Abstract
Converging lines of evidence suggest an adverse effect of heavy cannabis use on adolescent brain development, particularly on the hippocampus. In this preliminary study, we compared hippocampal morphology in 14 “treatment-seeking” adolescents (aged 18-20) with a history of prior heavy-cannabis use (5.8 joints/day) after an average of 6.7 months of drug abstinence, and 14 demographically matched normal controls. Participants underwent a high-resolution 3D MRI as well as cognitive testing including the California Verbal Learning Test (CVLT). Heavy-cannabis users showed significantly smaller volumes of the right (p< .04) and left (p< .02) hippocampus, but no significant differences in the amygdala region compared to controls. In controls, larger hippocampus volumes were observed to be significantly correlated with higher CVLT verbal learning and memory scores, but these relationships were not observed in cannabis users. In cannabis users, a smaller right hippocampus volume was correlated with a higher amount of cannabis use (r= - .57, p< .03). These data support a hypothesis that heavy-cannabis use may have an adverse effect on hippocampus development. These findings, after an average 6.7 month of supervised abstinence, lend support to a theory that cannabis use may impart long-term structural and functional damage. Alternatively, the observed hippocampal volumetric abnormalities may represent a risk factor for cannabis dependence. These data have potential significance for understanding the observed relationship between early cannabis exposure during adolescence and subsequent development of adult psychopathology reported in the literature for schizophrenia and related psychotic disorders.
Keywords: hippocampus, cannabis, adolescence, magnetic resonance imaging, CVLT, learning and memory
1. Introduction
Adolescence is a period of increased risk-taking and thrill-seeking, often including drug abuse (Ernst et al., 2006; Casey et al., 2008; Steinberg, 2008; Somerville et al., 2010). According to the 2007 National Survey on Drug Use and Health (NSDUH, United States Department of Health and Human Services) young people show higher rates of drug use as compared with older age groups. U.S. school survey data (Johnston et al., 2008) shows that 15% of 8th graders have tried marijuana at least once and 43% have tried marijuana by 12th grade. Another national survey also reported a history of cannabis use in approximately 45% of 12th graders in the U.S., with 5% reporting current daily use (Terry-McElrath et al., 2005). Further, other studies support the hypothesis that adolescent cannabis use is a gateway to illicit drug use in early adulthood (Fergusson et al., 2006; Luengo et al., 2008).
Adolescence is a period during which the brain undergoes dramatic developmental changes. Maturation of the human brain is a complex and comprehensive process, with critical changes occurring at key points throughout development. Contrary to the once-held belief that the brain completes its development by the end of the childhood years (Kretschmann et al., 1986; Durston et al., 2001), the brain continues to undergo a substantial amount of development throughout adolescence and into early adulthood. Gray matter in the cerebral cortex shows a characteristic “rise and fall” pattern (Geidd et al., 1999; Sowell et al., 2001; Gogtay et al., 2004; Thompson et al., 2005; Hua et al., 2009), while white matter connecting gray matter areas increases steadily from birth to adulthood (Paus et al., 1999; De Bellis et al., 2001; Schmithorst et al., 2002; Suzuki et al., 2003; Nagy et al., 2004; Barnea-Goraly et al., 2005; Schmithorst et al., 2008; Olson et al., 2009). In a previous study conducted by our group, we compared normal brain development in a group of early adolescents (average age 12 years) as compared with a group of late adolescents (average age 18 years old) and demonstrated accelerated brain development during late adolescence (Ashtari et al., 2007). Results from these studies suggest that in addition to birth and early childhood, adolescence is a key period for neuronal maturation and a critical time for brain development.
In addition to the overall normal brain development, other important processes occur during adolescence such as neurogenesis (the birth of new neurons) in the hippocampus (Benes et al., 1989). Similar processes have been studied in animals and studies report neurogenesis in the dentate gyrus of the hippocampus throughout the lifetime of the animal (Eriksson et al., 1998; Ciaroni et al., 1999; Eisch et al., 2000). In a recent study, Eisch and colleagues (2008) present evidence that adult hippocampal neurogenesis is strongly implicated in psychiatric disorders, particularly addiction. This data suggests that neurogenesis is regulated by drugs of abuse (Eisch et al., 2000; Abrous et al., 2002; Nixon and Crews, 2002; Noonan et al., 2008) and that the hippocampus influences both drug taking and drug-seeking behaviors (Taepavarapruk et al., 2000; Floresco et al., 2001; Lodge and Grace, 2006). While much is still being learned about the precise role of the hippocampus in cognitive function, it is generally accepted that this region includes “a system of anatomically related structures that are essential for memory functions” (Squire et al., 2004). Thus, chronic administration of drugs of abuse such as cannabis may decrease hippocampal function and in particular may affect memory performance.
The neurocognitive effects of cannabis use in adults include poor performance on tests of learning, memory, and executive functions (Varma et al., 1988; Pope et al., 1996; 1997; Croft et al., 2001; Bolla et al., 2002; Solowij et al., 2002; Lyons et al., 2004; Bava and Tapert, 2010) when compared to matched controls. Despite the prevalence of marijuana use in adolescence, few reports have focused on the neurocognitive impact of heavy cannabis use during this time. An earlier study by Schwartz and colleagues studied adolescents ages 14-16 with cannabis dependence and found verbal and non-verbal short-term memory impairments as compared to controls (Schwartz, 1989).
Tapert et al (2002), in an eight year follow up longitudinal study, examined adolescents with a history of substance use disorders and showed accumulative, diminished performance over time on tests of cognitive performance among cannabis users. Harvey and colleagues (2007) found that adolescents who are regular cannabis users performed worse on tests of attention, learning, and memory and that a greater amount of cannabis use predicted poorer executive functioning and performance on tests of working memory. Schweinsburg et al (2005) showed abnormalities in brain response using fMRI during a spatial working memory task after 1 month of abstinence for a group of adolescent cannabis users as compared with controls. They observed different patterns of activation in cannabis users when performing the task and attributed these differences to a compensatory brain mechanism or persisting brain abnormalities associated with heavy cannabis use during adolescence (Schweinsburg et al., 2008a). These and other studies (Freedland et al., 2002; Iversen, 2003; Pontieri et al., 1999; Quickfall and Crockford, 2006) show that the brain regions such as the frontal lobe, hippocampus, amygdala, basal ganglia and striatum, which are rich in cannabinoid receptors, are more susceptible to the effects of cannabis.
