (0.223–6.932) | 0.805 | ||
| MO | 43 | 0.672 (0.356–1.270) | 0.221 | 0.560 (0.256–1.224) | 0.146 | 0.885 (0.200–3.913) | 0.872 | ||
| Pappa 2010 (22) | all | controls | 178 | Referent | Referent | Referent | |||
| MO | 103 | 0.852 (0.559–1.299) | 0.457 | 0.853 (0.513–1.417) | 0.539 | 0.679 (0.207–2.222) | 0.522 | ||
| females | controls | 60 | Referent | Referent | Referent | ||||
| MO | 57 | 0.805 (0.416–1.559) | 0.520 | 0.864 (0.404–1.849) | 0.706 | 0.339 (0.034–3.361) | 0.356 | ||
| males | controls | 118 | Referent | Referent | Referent | ||||
| MO | 46 | 0.927 (0.525–1.636) | 0.793 | 0.865 (0.425–1.762) | 0.690 | 1.106 (0.273–4.475) | 0.887 | ||
| TNF-alpha -238G>A (rs361525) polymorphism | |||||||||
| additive | dominant | recessive | |||||||
| Author | Participants | Disease status | Study size | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value |
| Schürks 2009 (17) | all/females | controls | 20,425 | Referent | Referent | Referent | |||
| migraine | 4577 | 0.967 (0.872–1.073) | 0.528 | 0.983 (0.882–1.096) | 0.763 | 0.495 (0.238–1.028) | 0.059 | ||
| MA | 1275 | 0.990 (0.826–1.187) | 0.916 | 1.011 (0.837–1.223) | 0.907 | 0.444 (0.109–1.812) | 0.258 | ||
| MO | 1951 | 0.866 (0.740–1.014) | 0.074 | 0.869 (0.737–1.025) | 0.095 | 0.581 (0.212–1.591) | 0.291 | ||
| Pappa 2010 (22) | all | controls | 178 | Referent | Referent | Referent | |||
| MO | 103 | 1.549 (0.545–4.405) | 0.411 | 1.549 (0.545–4.405) | 0.411 | --- | --- | ||
| females | controls | 60 | Referent | Referent | Referent | ||||
| MO | 57 | 3.278 (0.331–32.467) | 0.310 | 3.278 (0.331–32.467) | 0.310 | --- | --- | ||
| males | controls | 118 | Referent | Referent | Referent | ||||
| MO | 46 | 1.510 (0.420–5.425) | 0.527 | 1.510 (0.420–5.425) | 0.527 | --- | --- | ||
| TNF-alpha -376G>A (rs1800750) polymorphism | |||||||||
| additive | dominant | recessive | |||||||
| Author | Participants | Disease status | Study size | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value |
| Schürks 2009 (17) | all/females | controls | 20,425 | Referent | Referent | Referent | |||
| migraine | 4578 | 1.132 (0.943–1.358) | 0.182 | 1.144 (0.949–1.379) | 0.159 | 0.811 (0.180–3.661) | 0.786 | ||
| MA | 1276 | 1.352 (1.009–1.811) | 0.044 | 1.368 (1.010–1.852) | 0.043 | 1.458 (0.188–11.286) | 0.718 | ||
| MO | 1951 | 0.942 (0.708–1.253) | 0.682 | 0.960 (0.718–1.283) | 0.782 | --- | --- | ||
| Pappa 2010 (22) | all | controls | 178 | Referent | Referent | Referent | |||
| MO | 103 | --- | --- | --- | --- | --- | --- | ||
| females | controls | 60 | Referent | Referent | Referent | ||||
| MO | 57 | --- | --- | --- | --- | --- | --- | ||
| males | controls | 118 | Referent | Referent | Referent | ||||
| MO | 46 | --- | --- | --- | --- | --- | --- | ||
TNF: tumor necrosis factor; OR: odds ratio; MA: migraine with aura; MO: migraine without aura.
Tables 4 and 5 summarize the pooled effect estimates, measures of heterogeneity, and tests for small study effects for the TNF-alpha -308G>A and TNF-beta 252A>G polymorphisms.
Table 4.
Association between the TNF-alpha -308G>A (rs1800629) polymorphisms and migraine from random effects model, heterogeneity, and small study effects
| Migraine | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Heterogeneity | Small study effects p-value |
||||||||
| Genetic model |
Participants | No of studies |
Pooled OR (95% CI) |
Q | df | p-value |
I2 in % |
Begg test | Egger’s test |
| additive | All (14–23) | 10 | 1.16 (0.80–1.68) | 78.6 | 9 | <0.0001 | 88.5 | 0.66 | 0.68 |
| European descent (16, 17, 19, 20, 22) | 5 | 0.75 (0.46–1.22) | 30.3 | 4 | <0.0001 | 86.8 | 1.00 | 0.30 | |
| Asian descent (14, 21) | 2 | 1.35 (0.96–1.89) | 1.1 | 1 | 0.29 | 12.5 | 0.32 | ---- | |
| Heterogeneous descent (15, 18, 23) | 3 | 2.32 (1.18–4.56) | 6.7 | 2 | 0.04 | 70.3 | 0.12 | 0.37 | |
| Females | 9 | 1.29 (0.83–2.00) | 73.4 | 8 | <0.0001 | 89.1 | 0.53 | 0.51 | |
| European descent (16, 17, 19, 20, 22) | 5 | 0.77 (0.44–1.32) | 23.4 | 4 | <0.0001 | 82.9 | 1.00 | 0.38 | |
| Asian descent (14, 21) | 2 | 1.34 (0.93–1.92) | 1.2 | 1 | 0.28 | 12.8 | 0.32 | ---- | |
| Heterogeneous descent (15, 18) | 2 | 4.02 (2.71–5.94) | 0.2 | 1 | 0.65 | 0.0 | 0.32 | ---- | |
| Males (14–16, 18–20, 22) | 7 | 0.86 (0.52–1.42) | 9.6 | 6 | 0.14 | 37.6 | 0.18 | 0.18 | |
| European descent (16, 19, 20, 22) | 4 | 0.51 (0.32–0.83) | 1.1 | 3 | 0.78 | 0.0 | 0.04 | 0.002 | |