The neurological effects of cannabis are largely mediated by the binding of its active ingredient, delta9-tetrahydrocannabinol (9-THC), to cannabinoid receptors (Matsuda et al., 1990; Munro et al., 1993; Martin, 1995; Puighermannal et al., 2009) localized in the various brain regions. Cannabinoids appear particularly neurotoxic to hippocampal neurons (Chan et al., 1998; Hoffman and Lupica, 2000; Kim and Thayer, 2001; Carlson et al., 2002). Short-term memory dysfunction from cannabis could occur because THC may alter the way in which information is processed by the hippocampus. Laboratory rats treated with THC display the same reduced ability to perform tasks requiring short-term memory as other rats whose hippocampal neurons were destroyed (Heyser et al., 1993). A recent study by Rubino et al (2009) employed an animal model of adolescent rats with chronic exposure to THC to examine the long-term effects of THC on learning and memory. Adolescent rats were treated with THC or its vehicle from 35 to 45 postnatal days (PND) and left undisturbed until adulthood (75 PND), at which point spatial memory was assessed using the radial maze task. THC pre-exposed animals exhibited worse performance than vehicles, suggesting a specific deficit in spatial working memory (Rubino et al., 2009). Post-mortem analysis of pre-exposed rats revealed a significantly lower overall total dendritic length and spine density than vehicles. The authors suggested that THC pre-exposed rats may establish fewer synaptic contacts and/or less efficient synaptic connections throughout the hippocampus and concluded that this may represent the molecular underpinnings of the cognitive deficit induced by adolescent THC exposure (Rubino et al., 2009). The main conclusion of this work was that chronic and heavy exposure to THC during adolescence produced impairments in spatial working memory in adult animals along with reduced levels of markers of neuroplasticity in the hippocampus and morphological alterations in the dentate gyrus (Rubino et al., 2009).
The present cross-sectional study was designed to examine the human hippocampus, an area of the brain rich in cannabis receptors which also undergoes neurogenesis during adolescence, in a group of adolescents with a history of heavy cannabis use and demographically matched healthy controls. 3D high-resolution MR imaging was performed to evaluate volume differences in the hippocampus and amygdala structures. Although studies reporting the effect of cannabis on amygdala structure are scarce, based on the findings reported by Yücel et al (2008) and the fact that the amygdala is also rich in cannabinoid receptors, we also evaluated amygdala volume. Since cognitive impairment is one of the most prominent negative consequences associated with cannabis consumption (Fried et al., 2002; Grant et al., 2003; Pope et al., 2003; Jacobsen et al., 2004; Solowij et al., 2008; Battisti et al., 2010), we evaluated all subjects utilizing the California Verbal Learning Test (CVLT) to further study the chronic effect of heavy cannabis use on brain structure and cognition.
2. Materials and Method
2.1 Study Subjects
Fourteen treatment-seeking male adolescents (mean age = 19.3; SD = 0.8) who claimed cannabis as their drug of choice and met DSM-IV diagnostic criteria for cannabis dependence, in early full remission (American Psychiatric Association, 1994) were recruited from a therapeutic community (Aurora Concept Inc., Queens, NY). Each reported smoking three or more “joints” per day for at least one year prior to commencement of treatment (mean = 5.8 joints per day; SD = 2.1) and were drug-free for at least 30 days and an average of 6.7 months (see Table 1) prior to the time of the MRI. The exact amount of THC in each joint consumed by each subject is hard to accurately quantify (Gray et al, 2009). However, 0.5 grams/joint and 4 joints/blunt are the estimates reported by our study participants. Published reports estimate an average of 0.4-0.5 grams/joint, although the exact estimate varies among countries (European Commission, 2009; Gray et al., 2009).
Table 1.
Subject Demographics
| Patients with Cannabis Use (n=14) | Healthy Controls (n=14) | Test Statistic | p | |
|---|---|---|---|---|
| Mean Age (SD), years | 19.3 (0.8) | 18.5 (1.4) | t = -1.88 | .07 |
| Sex (M/F) | 14 / 0 | 14 / 0 | ||
| Ethnicity (Caucasian/non-Caucasian)* | 0 / 14 | 3 / 11 | .22 | |
| Handedness (dextral/non-dextral)* | 10 / 3 | 12 / 1 | .59 | |
| Parental SES (high/low)1 | 9 / 1 | 13 / 0 | .44 | |
| Mean WRAT3 score (SD)2 | 84.3 (13.9) | 104.0 (17.6) | t = 3.29 | <.005 |
| Brain Volume (mm3) | 1553.5 (110) | 1426.9 (144) | t = 2.6 | <..01 |
| Mean Age at Onset of Cannabis Use (SD), years | 13.1 (1.6) | |||
| Mean Duration of Use (SD), years | 5.3 (2.1) | |||
| Mean Amount Used (SD), joints per day | 5.8 (2.6) | |||
| Mean Time since Last Use (SD), months | 6.7 (3.7) |
Note: Independent samples t – tests and Fisher's Exact tests utilized as appropriate.
One missing data point for patients and one for controls.
Socioeconomic status (SES) (Hollingshead 1975)
Wide Range Achievement Test (WRAT3) Reading subtest (Wilkinson 1993)
Since these subjects were housed in a controlled environment, toxicology tests were administered on a regular and random basis for the duration of their stay at the facility. Participants in this study were required to have a negative toxicology test on the day of MRI. Similar to previous reports (Solowij et al., 2002) cannabis users were excluded if they reported a history of dependence on any drug besides cannabis or nicotine, or if they had a history of any psychotic disorder, bipolar disorder, or major depressive episode. Fourteen healthy male comparison subjects (mean age = 18.5; SD = 1.4) were recruited from a nearby adolescent medicine clinic, from responses to fliers that were placed in the community and by word of mouth. These subjects were recruited from geographic areas similar to cannabis users in terms of demographic and socioeconomic makeup. Controls were excluded if they had any current or past DSMIV diagnosis, a history of psychological counseling or reported a history of more than 5 lifetime exposures to any illicit drug, tobacco, or alcohol. Additional exclusion criteria for all subjects included mental retardation (as evidenced by intelligence test scores or DSM-IV diagnosis), known neurological illness, a history of head injury with loss of consciousness for more than 30 seconds, any focal findings revealed by routine clinical scan at the time of research MRI, or current use of psychotropic medications.