| Asian descent (14) | 1 | 1.51 (0.54–4.25) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Heterogeneous descent (15, 18) | 2 | 1.62 (0.77–3.43) | 0.4 | 1 | 0.53 | 0.0 | 0.32 | ---- | |
| dominant | All (14–23) | 10 | 1.21 (0.81–1.80) | 79.5 | 9 | <0.0001 | 88.7 | 0.66 | 0.65 |
| European descent (16, 17, 19, 20, 22) | 5 | 0.77 (0.46–1.29) | 29.8 | 4 | <0.0001 | 86.6 | 1.00 | 0.35 | |
| Asian descent (14, 21) | 2 | 1.44 (1.03–2.00) | 1.0 | 1 | 0.31 | 1.5 | 0.32 | ---- | |
| Heterogeneous descent (15, 18, 23) | 3 | 2.35 (1.02–5.42) | 8.3 | 2 | 0.02 | 75.9 | 0.12 | 0.41 | |
| Females (14–22) | 9 | 1.36 (0.86–2.15) | 70.8 | 8 | <0.0001 | 88.7 | 0.53 | 0.46 | |
| European descent (16, 17, 19, 20, 22) | 5 | 0.79 (0.45–1.40) | 22.7 | 4 | <0.0001 | 82.4 | 1.00 | 0.44 | |
| Asian descent (14, 21) | 2 | 1.45 (0.99–2.11) | 1.2 | 1 | 0.28 | 12.9 | 0.32 | ---- | |
| Heterogeneous descent (15, 18) | 2 | 4.25 (2.81–6.44) | 0.0 | 1 | 0.97 | 0.0 | 0.32 | ---- | |
| Males (14–16, 18–20, 22) | 7 | 0.90 (0.51–1.58) | 10.3 | 6 | 0.11 | 41.6 | 0.65 | 0.20 | |
| European descent (16, 19, 20, 22) | 4 | 0.51 (0.30–0.85) | 1.5 | 3 | 0.69 | 0.0 | 0.04 | 0.01 | |
| Asian descent (14) | 1 | 1.51 (0.54–4.25) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Heterogeneous descent (15, 18) | 2 | 2.04 (0.85–4.86) | 0.0 | 1 | 0.89 | 0.0 | ---- | ---- | |
| recessive | All (15–19, 21) | 6* | 0.95 (0.36–2.52) | 12.5 | 5 | 0.03 | 59.9 | 0.85 | 0.99 |
| European descent (16, 17, 19) | 3* | 0.50 (0.11–2.23) | 5.1 | 2 | 0.08 | 60.6 | 0.60 | 0.51 | |
| Asian descent (21) | 1* | 0.29 (0.03–2.79) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Heterogeneous descent (15, 18) | 2* | 3.83 (0.73–19.95) | 1.8 | 1 | 0.18 | 44.7 | 0.32 | ---- | |
| Females (17, 18, 21) | 3* | 1.28 (0.29–5.72) | 5.6 | 2 | 0.06 | 64.0 | 0.60 | 0.79 | |
| European descent (17) | 1* | 0.94 (0.78–1.14) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Asian descent (21) | 1* | 0.29 (0.03–2.79) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Heterogeneous descent (18) | 1* | 9.64 (1.13–82.42) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Males (16) | 1* | 0.21 (0.02–1.96) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Migraine with aura | |||||||||
| Heterogeneity | Small study effects p-value |
||||||||
| Genetic model |
Participants | No of studies |
Pooled OR (95% CI) |
Q | df | p-value |
I2 in % |
Begg test | Egger’s test |
| additive | All (14, 16, 17, 20, 21, 23) | 6 | 1.20 (0.92–1.55) | 7.0 | 5 | 0.22 | 28.3 | 0.57 | 0.80 |
| European descent (16, 17, 20) | 3 | 1.10 (0.83–1.46) | 2.6 | 2 | 0.27 | 23.1 | 0.60 | 0.86 | |
| Asian descent (14, 21) | 2 | 1.71 (1.07–2.71) | 0.7 | 1 | 0.41 | 0.0 | 0.32 | ---- | |
| Heterogeneous descent (23) | 1 | 0.65 (0.19–2.29) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Females (14, 17, 20, 21) | 4 | 1.42 (0.98–2.07) | 5.3 | 3 | 0.15 | 43.8 | 0.17 | 0.13 | |
| European descent (17, 20) | 2 | 1.14 (1.03–1.26) | 0.8 | 1 | 0.36 | 0.0 | 0.32 | ---- | |
| Asian descent (14, 21) | 2 | 1.81 (1.05–3.14) | 1.3 | 1 | 0.26 | 20.5 | 0.32 | ---- | |
| Males (14, 20) | 2 | 1.04 (0.29–3.71) | 0.1 | 1 | 0.73 | 0.0 | 0.32 | ---- | |
| European descent (20) | 1 | 1.37 (0.18–10.20) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Asian descent (14) | 1 | 0.86 (0.16–4.87) | ---- | ---- | ---- | ---- | ---- | ---- | |
| dominant | All (14, 16, 17, 20, 21, 23) | 6 | 1.29 (0.94–1.78) | 8.0 | 5 | 0.16 | 37.5 | 0.85 | 0.61 |
| European descent (16, 17, 20) | 3 | 1.16 (0.80–1.68) | 2.9 | 2 | 0.23 | 31.4 | 0.60 | 0.95 | |
| Asian descent (14, 21) | 2 | 1.90 (1.17–3.09) | 0.5 | 1 | 0.48 | 0.0 | 0.32 | ---- | |
| Heterogeneous descent (23) | 1 | 0.65 (0.19–2.29) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Females (14, 17, 20, 21) | 4 | 1.65 (1.02–2.68) | 7.2 | 3 | 0.07 | 58.1 | 0.17 | 0.08 | |
| European descent (17, 20) | 2 | 1.42 (0.68–2.98) | 1.7 | 1 | 0.19 | 41.9 | 0.32 | ---- | |
| Asian descent (14, 21) | 2 | 2.06 (1.19–3.58) | 1.1 | 1 | 0.29 | 12.4 | 0.32 | ---- | |
| Males (14, 20) | 2 | 1.09 (0.28–4.20) | 0.2 | 1 | 0.62 | 0.0 | 0.32 | ---- | |
| European descent (20) | 1 | 1.75 (0.17–17.99) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Asian descent (14) | 1 | 0.86 (0.16–4.87) | ---- | ---- | ---- | ---- | ---- | ---- | |
| recessive | All (17) | 1* | 1.12 (0.82–1.54) | ---- | ---- | ---- | ---- | ---- | ---- |