2.2 Study Procedures
After providing a complete description of the study, written informed consent (and when necessary, parental consent and child assent) was obtained from all subjects. Procedures were approved by the Institutions Review Board. All adolescents were interviewed by a research psychologist or trained psychometrician under the supervision of a board-certified child and adolescent psychiatrist using the Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS) (Kaufman et al., 1997). Since this study was an extension of existing studies in childhood and adolescent psychiatric patients, the K-SADS (designed for individuals 18 years and younger) was used in order to provide consistency in defining disorders and characterizing illness across samples. Additionally, consent was obtained from cannabis users to review the in-patient rehabilitation charts in order to collect more detailed information about history of drug use and psychological functioning and to corroborate self-reports gathered during the clinical interview.
2.2.1 Magnetic Resonance Imaging
MRI scans were conducted at the Long Island Jewish Medical Center on a 1.5T GE Neuro Vascular Interactive (NV/i) system. This unit was equipped with a high strength (50 mT/m) and high-speed gradient system (Slew Rate = 150 T/m/sec). All scans were carried out by a single operator and monitored to be free of artifacts at the time of acquisition. In addition to the routine clinical protocol to rule out incidental pathological findings, a 3D spoiled gradient-echo (SPGR) sequence with inversion preparation pulse (IR-Prep) (TR = 10.1 ms, TE = 4.2 ms; bandwidth = 21 kHz, matrix size = 256×192, FOV = 22×22 cm2, inversion time = 600 ms, NEX = 1) was performed. This sequence generated 124 coronal slices, 1.5 mm in thickness that continuously covered the whole brain in 7:44 minutes.
2.2.2 Image Processing
We used a semi-automated brain processing pipeline to maximize reliability. Our pipeline consisted of, first, semi-automated brain extraction; second, automated group template estimation; and finally, a semi-automated extraction of the hippocampus and amygdala based on established anatomical criteria. Brain Extraction: We performed initial skull stripping with Brain Extraction Tool (BET) software (Smith, 2002). Subsequently, we manually edited each subject's BET extraction to include brain parenchyma and remove residual tissues such as eye sockets and exterior brain areas such as the neck and scalp area. Two raters visually assessed these results in three dimensions using the ITK-SNAP interactive segmentation tool. Group Template Estimation: We used the well-evaluated (Klein et al., 2009; 2010) Advanced Normalization Tools (ANTS, http://www.picsl.upenn.edu/ANTS/) (Avants et al., 2008) for diffeomorphic image registration to estimate an unbiased group template from the brain extracted dataset (Avants et al., 2010). This toolkit is used in related pediatric studies to automatically determine significant patterns of statistical difference in both the cortex (Hanson et al., 2010a) and hippocampus (Rao et al., 2010). In this study, we used the template to perform a second round of semi-automatic image registrations based on user-guidance. That is, we labeled the group template and the subject data to further improve normalization accuracy specifically within the hippocampus-amygdala region. Hippocampus and Amygdala Delineation Criteria: The hippocampus and amygdala complex were delineated using ITK-SNAP (Yushkevich et al., 2006; www.itksnap.org) by a trained rater (MA) (Ashtari et al., 1991; Ashtari et al., 1999; Bogerts et al., 1993, Bogerts et al., 1990a,b) using the 1 mm isotropic group brain atlas constructed by Advanced Normalization Tools (ANTS) from the 3D SPGR images of the study participants. Measurements of the hippocampal formation included all CA segments (CA1, CA2, CA3, and CA4), the dentate gyrus, the alveus, and the subicular region. Neuroanatomical boundaries were primarily based on criteria from postmortem histological work (Bogerts et al., 1985; Falkai and Bogerts, 1986) and prior published studies (Ashtari et al., 1991; 1999). However, owing to the clarity of the atlas, the most posterior section of the hippocampus was defined as the slice where the fornix was not interrupted by the pulvinar and clearly observed as one piece. Medially, hippocampal boundaries were clearly demarcated. The anterior delineation of hippocampal border is the most challenging part of identifying the hippocampus consistently. The operator used the neuroanatomical information available in each orthogonal plane and indexed between previous and next slices to facilitate measurement of complex regions to distinguish them from surrounding structures. The temporal horns and alveus landmarks were used to anteriorly differentiate the hippocampal head (pes hippocampus) from the amygdala. The amygdala was extracted posteriorly where the amygdala structure appeared between the temporal horn and choroidal fissure and carefully differentiated superiorly from the tail of the caudate and inferior portion of the putamen, superolaterally from the claustrum. Medially, the amygdala appears as an ovoid gray matter structure surrounded by the temporal horn inferiorly and choroidal fissure superiorly. Anteriorly, amygdala extraction terminated where the amygdaloid complex lost its typical oval shape. To ensure consistent delineation, structures were measured by one operator (MA), who is an experienced medial temporal morphometrist in both young and old subject populations, with established high intra-rater reliability (intraclass correlation (ICC) range from .85 to .95) (Ashtari et al., 1991; Bogerts et al., 1993; Barr et al., 1997; Ashtari et al., 1999). Semi-automatic Hippocampus/Amygdala Extraction in Subject Space: The above delineation criteria and the atlas constructed using the symmetric diffeomorphic image registration (Avants and Gee, 2004; Avants et al., 2006; 2007) were used to extract the medial temporal structures (Figure 1). To extract these structures in the subject space, a well-defined template-based landmarking protocol that requires only six landmarks per hippocampus and four per amygdala (placed on only three slices of the individual brain) was used to manually landmark the hippocampus/amygdala in each subject. This protocol is outlined in Pluta et al (2009) and is easily modified for specific applications. In the case of medial temporal lobe structures, the protocol takes approximately one minute per hippocampus/amygdala complex by leveraging the interface available in ITK-SNAP for landmark placement. A key benefit of this approach is that the landmarks enable the image registration to avoid local minima and guide the alignment of the hippocampus/amygdala complex specifically within areas that are challenging to match, for example, the boundary between the head of the hippocampus and the amygdala. While these landmarks provide overall robustness, fine structural alignment is guided by a combination of image and landmark features, thus allowing the original image information to refine the mapping once the landmarks are approximately aligned. This reliable image registration algorithm, which has been shown to perform similar to an expert user (Pluta et al., 2009), was then used to map the template extracted medial temporal structures to each individual's brain, thereby labeling the hippocampus and amygdala regions of interest (ROI) with separate values. The automated extraction of the hippocampus and amygdala structures for each subject was carefully examined and edited based on the above mentioned delineation criteria. The volume of the right and left hippocampus and amygdala for each individual subject were calculated using ITK-SNAP.