| Migraine without aura | |||||||||
| Heterogeneity | Small study effects p-value |
||||||||
| Genetic model |
Participants | No of studies |
Pooled OR (95% CI) |
Q | df | p-value |
I2 in % |
Begg test | Egger’s test |
| additive | All (14–23) | 10 | 1.11 (0.74–1.67) | 78.6 | 9 | <0.0001 | 88.6 | 0.66 | 0.68 |
| European descent (16, 17, 19, 20, 22) | 5 | 0.68 (0.41–1.13) | 27.6 | 4 | <0.0001 | 85.5 | 1.00 | 0.28 | |
| Asian descent (14, 21) | 2 | 1.24 (0.88–1.74) | 0.4 | 1 | 0.55 | 0.0 | 0.32 | ---- | |
| Heterogeneous descent (15, 18, 23) | 3 | 2.87 (1.86–4.43) | 2.8 | 2 | 0.25 | 28.0 | 0.12 | 0.33 | |
| Females (14, 15, 17–22) | 8 | 1.44 (0.91–2.27) | 49.4 | 7 | <0.0001 | 85.8 | 1.00 | 0.22 | |
| European descent (17, 19, 20, 22) | 4 | 0.97 (0.89–1.06) | 0.5 | 3 | 0.91 | 0.0 | 0.50 | 0.37 | |
| Asian descent (14, 21) | 2 | 1.18 (0.83–1.70) | 0.1 | 1 | 0.80 | 0.0 | 0.32 | ---- | |
| Heterogeneous descent (15, 18) | 2 | 4.02 (2.71–5.94) | 0.2 | 1 | 0.65 | 0.0 | 0.32 | ---- | |
| Males (14, 15, 18–20, 22) | 6 | 1.11 (0.69–1.77) | 4.9 | 5 | 0.43 | 0.0 | 0.57 | 0.89 | |
| European descent (19, 20, 22) | 3 | 0.61 (0.29–1.27) | 0.1 | 2 | 0.96 | 0.0 | 0.60 | 0.81 | |
| Asian descent (14) | 1 | 1.86 (0.62–5.58) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Heterogeneous descent (15, 18) | 2 | 1.62 (0.77–3.43) | 0.4 | 1 | 0.53 | 0.0 | 0.32 | ---- | |
| dominant | All (14–23) | 10 | 1.13 (0.73–1.76) | 80.4 | 9 | <0.0001 | 88.8 | 0.66 | 0.66 |
| European descent (16, 17, 19, 20, 22) | 5 | 0.68 (0.40–1.16) | 26.7 | 4 | <0.0001 | 85.0 | 1.00 | 0.34 | |
| Asian descent (14, 21) | 2 | 1.32 (0.92–1.90) | 0.3 | 1 | 0.57 | 0.0 | 0.32 | ---- | |
| Heterogeneous descent (15, 18, 23) | 3 | 2.94 (1.57–5.50) | 4.0 | 2 | 0.13 | 50.4 | 0.12 | 0.35 | |
| Females (14, 15, 17–22) | 8 | 1.52 (0.94–2.46) | 47.9 | 7 | <0.0001 | 85.4 | 0.81 | 0.18 | |
| European descent (17, 19, 20, 22) | 4 | 0.97 (0.87–1.07) | 0.5 | 3 | 0.91 | 0.0 | 1.00 | 0.71 | |
| Asian descent (14, 21) | 2 | 1.27 (0.86–1.86) | 0.1 | 1 | 0.78 | 0.0 | 0.32 | ---- | |
| Heterogeneous descent (15, 18) | 2 | 4.25 (2.81–6.44) | 0.0 | 1 | 0.97 | 0.0 | 0.32 | ---- | |
| Males (14, 15, 18–20, 22) | 6 | 1.18 (0.71–1.97) | 4.9 | 5 | 0.42 | 0.0 | 0.57 | 0.85 | |
| European descent (19, 20, 22) | 3 | 0.63 (0.29–1.34) | 0.1 | 2 | 0.95 | 0.0 | 0.60 | 0.76 | |
| Asian descent (14) | 1 | 1.86 (0.62–5.58) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Heterogeneous descent (15, 18) | 2 | 2.04 (0.85–4.86) | 0.0 | 1 | 0.89 | 0.0 | 0.32 | ---- | |
| recessive | All (15–19, 21) | 6* | 1.03 (0.40–2.62) | 11.2 | 5 | 0.047 | 55.4 | 0.85 | 0.99 |
| European descent (16, 17, 19) | 3* | 0.54 (0.13–2.27) | 4.6 | 2 | 0.10 | 56.1 | 0.60 | 0.50 | |
| Asian descent (21) | 1* | 0.39 (0.04–3.75) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Heterogeneous descent (15, 18) | 2* | 3.83 (0.73–19.95) | 1.8 | 1 | 0.18 | 44.7 | 0.32 | ---- | |
| Females (17, 18, 21) | 3* | 1.39 (0.34–5.70) | 5.0 | 2 | 0.08 | 59.6 | 0.60 | 0.74 | |
| European descent (17) | 1* | 0.99 (0.75–1.31) | ---- | ---- | ---- | ---- | ---- | ---- | |
| Asian descent (21) | 1* | 0.39 (0.04–3.75) | ---- | ---- | ---- | ---- | ---- | ---- | |
OR: odds ratio.
effect estimates for some studies could not be calculated due to small numbers
Table 5.
Association between the TNF-beta 252A>G (rs909253) polymorphisms and migraine from random effects model, heterogeneity, and small study effects
| Migraine | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Heterogeneity | Small study effects p-value |
||||||||||||||||
| Genetic model |
Participants | No of studies |
Pooled OR 95% CI) |
Q | df | p-value |
I2 in % |
Begg test | Egger’s test |
||||||||
| additive | All (14, 17, 19–22) | 6 | 1.02 (0.87–1.21) | 14.7 | 5 | 0.01 | 66.0 | 0.57 | 1.00 | ||||||||
| Females (14, 17, 19–22) | 6 | 1.02 (0.97–1.07) | 4.7 | 5 | 0.46 | 0.0 | 0.85 | 0.22 | |||||||||
| Males (14, 19, 20, 22) | 4 | 1.00 (0.59–1.69) | 7.7 | 3 | 0.05 | 60.8 | 0.50 | 0.86 | |||||||||
| dominant | All (14, 17, 19–22) | 6 | 1.02 (0.84–1.23) | 11.6 | 5 | 0.04 | 56.8 | 0.19 | 0.82 | ||||||||
| Females (14, 17, 19–22) | 6 | 1.03 (0.97–1.10) | 1.8 | 5 | 0.88 | 0.0 | 0.57 | 0.15 | |||||||||
| Males (14, 19, 20, 22) | 4 | 1.01 (0.53–1.94) | 7.9 | 3 | 0.047 | 62.2 | 0.50 | 0.96 | |||||||||
| recessive | All (14, 17, 19–22) | 6 | 1.06 (0.80–1.42) | 9.4 | 5 | 0.10 | 46.6 | 0.85 | 0.89 | ||||||||
| Females (14, 17, 19–22) | 6 | 1.13 (0.89–1.43) | 6.7 | 5 | 0.25 | 24.9 | 0.85 | 0.44 | |||||||||
| Males (14, 19, 22) | 3* | 1.25 (0.54–2.91) | 0.7 | 2 | 0.72 | 0.0 | 0.12 | 0.07 | |||||||||
| Migraine with aura | |||||||||||||||||
| Heterogeneity | Small study effects p-value |
||||||||||||||||
| Genetic model |
Participants | No of studies |
Pooled OR (95% CI) |
Q | df | p-value |
I2 in % |
Begg test | Egger’s test |
||||||||
| additive | All (14, 17, 20, 21) | 4 | 1.03 (0.95–1.12) | 1.7 | 3 | 0.64 | 0.0 | 0.50 | 0.97 | ||||||||
| Females (14, 17, 20, 21) | 4 | 1.09 (0.93–1.28) | 3.7 | 3 | 0.29 | 19.2 | 0.17 | 0.08 | |||||||||
| Males (14) | 1* | 0.95 (0.43–2.08) | --- | --- | --- | --- | --- | --- | |||||||||
| dominant | All (14, 17, 20, 21) | 4 | 1.05 (0.94–1.18) | 2.4 | 3 | 0.50 | 0.0 | 0.17 | 0.44 | ||||||||
| Females (14, 17, 20, 21) | 4 | 1.08 (0.96–1.21) | 2.0 | 3 | 0.58 | 0.0 | 0.17 | 0.25 | |||||||||
| Males (14) | 1* | 0.86 (0.32–2.30) | --- | --- | --- | --- | --- | --- | |||||||||
| recessive | All (14, 17, 20, 21) | 4 | 1.00 (0.84–1.19) | 2.4 | 3 | 0.50 | 0.0 | 0.50 | 0.21 | ||||||||
| Females (14, 17, 20, 21) | 4 | 1.24 (0.82–1.88) | 4.9 | 3 | 0.18 | 38.8 | 0.04 | 0.005 | |||||||||
| Males (14) | 1* | 1.24 (0.22–6.93) | --- | --- | --- | --- | --- | --- | |||||||||
| Migraine without aura | |||||||||||||||||
| Heterogeneity | Small study effects p-value |
||||||||||||||||
| Genetic model |
Participants | No of studies |
Pooled OR (95% CI) |
Q | df | p-value |
I2 in % |
Begg test | Egger’s test |
||||||||
| additive | All (14, 17, 19–22) | 6 | 1.00 (0.83–1.22) | 16.3 | 5 | 0.006 | 69.3 | 0.35 | 0.81 | ||||||||
| Females (14, 17, 19–22) | 6 | 1.02 (0.96–1.09) | 3.8 | 5 | 0.58 | 0.0 | 0.57 | 0.60 | |||||||||
| Males (14, 19, 20, 22) | 4 | 1.05 (0.63–1.78) | 6.9 | 3 | 0.07 | 56.8 | 0.50 | 0.96 | |||||||||
| dominant | All (14, 17, 19–22) | 6 | 1.01 (0.82–1.25) | 11.6 | 5 | 0.04 | 56.9 | 0.35 | 0.86 | ||||||||
| Females (14, 17, 19–22) | 6 | 1.01 (0.93–1.10) | 1.8 | 5 | 0.88 | 0.0 | 0.85 | 0.28 | |||||||||
| Males (14, 19, 20, 22) | 4 | 1.10 (0.55–2.20) | 8.0 | 3 | 0.046 | 62.5 | 1.0 | 0.89 | |||||||||
| recessive | All (14, 17, 19–22) | 6 | 1.07 (0.77–1.50) | 9.4 | 5 | 0.10 | 46.7 | 0.35 | 0.62 | ||||||||
| Females (14, 17, 19–22) | 6 | 1.09 (0.92–1.30) | 5.3 | 5 | 0.38 | 5.5 | 0.85 | 0.78 | |||||||||
| Males (14, 19, 22) | 3* | 1.23 (0.51–3.00) | 0.7 | 2 | 0.69 | 0.0 | 0.60 | 0.31 | |||||||||
effect estimates for some studies could not be calculated due to small numbers
Association between the TNF-alpha -308G>A (rs1800629) polymorphism and migraine
Genotype distributions in control and migraine populations were in HWE in all but one study (Table 2). Five studies suggested an increased risk of migraine assuming additive and dominant models (14, 15, 17, 18, 20) (Table 3). In contrast, one study suggested a reduced risk (16). The effects were stronger among females than males. The remaining four studies did not suggest an association between any of the genotypes and migraine (19, 21–23). Effect estimates for some of the associations assuming a recessive model could not be calculated, due to missing observations for the AA genotype in some studies.
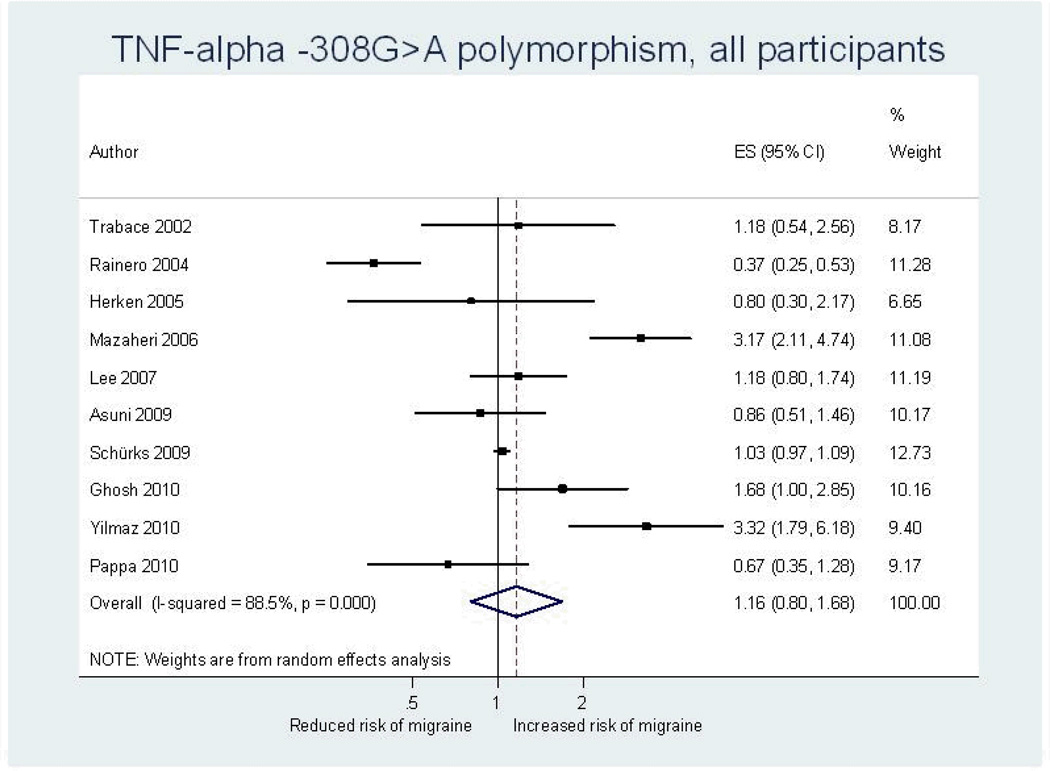
Our overall pooled analyses do not suggest an association between the TNF-alpha -308G>A polymorphism and migraine (Figure 2, Table 4). The pooled OR (95% CI) from an additive model for migraine was 1.16 (0.80–1.68). Effect estimates were similar when assuming a dominant model and lower in recessive models. There was high heterogeneity among all participants (additive model: I2=88.5%). Since meta-regression indicated that country of study origin had a significant modifying effect on the overall pooled OR (European populations: reference; Asian populations: p=0.18; populations of heterogeneous ethnic background: p=0.02) we performed pooled analyses among studies in these populations separately. The effect estimates were lower among Europeans than among Asians, a pattern most pronounced among males. Analyses among populations of heterogeneous descent suggested a significantly increased risk for migraine assuming an additive model (pooled OR=2.32; 95% CI 1.18–4.56), which was driven by a four-fold increased risk among females (pooled OR=4.02; 95% CI 2.71–5.94).