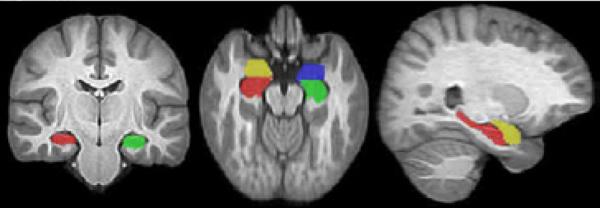
Figure 1.
An example of the automated extraction of the left (green) and right (red) hippocampi and left (blue) and right (yellow) amygdalae superimposed onto the brain template constructed from all 28 study participants.
2.2.3 Neurocognitive Evaluation
All subjects underwent a battery of neurocognitive tests; details regarding the tests and testing procedures can be found elsewhere (Rhinewine et al., 2005). Of relevance to this study, the Wechsler Adult Intelligence Scale-Third Edition (WAIS-3; Wechsler, 1997) was used to provide an estimate of current IQ level (Axelrod et al., 2001) and the Wide Range Achievement Test (WRAT-III; Wilkinson, 1993) was used to provide an estimate of premorbid intelligence (Wilkinson et al., 1993). WRAT-III (Wilkinson, 1993) reading scores have been shown to be a good estimate of general intelligence in individuals with neurological deficits, including patients with psychiatric disorders. In addition, similar to what has been reported in children with early-onset schizophrenia (Roofeh et al., 2006), we characterized the nature of verbal memory impairments in a group of adolescents with heavy cannabis use using the same dependent variables from the age-appropriate versions of the California Verbal Learning Test (CVLT) (Delis et al., 1987; 1994). The procedures for test administration have been described in detail elsewhere (Delis et al., 1987; 1994). The CVLT was chosen because this task has been sensitive to verbal memory deficits in substance abusing populations (Medina et al., 2006; 2007a) and has been associated with temporal lobe/hippocampus activation (Johnson et al., 2001).
2.3 Statistical Analyses
All analyses examining demographic and clinical characteristics of the study sample were conducted using SPSS (SPSS-18 Inc., Chicago, Illinois). Demographic differences between cannabis users and comparison subjects were analyzed using independent samples t-tests and Fisher's exact tests as appropriate. Group x Hemisphere x Region (hippocampus, amygdala) ANOVAs examined differences in structural volume. Post hoc statistical analyses of medial temporal structures were performed controlling for WRAT-III and total brain volume (TBV) separately and together. The relationship between WRAT-III scores and total brain volume was assessed by performing a Pearson correlation test. Pearson correlations were also performed to evaluate the relationship between hippocampus volumes, drug use, and the CVLT results.
3. Results
3.1 Sample Characteristics
Characteristics of the sample are summarized in Table 1. Cannabis users reported a mean age of first time cannabis use of 13.1 years (range: 9.0-15.0 years) with an average cannabis use of 5.8 joints per day. Cannabis users were drug-free for a median duration of 6.7 months (range: 3-11 months) prior to MRI scan. Numerous cannabis users had comorbid conditions, including post-traumatic stress disorder (n = 2), attention deficit/hyperactivity disorder (n = 2), oppositional defiant/conduct disorder (n = 4), and alcohol abuse (n = 5). In addition, eight cannabis patients reported nicotine abuse/dependence, four a history of ecstasy use (each on less than 3 occasions), and one of cocaine use (less than 3 occasions). There were no significant differences between cannabis users and control subjects in distributions of age, gender, parental socio-economic status (SES), ethnicity, or handedness. However, they significantly differed in WRAT-III scores and total brain volumes (see Table 1). Cannabis users had a median education level of 11th grade whereas the control group had a median education level of 1 year of college.
3.2 Group Comparison on the CVLT Performance
Using a general linear model (GLM) and controlling for total brain volume (TBV) and WRAT-III scaled scores, group performances on subcategories of the CVLT test were carried out. Heavy cannabis users did not differ significantly from the healthy controls on their performance on the CVLT total score (F =.13, p = .73), CVLT short delay free recall (F=.3, p=.590), short delay cued recall (F = 2.84, p = .1), long delay free recall (F = 0.0, p = 1.0) and long delay cued recall (F = .1, p = .76).
3.3 Group Comparisons on Medial Temporal Structural Volume
The Group (controls, cannabis) X Hemisphere X Region (hippocampus, amygdala) ANOVA showed a significant main effect of Group, F(1, 26) = 12.28, p < 0.015 and a significant Group X Region interaction, F(1, 26) = 4.92, p = 0.036, indicating greater volume in controls than patients only for the hippocampus, p = 0.001. None of the Group X Hemisphere interactions were significant. Within subject analyses showed a main effect of Region and Hemisphere. A significant Region X Hemisphere interaction indicated that the hippocampus was larger than the amygdala bilaterally, and that the hippocampus was larger in the right than in the left hemisphere, F(1, 26) = 8.50, p < 0.01.
3.4 Comparison of Heavy Cannabis Users with and without Comorbidities
To specify the effect of comorbidities (ADHD, CD, PTSD, etc) on medial temporal structures in our patient sample, we further split the participants into two groups, those with comorbidities (7, some with overlapping comorbidities) and cannabis users without any reported comorbidities (7). A two tailed student t-test showed no significant volume differences between the two groups. Though not significant, the group with comorbidities had higher volume averages for the hippocampus and amygdala. Analysis of variance covarying for TBV and WRAT-III scaled scores showed volume changes for the group with no comorbidities at a trend level (left hippocampus p =.1; right hippocampus p =.1; not significant for amygdala volumes) and no significant differences for the comorbid group and matched normal controls (left hippocampus p = .2; right hippocampus p =.3; not significant for amygdala volumes).
3.5 Total Brain Volume and WRAT-III Correlations
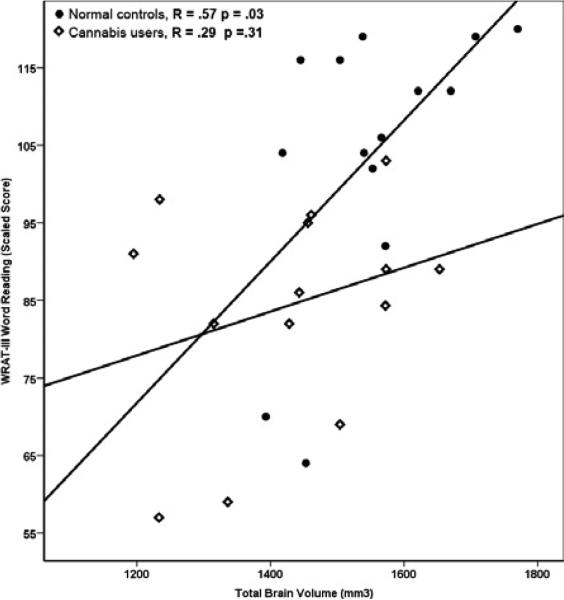
Total brain volume differed significantly between the heavy cannabis users and the healthy controls (F = 6.79, p = .015). This comparison was no longer significant when analysis was covaried for subjects’ WRAT-III scaled scores (F = 1.26, p = .27). Subsequent correlation analysis between the total brain volumes and WRAT-III scaled scores, for each group separately, showed a strong and significant positive correlation between WRAT-III and TBV (Figure 3) for the normal control group (R = .57, p = .03) but not for heavy cannabis users (R = .29, p = .31).
Figure 3.
Correlation between scaled scores of WRAT-III and total brain volume. There was a significant correlation among the normal control group but no correlations for the heavy cannabis users.
3.6 Post Hoc Analysis
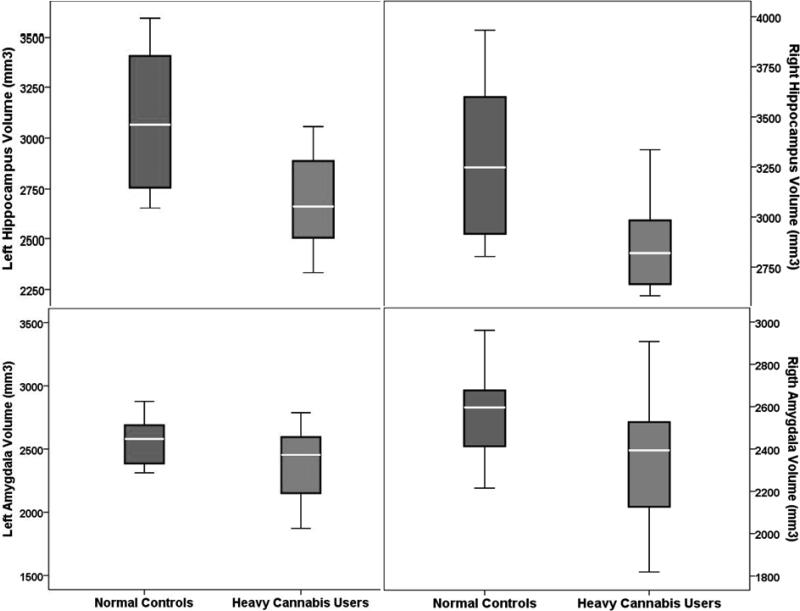
A general linear model (GLM) was used to determine regional differences in medial temporal structure volumes controlling for TBV and WRAT-III scaled scores separately and together. Results are summarized in Table 2 and illustrated in Figure 2. Significant differences were observed for hippocampus volume bilaterally, after controlling for WRAT- III and TBV separately. However, when both variables were controlled for simultaneously, only the left hippocampus volume was significant (F = 4.51, p = .04), while the right hippocampus volume was significant at a trend level (F = 3.45, p = .07). No significant amygdala volume differences were observed between heavy cannabis and matched control groups.
Table 2.
General linear model (GLM) statistical results for hippocampus and amygdala volumes.
| Structure Volume (mm)3 | Controls Mean ± SD | HCU Mean ± SD | Controlling for TBV* | Controlling for WRAT-3 | Controlling for TBV and WRAT-3 | |||
|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | |||
| Left hippocampus | 3112.57 ±340.10 | 2670.71 ±234.18 | 7.605 | .01 | 6.13 | .02 | 4.51 | .04 |
| Right hippocampus | 3270.43 ±394.57 | 2858.07 ±239.84 | 5.213 | .03 | 4.73 | .04 | 3.45 | .07 |
| Left amygdala | 2518.93 ±251.53 | 2460.00 ±380.35 | .007 | .93 | .32 | .57 | .417 | .53 |
| Right amygdala | 2533.29 ±249.16 | 2368.64 ±299.30 | .530 | .47 | .81 | .38 | .36 | .55 |
TBV Total Brain Volume.
Figure 2.
Results for hippocampus and amygdala volumes in heavy cannabis users and controls. Significant differences were observed for hippocampus volume bilaterally, after controlling for WRAT-III and TBV separately. However, when both variables were controlled for at the same time, only the left hippocampus volume was significant (F = 4.51, p = .04) and the right hippocampus volume was significant at a trend level (F = 3.45, p = .07). No significant volumetric differences for the amygdala were found between cannabis users and controls.