Figure 2.
Odds ratios for the association between the TNF-alpha -308G>A polymorphism and migraine assuming an additive model from individual studies and from the pooled analysis. ES=estimate (indicates odds ratio). CI=confidence interval.
Analyses stratified by clinical phenotype also do not suggest an association between the TNF-alpha -308G>A polymorphism and MA (all participants, additive model: pooled OR=1.20; 95% CI 0.92–1.55) or MO (all participants, additive model: pooled OR=1.11; 95% CI 0.74–1.67) (Table 4). This agrees with results from meta-regression indicating that clinical phenotype is not a significant source of heterogeneity (all p>0.23). While heterogeneity among studies investigating MO was high (all participants, additive model: I2=88.6%), it was low among those investigating MA (all participants, additive model: I2=28.3%). Pooled results of the two studies among Asian populations suggested an increased risk assuming additive and dominant models, specifically for MA and were most pronounced among females assuming a dominant model (pooled OR=2.06; 95% CI 1.19–3.58).
For migraine analyses among males suggested potential small study effects (additive model: Begg’s test: p=0.04; Egger’s test: p=0.002). When considering gender stratified results meta-regression did not indicate that gender significantly impacts pooled results for migraine, MA or MO in additive or dominant models (all p-values ≥0.34). Meta-regression among effect estimates from recessive models could not be performed.
Association between the TNF-beta 252A>G (rs909253) polymorphism and migraine
Genotype distributions in all control and migraine populations were in HWE (Table 2). One study suggested a decreased risk of migraine assuming additive and dominant models for MO (20) (Table 3). In contrast, effect estimates suggested an increased risk for MA among females. Effect estimates for some of the associations could not be calculated due to missing observations for some of the genotypes. Two studies suggested an increased risk for migraine (19, 21). Three studies did not suggest an altered risk for migraine for any of the genotypes (14, 17, 22).
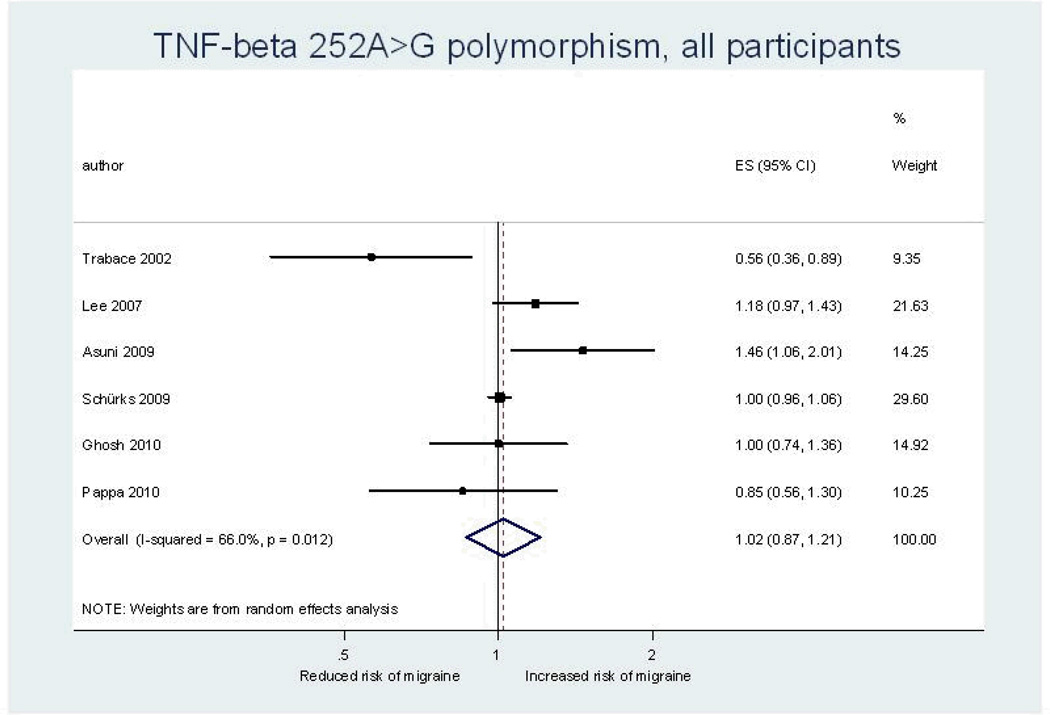
Our overall pooled analyses do not suggest an association between the TNF-beta 252A>G polymorphism and migraine (Figure 3, Table 5). The pooled OR (95% CI) from an additive model for migraine was 1.02 (0.87–1.21). The effect estimates were similar for females and males and results did not change in dominant or recessive models. There was moderate heterogeneity among all participants (additive model: I2=66.0%), which only appeared among males (additive model: I2=60.8%), but not females (additive model: I2=0.0%).
Figure 3.
Odds ratios for the association between the TNF- beta 252A>G polymorphism and migraine assuming an additive model from individual studies and from the pooled analysis. ES=estimate (indicates odds ratio). CI=confidence interval.
Study results stratified by clinical phenotype also do not suggest an association between the TNF-beta 252A>G polymorphism and MA (all participants, additive model: pooled OR=1.03; 95% CI 0.95–1.12) or MO (all participants, additive model: pooled OR=1.00; 95% CI 0.83–1.22) (Table 5). The results were similar in dominant and recessive models as well as for females and males. This agrees with results from meta-regression on clinical phenotype (all p>0.39). Studies among participants with MA did not suggest heterogeneity (all participants, additive model: I2=0.0%). In contrast, for MO there was moderate heterogeneity (all participants, additive model: I2=69.3%), which only appeared for males (additive model: I2=56.8%), but not females (additive model: I2=0.0%).
For MA there was indication for a small study effect among females assuming a recessive model (Begg’s test: p=0.04; Egger’s test: p=0.005). Meta-regression did not indicate that country of study origin significantly impacts the pooled results for migraine, MA, or MO (all p-values>0.10). In addition, when considering gender stratified study results meta-regression did not indicate that gender significantly impacts the pooled results for migraine or MO (all p-values≥0.68). Meta-regression among effect estimates from gender stratified analyses could not be performed.