3.7 Hippocampus and Amygdala Volumes and Correlations with Cannabis Use
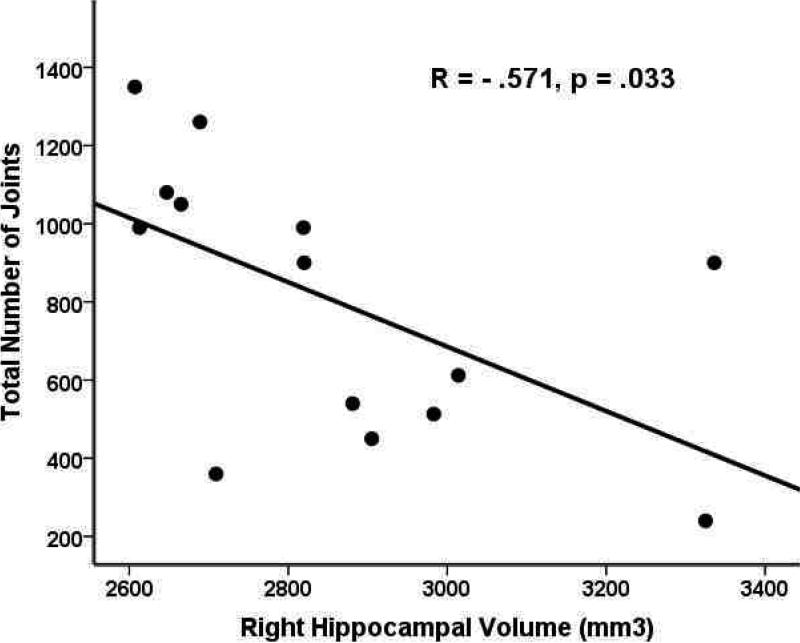
Results from a set of post-hoc exploratory analyses to examine the relationship between age at onset of cannabis use, total amount of drug use, and abstinence time and volume of medial temporal structures revealed significant negative correlations (R =-.57, p = .03) between the right hippocampus volume and the total number of joints smoked, shown in Figure 4.
Figure 4.
Correlation between the right hippocampal volume (mm3) and total number of joints smoked.
3.8 Hippocampus and Amygdala Volumes and Correlations with Verbal Learning and Memory
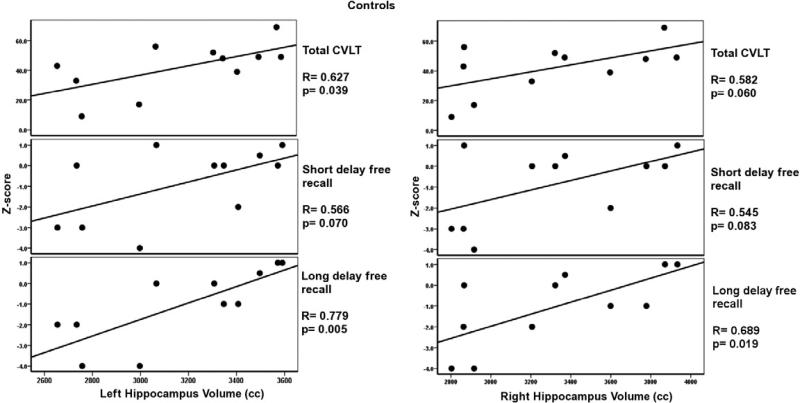
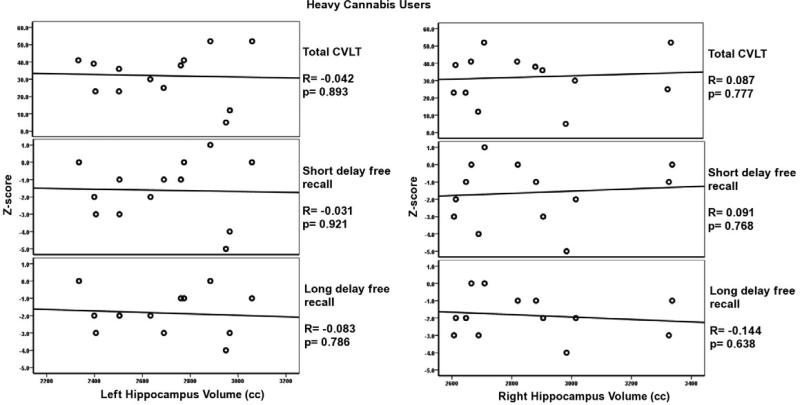
Significant correlations were observed only in the normal control group between the multiple subcomponents of the California Verbal Learning Test (CVLT), and left and right hippocampus volume. These correlations are highlighted in bold in Table 3. No correlations were observed between CVLT test results and hippocampus volumes for heavy cannabis users (see Table 3). Correlations for three main components of the CVLT tests, total CVLT score (total score for free recall trials 1-5), short term free recall, and long term free recall, are presented in Figures 5 and 6 for normal controls and heavy cannabis users, respectively. There were no significant correlations between the CVLT tests and amygdala volumes among normal controls and heavy cannabis users.
Table 3.
Pearson correlations between hippocampal volumes and CVLT measures.
| (Scaled Scores) for Age & Education | Controls | Heavy Cannabis Users | ||||||
|---|---|---|---|---|---|---|---|---|
| Rt. Hippocampus | Lt. Hippocampus | Rt. Hippocampus | Lt. Hippocampus | |||||
| Correlation Coefficient | P Value | Correlation Coefficient | P Value | Correlation Coefficient | P Value | Correlation Coefficient | P Value | |
| CVLT Trials 1-5 | 0.582 | .060 | 0.627 | 0.039 | 0.087 | 0.777 | -0.042 | 0.893 |
| Trial 1 Free Recall | 0.628 | 0.039 | 0.766 | 0.006 | 0.066 | 0.830 | 0.213 | 0.484 |
| Trial 5 Free Recall | 0.599 | 0.052 | 0.688 | 0.019 | 0.137 | 0.656 | 0.005 | 0.987 |
| Short Delay Free Recall | 0.545 | 0.083 | 0.566 | 0.070 | 0.091 | 0.768 | -0.031 | 0.921 |
| Long Delay Free Recall | 0.689 | 0.019 | 0.779 | 0.005 | -0.144 | 0.638 | -0.083 | 0.786 |
| Short Delay Cued Recall | 0.666 | 0.025 | 0.699 | 0.017 | -0.042 | 0.893 | 0.106 | 0.732 |
| Long Delay Cued Recall | 0.473 | 0.142 | 0.631 | 0.037 | -0.072 | 0.816 | -0.107 | 0.727 |
Figure 5.
Correlations between the left and right hippocampus volumes and the CVLT subtests for normal controls. Note the more robust and significant correlations of the CVLT tests (verbal test) with left as opposed to the right hippocampus volume.
Figure 6.
Results for the left and right hippocampus volumes and the CVLT subtests for heavy cannabis users. Note the lack of significant correlations for either the left or right hippocampus volumes with the CVLT measures among the cannabis users.
4. Discussion
4.1 Effect of Cannabis on Hippocampus Structures
The primary findings of this study showed that adolescents engaging in heavy cannabis use had significantly smaller left (14.2%, p = 0.02) and right (12.6%, p = 0.04) hippocampus volumes as compared to their matched controls. No significant changes were observed in the left and right amygdala volume. These results were obtained subsequent to a relatively long period of documented abstinence (average 6.7 months, see Table 1). As such, these findings lend support to a theory that the influences of cannabis may not be just short-term in nature but that the brain may endure long-term structural and functional damage. To our knowledge, this is the first study reporting alteration of brain structures in a group of heavy cannabis users after a long period of drug abstinence. The average period of abstinence in previous studies on the potential harmful effects of cannabis on the brain does not exceed 30 days (Medina et al. 2007a,b; Tapert et al. 2007; Schweinsburg et al. 2008b; Bava et al, 2010; Hanson et al. 2010b; Schweinsburg et al, 2010).
In addition to volumetric differences, the right hippocampus among heavy cannabis users showed a negative significant correlation with the total number of joints smoked (r = -0.57; p = .03, see Figure 4), indicating lower hippocampus volumes were associated with greater cannabis exposure during adolescence. It is again important to note that this robust correlation between smaller right hippocampus volume and total cannabis exposure are from this unique sample of treatment-seeking patients that had a relatively long period of supervised drug abstinence prior to their brain scan. Therefore, our results are possibly more reflective of persistent brain and cognitive anomalies after cessation of drug use and not an acute effect. Findings from our study also support previous animal studies reporting hippocampus damage due to long-term cannabis exposure (Scallet et al., 1987; Landfield et al., 1988; Chan et al., 1998; Lawston et al., 2000).
Our results showed right>left asymmetry for hippocampus volumes for both normal and heavy cannabis users. These results are in agreement with those reported in two other studies evaluating hippocampus volumes in adolescent normal control groups (Giedd et al., 1996; Suzuki et al., 2005). However, our findings are in conflict with results reported by Medina and colleagues (Medina et al., 2007b) who reported left>right hippocampus asymmetry in a group of adolescent cannabis plus alcohol users and their matched normal controls.
In addition to hippocampal volume differences, a significant difference in total brain volume between the two groups was also detected (see Table 1). However, this difference seems to arise due to strong correlations detected between the measures of intelligence (WRAT-III scores) and total brain volume (see Figure 3). The relationship between brain volumes and factors of intelligence has previously been reported for children (Reiss et al., 1996) and adults (Wickett et al., 2000), although it is also reported to have genetic origins as well (Posthuma et al., 2002). A meta-analysis of the relationship between in vivo brain volume and intelligence conducted by McDaniel and colleagues (2005) also reported that for all age and sex groups, brain volume is positively correlated with intelligence.
Abnormalities of medial temporal structures in heavy cannabis users have been recently reported by Yücel et al (2008). Our results are in agreement with this report suggesting that long-term heavy cannabis use is associated with significant hippocampus atrophy that may be related to cumulative cannabis use. However, unlike Yücel and colleagues, we did not find significant changes in amygdala volumes for the heavy cannabis users as compared to their matched controls. This discrepancy may be due to the fact that our study participants were much younger (mean age = 19.3; SD = 0.8) than those studied by Yücel et al (mean age = 39.8; SD = 8.9). A lack of findings in amygdala volumes may also be attributed to the small sample size of this study and shorter duration of cannabis use. The mean duration of cannabis use among users in this study was 5.3 years as opposed to the mean cannabis use of around 20 years reported by Yücel et al (2008).
Although mounting evidence from recent and other studies suggests that heavy cannabis use is associated with alterations in hippocampus morphology, there are conflicting data regarding the long-term effects of cannabis use, as a handful of other studies have reported no association between prior cannabis use and hippocampus morphology. A recent functional MRI (fMRI) report by Jager et al (2010) reported normal activation in bilateral hippocampi and para hippocampal gyri with no associative memory dysfunction in a group of regular cannabis users. This may be due to the fact that the subjects reported in the Jager et al (2010) study were relatively younger and were not considered heavy users as compared to the subjects in our study. Although, Tzilos et al (2005) studied the effect of cannabis in a group of older, long-term users and reported no significant volume differences in the left or right hippocampus. Moreover, Tzilos and colleagues also reported no association between hippocampus volume and the amount or period of cannabis use. The lack of findings reported by Tzilos et al (2005) may have been due to the later age of initiation for drug use among their study population. On the other hand, Yücel and colleagues conducted a study on 15 men (average age 39.8 years) who smoked more than five joints daily for approximately 20 years and reported a significant 12% reduction in hippocampus volume and a smaller but still significant 7% reduction in amygdala volume compared to controls (Yücel et al., 2008). In addition to these studies, there are a number of studies that report no effect of cannabis use on neuropsychological and neurocognitive functions (Fried et al., 2002; Grant et al., 2003; Lyons et al., 2004).