Association between the TNF-alpha -238G>A (rs361525) polymorphism and migraine
The genotype distribution among controls deviated from HWE in one study (p=0.006) (17) (Table 2). In the second study, the Hardy-Weinberg principle was given among migraineurs and controls (22). The minor allele frequency (MAF) in both studies was low (≤5.1%).
Results from both studies do not suggest a statistically significant association between the TNF-alpha -238G>A polymorphism and migraine (Table 3).
Pooled results did not suggest an association for migraine (all participants, additive model: pooled OR=0.97; 95% CI 0.88–1.08) or MO (all participants, additive model: pooled OR=0.91; 95% CI 0.66–1.26). There was no indication for heterogeneity among the studies (additive model, migraine: I2=0.0%; MO: I2=14.0%). Only one study investigated associations in MA (17), suggesting no association (Table 3).
Association between the TNF-alpha -376G>A (rs1800750) polymorphism and migraine
In one study HWE and effect estimates could not be calculated (22) (Tables 2 and 3). In the second study the genotype distribution among controls was not in HWE (p=0.002) and the MAF was <2% (17). Results suggested an increased risk for all three models. Pooled analyses could not be performed.
Sensitivity analyses
Galbraith plots
For some of our analyses, Galbraith plots identified individual studies as important sources of heterogeneity. Hence, we performed sensitivity analyses by excluding studies that fell outside the margin set by the z score ±2 standard deviations.
With regard to the TNF-alpha -308G>A polymorphism Galbraith plots identified studies introducing heterogeneity for migraine, MO, and among females only for MA. After excluding these studies, associations remained statistically insignificant. For example, among all participants and assuming an additive model the pooled OR (95% CI) was 1.04 (0.94–1.15) for migraine and 0.98 (0.90–1.06) for MO after excluding three studies (15, 16, 18) and 1.15 (1.04–1.27) for females with MA after excluding one study (14).
With regard to the TNF-beta 252A>G polymorphism Galbraith plots identified studies introducing heterogeneity for migraine and MO. Excluding these studies did not change the associations. For example, among all participants and assuming an additive model the pooled OR (95% CI) for migraine was 1.02 (0.96–1.08) and for MO 1.02 (0.94–1.10) after excluding two studies (19, 20).
Excluding other studies
We further investigated if study design and age of the study population impacts the pooled results by excluding the cohort study (17) and the study among children/adolescents (22), respectively. For the TNF-alpha -308G>A and the TNF-beta 252A>G polymorphisms this did not change the associations with migraine, MA, or MO for any of the models assumed (data not shown). Further, excluding the study where genotype distribution of the TNF-alpha -308G>A polymorphism deviated from HWE (15) did not change the results (e.g. additive model, all participants: OR=1.02, 95% CI 0.72–1.44).
Discussion
The overall results from this meta-analysis do not indicate an association between any of the investigated polymorphisms and migraine, which did not change in sensitivity analyses. However, among studies investigating the TNF-alpha -308G>A polymorphism the association differs by country of origin and clinical phenotype has a modifying effect. Compared to studies among European and Asian populations those among populations of heterogeneous ethnic background (Turkey and Iran) suggested an increased risk for migraine and MO. The effect was stronger among females than males translating into a four-fold increased risk. In addition, results for MA suggested a two-fold increased risk among Asian populations which may be stronger in females than males. The results from these subgroup analyses, however, need to be treated with caution due to potential population stratification and small study numbers.
An inflammatory process is important in the pathophysiology of migraine and has been named “neurogenic inflammation” (4). TNF-alpha is an inflammatory cytokine with a plausible link to migraine. First, an increased release of TNF-alpha has been reported in MO (5, 6) and TNF-alpha plasma levels are higher during than between migraine attacks (7). Further, lower serum concentrations of the soluble receptor for TNF-alpha have been reported in migraineurs compared to controls, while the serum concentrations of TNF-alpha did not differ (9). In contrast, among women with menstrual migraine urine secretion of TNF-alpha appears to be markedly decreased (8). Second, TNF-alpha was shown to stimulate CGRP gene transcription and CGRP plays a key role in the pathophysiology of migraine (10). Third, TNF-alpha is crucial in the development of inflammatory hyperalgesia (32), a phenomenon related to allodynia experienced by some migraine patients.
Levels of TNF-alpha and the closely related TNF-beta are under genetic control. The TNF-alpha -308G>A polymorphism modulates transcriptional activity of the TNF gene (12) and TNF-alpha and TNF-beta polymorphisms have been shown to determine secretion of TNF-alpha and TNF-beta (13). Hence, studies have investigated the role of gene polymorphisms in the TNF-alpha and the TNF-beta gene in migraine (Table 1). However, despite biological plausibility, study results were conflicting and our meta-analysis does not support an overall association between any of the investigated gene variants and migraine including its subtypes.
There are two possible explanations for the observed overall lack of associations. Either, there may be truly no association and in some studies positive results may have occurred due to chance or certain study characteristics or the pattern of association may be more complex involving additional factors. In this context the following considerations need to be discussed.
First, pooling study results on the TNF-alpha -308G>A and TNF-beta 252A>G polymorphisms indicated remaining moderate to high heterogeneity for migraine and MO which may be due to differences between subgroups. This is plausible given that migraine susceptibility differs for example by gender (33) and ethnicity (34). For the TNF-alpha -308G>A variant analyses stratified by country of study origin eliminate part of that heterogeneity. We observed that Asian populations are at increased risk for MA, the effects being stronger among females than males. We also found that populations with a heterogeneous ethnic background have an increased risk for migraine and MO compared to European and Asian populations. We cannot exclude that this is a spurious finding due to population stratification. Population stratification denotes a systematic difference in allele frequencies between subpopulations in a population for example due to different ancestry, nonrandom mating, and physical separation. If cases and controls stem from different subpopulations and a marker under investigation has different allele frequencies in these subpopulations, an association (or lack of association) may occur reflecting the ancestral difference rather than the disease susceptibility.
Second, these differential associations were based on small numbers of studies. While we made the effort to gather the maximum amount of information including data stratified by gender and clinical phenotype, additional information was unavailable from two studies for the TNF-alpha -308G>A polymorphism (16, 23). This information would have been valuable in sketching more clearly associations with migraine among populations of different descent.
Third, although all studies used strict or modified IHS classification criteria (25, 26), there is the potential for migraine as well as MA and MO to be misclassified given its biologically heterogeneity and wide clinical spectrum (35).
Fourth, the investigated gene variants may be associated with certain migraine traits or measures of migraine severity like attack frequency rather than IHS defined migraine status.
Fifth, we have included all available genetic association studies and not prematurely excluded any study based on HWE. This approach increases the total sample size and thus the power to detect a potential association and also allows for greater flexibility at the analysis level by performing sensitivity analyses. This is in keeping with recommendations for performing meta-analyses (36). Our analyses indicate that excluding studies where HWE among controls is deviated does not change the results.
Sixth, the available studies varied in sample size. In particular one cohort study (17) was much larger than the other case-control studies. While a very large study may dominate the overall pooled results in a meta-analysis, there was no such indication from our analyses. Results from sensitivity analyses excluding the cohort study were very similar to the overall results for the TNF-alpha -308G>A and TNF-beta 252A>G polymorphisms.
Seventh, almost 200 known single nucleotide polymorphisms (SNPs) have been reported in the TNF-alpha and TNF-beta genes (37, 38), which may affect gene transcription and TNF level. Some SNPs may have a low MAF as seen in some of the included studies (Table 2), obviating reliable analyses; however, such variants are unlikely to strongly influence a common and complex disorder like migraine. While there are single reports suggesting an association between other SNPs with migraine, no follow-up studies are available yet (Table 1b). Many of the gene variants, however, including gene interactions, have not been investigated yet.
Eighth, TNF-alpha’s and TNF-beta’s role in migraine is likely not just determined by their levels, but also by their functional effect at the receptor level, which is likewise under genetic control and unaccounted for in all studies.
Finally, many neurotransmitters like CGRP, serotonin, dopamine, orexin, and glutamate are important for migraine pathophysiology (39). While a functional interaction between TNF-alpha and CGRP has been shown (10), many more interactions between inflammatory markers and neurotransmitters are likely involved in migraine.
Additional targeted research is warranted to further clarify a potential role of TNF-alpha and TNF-beta gene variants in migraine. An important next step is performing genome-wide association studies (GWAS) in large populations. The first one has recently been published implicating the glutamate pathway in a population primarily suffering from MA (40). While other GWAS are under way, these are unlikely to answer questions raised by our meta-analysis namely a possible genetic effect among non-European populations, since all are performed in populations of European descent. Hence, additional large studies such as GWAS need to be performed in other populations that also account for potential population stratification, in order to discern potential genetic differences in migraine susceptibility.
Acknowledgements
We would like to gratefully acknowledge the following colleagues and researchers, who supported this meta-analysis by kindly providing us with additional data from their published studies (in alphabetical order):
Greece: Maria Hatzistilianou (MD), School of Medicine, Aristotle University, Thessaloniki.
Italy (Cagliari, Sardinia): Carlo Asuni (MD), Unit of Clinical Pharmacology, Teaching Hospital of Cagliari; Donatella Congiu (MSc) and Maria Del Zompo (MD), Department of Neurosciences "B.B. Brodie"; University of Cagliari.
Italy (Rome): Patrizia Lulli (PhD) and Paolo Martelletti (MD), Department of Clinical and Molecular Medicine; Simonetta Trabace (PhD), Department of Molecular Medicine; Sapienza University of Rome.
South Korea: Jong-Won Kim (MD, PhD), Department of Laboratory Medicine, Samsung Medical Center, Sungkyankwan University School of Medicine, Seoul.
Turkey: Sema Erol Çakmak (MD), Aynur Özge (MD, PhD), Osman Özgür Yalin (MD), İbrahim Arda Yilmaz (MD), Department of Neurology; M. Emin Erdal (PhD), Department of Medical Biology and Genetics; Mersin University Medical Faculty. Tuba Gökdogan Edgünlü (PhD), Department of Medical Genetics, Mugla University School of Medical Healthy Science.
Full Disclosures for the last 2 years
Dr. Schürks has received an investigator-initiated research grant from the Migraine Research Foundation. He has received honoraria from L.E.K. Consulting for telephone surveys and from the American Academy of Neurology for educational material.
P. Rist is funded by a training grant from the National Institute of Aging (AG00158).
Dr. Zee has received research support from the National Heart, Lung, and Blood Institute, the Doris Duke Charitable Foundation, the Leducq Foundation, the Donald W. Reynolds Foundation, and Roche.
Dr. Chasman has received research support from Celera.
Dr. Kurth has received investigator-initiated research funding from the National Institutes of Health, McNeil Consumer & Specialty Pharmaceuticals, Merck, and Wyeth Consumer Healthcare; he is a consultant to i3 Drug Safety and World Health Information Science Consultants, LLC, and he has received honoraria from the American Academy of Neurology, Genzyme, Merck, and Pfizer for educational lectures; and from MAP Pharmaceutical for contributing to an scientific expert panel.
Footnotes
None of the disclosures for any of the authors constitutes a conflict of interest with regard to this meta-analysis.
References
- 1.Haut SR, Bigal ME, Lipton RB. Chronic disorders with episodic manifestations: focus on epilepsy and migraine. Lancet Neurol. 2006;5:148–157. doi: 10.1016/S1474-4422(06)70348-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silberstein SD. Migraine. Lancet. 2004;363:381–391. doi: 10.1016/S0140-6736(04)15440-8. [DOI] [PubMed] [Google Scholar]
- 3.Pietrobon D, Striessnig J. Neurobiology of migraine. Nat Rev Neurosci. 2003;4:386–398. doi: 10.1038/nrn1102. [DOI] [PubMed] [Google Scholar]
- 4.Moskowitz MA. Pathophysiology of headache-past and present. Headache. 2007;47 Suppl 1:S58–S63. doi: 10.1111/j.1526-4610.2007.00678.x. [DOI] [PubMed] [Google Scholar]
- 5.Covelli V, Munno I, Pellegrino NM, Altamura M, Decandia P, Marcuccio C, et al. Are TNF-alpha and IL-1 beta relevant in the pathogenesis of migraine without aura? Acta Neurol (Napoli) 1991;13:205–211. [PubMed] [Google Scholar]
- 6.Covelli V, Munno I, Pellegrino NM, Di Venere A, Jirillo E, Buscaino GA. Exaggerated spontaneous release of tumor necrosis factor-alpha/cachectin in patients with migraine without aura. Acta Neurol (Napoli) 1990;12:257–263. [PubMed] [Google Scholar]
- 7.Perini F, D'Andrea G, Galloni E, Pignatelli F, Billo G, Alba S, et al. Plasma cytokine levels in migraineurs and controls. Headache. 2005;45:926–931. doi: 10.1111/j.1526-4610.2005.05135.x. [DOI] [PubMed] [Google Scholar]
- 8.Mueller L, Gupta AK, Stein TP. Deficiency of tumor necrosis factor alpha in a subclass of menstrual migraineurs. Headache. 2001;41:129–137. doi: 10.1046/j.1526-4610.2001.111006129.x. [DOI] [PubMed] [Google Scholar]
- 9.Empl M, Sostak P, Riedel M, Schwarz M, Muller N, Forderreuther S, Straube A. Decreased sTNF-RI in migraine patients? Cephalalgia. 2003;23:55–58. doi: 10.1046/j.1468-2982.2003.00453.x. [DOI] [PubMed] [Google Scholar]
- 10.Durham PL. Calcitonin gene-related peptide (CGRP) and migraine. Headache. 2006;46 Suppl 1:S3–S8. doi: 10.1111/j.1526-4610.2006.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carroll MC, Katzman P, Alicot EM, Koller BH, Geraghty GE, Orr HT, et al. Linkage map of the human major histocompatibility complex including the tumor necrosis factor genes. Proc Natl Acad Sci. 1987;84:8535–8539. doi: 10.1073/pnas.84.23.8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson AG, Symons JA, McDowell TL, McDevitt HO, Duff GW. Effects of a polymorphism in the human tumor necrosis factor alpha promoter on transcriptional activation. Proc Natl Acad Sci. 1997;94:3195–3199. doi: 10.1073/pnas.94.7.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouma G, Crusius JB, Oudkerk Pool M, Kolkman JJ, von Blomberg BM, Kostense PJ, et al. Secretion of tumour necrosis factor alpha and lymphotoxin alpha in relation to polymorphisms in the TNF genes and HLA-DR alleles. Relevance for inflammatory bowel disease. Scand J Immunol. 1996;43:456–463. doi: 10.1046/j.1365-3083.1996.d01-65.x. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh J, Joshi G, Pradhan S, Mittal B. Investigation of TNFA 308G > A and TNFB 252G > A polymorphisms in genetic susceptibility to migraine. J Neurol. 2010;257:898–904. doi: 10.1007/s00415-009-5430-x. [DOI] [PubMed] [Google Scholar]
- 15.Mazaheri S, Hajilooi M, Rafiei A. The G-308A promoter variant of the tumor necrosis factor-alpha gene is associated with migraine without aura. J Neurol. 2006;253:1589–1593. doi: 10.1007/s00415-006-0270-4. [DOI] [PubMed] [Google Scholar]
- 16.Rainero I, Grimaldi LM, Salani G, Valfre W, Rivoiro C, Savi L, Pinessi L. Association between the tumor necrosis factor-alpha-308 G/A gene polymorphism and migraine. Neurology. 2004;62:141–143. doi: 10.1212/01.wnl.0000101717.16799.8f. [DOI] [PubMed] [Google Scholar]
- 17.Schürks M, Kurth T, Buring JE, Zee RY. A candidate gene association study of 77 polymorphisms in migraine. J Pain. 2009;10:759–766. doi: 10.1016/j.jpain.2009.01.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yilmaz IA, Özge A, Erdal ME, Edgünlü TG, Cakmak SE, Yalin OÖ. Cytokine polymorphism in patients with migraine: some suggestive clues of migraine and inflammation. Pain Med. 2010;11:492–497. doi: 10.1111/j.1526-4637.2009.00791.x. [DOI] [PubMed] [Google Scholar]
- 19.Asuni C, Stochino ME, Cherchi A, Manchia M, Congiu D, Manconi F, et al. Migraine and tumour necrosis factor gene polymorphism. An association study in a Sardinian sample. J Neurol. 2009;256:194–197. doi: 10.1007/s00415-009-0961-8. [DOI] [PubMed] [Google Scholar]
- 20.Trabace S, Brioli G, Lulli P, Morellini M, Giacovazzo M, Cicciarelli G, Martelletti P. Tumor necrosis factor gene polymorphism in migraine. Headache. 2002;42:341–345. doi: 10.1046/j.1526-4610.2002.02104.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee KA, Jang SY, Sohn KM, Won HH, Kim MJ, Kim JW, Chung CS. Association between a polymorphism in the lymphotoxin-a promoter region and migraine. Headache. 2007;47:1056–1062. doi: 10.1111/j.1526-4610.2007.00847.x. [DOI] [PubMed] [Google Scholar]
- 22.Pappa S, Hatzistilianou M, Kouvatsi A, Pantzartzi C, Sakellaropoulou A, Pavlou E, et al. Tumour necrosis factor gene polymorphisms and migraine in greek children. Arch Med Sci. 2010;6:430–437. doi: 10.5114/aoms.2010.14267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herken H, Erdal ME, Yilmaz M, Savasoglu K, Bayazit YA. The -308 G/A polymorphism of tumor necrosis factor alpha gene is not associated with migraine. Pain Clinic. 2005;17:389–393. [Google Scholar]
- 24.Sagoo GS, Little J, Higgins JP. Systematic reviews of genetic association studies. PLoS Med. 2009;6:e28. doi: 10.1371/journal.pmed.1000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The International Classification of Headache Disorders: 2nd edition. Vol. 24. Cephalalgia: 2004. pp. 9–160. [DOI] [PubMed] [Google Scholar]
- 26.Headache Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia. 1988;8 Suppl 7:1–96. [PubMed] [Google Scholar]
- 27.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 29.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asuni C, Stochino ME, Cherchi A, Manconi M, Congiu D, Del Zompo M. Migraine and tumor necrosis factor gene polymorphism: An association study in a Sardinian sample. Am J Med Genet Part B. 2006;141B:785. doi: 10.1007/s00415-009-0961-8. [DOI] [PubMed] [Google Scholar]
- 31.Martelletti P, Brioli G, Lulli P, Morellini M, Giacovazzo M, Trabace S. Tumor necrosis factor B gene polymorphism contributes to susceptibility to migraine without aura. J Headache Pain. 2000;2:119–122. [Google Scholar]
- 32.Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br J Pharmacol. 1992;107:660–664. doi: 10.1111/j.1476-5381.1992.tb14503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lipton RB, Bigal ME. The epidemiology of migraine. Am J Med. 2005;118 Suppl 1:3S–10S. doi: 10.1016/j.amjmed.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 34.Scher AI, Stewart WF, Lipton RB. Migraine and headache: a meta-analytic approach. In: Crombie IK, editor. Epidemiology of pain. Seattle, WA: IASP Press; 1999. pp. 159–170. [Google Scholar]
- 35.Wessman M, Terwindt GM, Kaunisto MA, Palotie A, Ophoff RA. Migraine: a complex genetic disorder. Lancet Neurol. 2007;6:521–532. doi: 10.1016/S1474-4422(07)70126-6. [DOI] [PubMed] [Google Scholar]
- 36.Jüni P, Altman DG, Egger M. Assessing the quality of randomised controlled trials. In: Egger M, Smith GD, Altman DG, editors. Systematic reviews in health care: meta-analysis in context. London: BMJ Publishing Group; 2001. pp. 87–108. [Google Scholar]
- 37. http://genome.ucsc.edu/
- 38. http://www.ncbi.nlm.nih.gov/projects/SNP/.
- 39.Goadsby PJ, Charbit AR, Andreou AP, Akerman S, Holland PR. Neurobiology of migraine. Neuroscience. 2009;161:327–341. doi: 10.1016/j.neuroscience.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 40.Anttila V, Stefansson H, Kallela M, Todt U, Terwindt GM, Calafato MS, et al. Genome-wide association study of migraine implicates a common susceptibility variant on 8q22.1. Nat Genet. 2010;42:869–873. doi: 10.1038/ng.652. [DOI] [PMC free article] [PubMed] [Google Scholar]