4.2 Effect of Cannabis on Memory Function
Abnormal hippocampus morphology has been primarily associated with a decline in memory function. To characterize the nature of memory impairments of the heavy cannabis users as compared to their matched controls, we used age-appropriate versions of the California Verbal Learning Test (CVLT). Relationships between hippocampus volume and all the CVLT sub-tests of verbal memory functioning were examined for controls and heavy cannabis users. Results showed significant and strong positive correlations between the left and right hippocampus volume and CVLT performance in the control group only, with a stronger correlation between CVLT performance and left hippocampus volume in the control group. This is an interesting result, as the left hippocampus is known to be more engaged in verbal memory function (Rausch et al., 1993; Kilpatrick et al., 1997; Sass et al., 2005; Meador et al., 2006). No correlation between hippocampus volume and the CVLT were found among adolescent heavy cannabis users (see Figures 5 and 6).
Heavy cannabis users did not differ in their performance on the CVLT cognitive memory tests as compared to their matched normal controls. These results were obtained despite the fact that there were no correlations between hippocampus volume and CVLT scores. Although there is no precise explanation, as suggested by a recent study (Jager et al., 2010) this discrepancy may be due to unknown compensatory mechanisms whereby cannabis users may utilize other brain areas or more of the same brain regions to perform tasks at hand. Jager et al (2010) used fMRI to test working memory function in a group of cannabis users and matched controls and reported increased frontal lobe activation among the cannabis users while there were no differences in task performance between groups. Based on these results, Jager and colleagues (2010) suggested that a functional compensatory mechanism may have been used by the cannabis users, resulting in equal task performance as compared to their matched controls. Although Jager and colleagues were primarily evaluating working memory and the frontal lobe, their hypothesis may also be a plausible explanation for our heavy cannabis users’ hippocampus findings and their behavioral performance reported here. However, there is no evidence for this hypothesis and it warrants careful and comprehensive future research.
Numerous studies have reported a relationship between memory function, the hippocampus, and drug abuse. In a recent comprehensive review of the chronic effects of cannabis on memory in humans, Solowij et al (2008) presents clear evidence for impaired encoding, storage, manipulation, and retrieval mechanisms in long-term or heavy cannabis users. Based on this report, sufficient evidence has accumulated to conclude that long-term heavy cannabis use is associated with impaired memory. Particular to adolescents is an earlier study by Pope et al (2003), which reported greater verbal and memory impairment in individuals who initiated heavy cannabis use during adolescence than those who began use later in life. An fMRI study by Jacobsen and colleagues (2004) also reported that adolescent cannabis users showed increased right hippocampal response during a 2-back verbal working memory task as compared to non-users. The most recent study of long-term cannabis effects on memory function was reported by Battisti et al (2010). The authors concluded that relative to non-using controls, chronic users of cannabis had altered memory-related brain activation in the form of dysfunctional subsequent memory effect (SME) production and/or poorer neural efficiency, which was associated with deficits in memory recall (Battisti et al., 2010). This study also reported a significant correlation with a longer history of cannabis use and earlier onset of use with greater brain alteration. These studies are again suggestive that adolescence is a period sensitive to drug use and damage to the hippocampus results in short- and long-term memory dysfunction.
4.3 Study Limitations
There are a number of limitations to the study reported here, many of which are also present in other investigations of this type. The sample size in this study was small, partially due to the difficulty in recruiting a sample that would meet all necessary inclusion/exclusion criteria. This sample included only clinically-referred male patients from mid-to-low socio-economic backgrounds, and with low IQ scores. As mentioned previously, although we have documented and quantified many aspects of marijuana use/abuse in our sample (e.g. the amount of use, initial age and length of use, and period of abstinence), these data are highly dependent on the veracity, validity, and reliability of self-reports. However, we corroborated self-reported information with all available sources of information (treatment charts, clinical staff, and significant others when available).
We did not control for the effects of alcohol and tobacco smoking. The effect of heavy alcohol use on hippocampus volume has been reported by Medina and colleagues (2007b). Although this may be considered a limitation, it should be mentioned that only 5/14 participants were alcohol abusers. Since five of the heavy cannabis users were alcohol abusers and since our controls were not matched for cigarette smoking to the cannabis group, conclusions from this study should be considered preliminary and the differences in hippocampus volume reported here may be due to a combination of alcohol, nicotine, and cannabis use.
Hippocampal volume differences have been found in a variety of psychiatric populations, e.g., depression (Kronmüller et al., 2009; Cole et al., 2010), PTSD (Shin et al., 2004), autism (Nicolson et al., 2006), schizophrenia (Smith et al., 2003), and epilepsy (Scott et al., 2003; Auer et al., 2008). As such, the findings reported here for heavy cannabis users may be nonspecific. However, our preliminary analysis comparing heavy cannabis users with one or more comorbidities to those with no comorbidities showed no significant differences in hippocampus volumes between the two groups. In fact, in a separate analysis comparing each of these groups with normal controls the group with no comorbidities showed more significant bilateral hippocampal volumes differences. Additional studies in at-risk populations prior to the initiation of cannabis use are needed to more fully resolve this issue.
It is also important to mention that findings reported here are from a group of heavy cannabis users and may not extend to recreational users of cannabis with more limited exposure to cannabis. Additionally, these findings may only pertain to a specific subgroup of patients who were treatment seeking with a supervised prolonged period of sustained abstinence. Longitudinal studies are needed to determine if the findings reported here represent the effect of drugs on the developing brain or preexisting abnormalities predisposing the individuals to substance use. Due to the cross-sectional nature of the study, we cannot establish causality between cannabis use and reduced hippocampus volume. A more extensive cognitive evaluation is also needed to draw more solid conclusions regarding the relationship between cannabis, brain structure, and cognition. It is important to note that the control sample and heavy cannabis users had different levels of education, a limitation that should be addressed in future studies by matching groups on education status.
In conclusion, there is growing evidence suggesting that adolescence is a key period for neuronal maturation. We have demonstrated bilateral hippocampal atrophy in a group of adolescents with heavy cannabis use after an average of 6.7 months of abstinence. To our knowledge, this is the first study reporting alteration of brain structures in a group of heavy cannabis users after a long period of abstinence. These results may indicate that heavy cannabis use during adolescence may cause long-term brain alteration in areas known to be involved in neurogenesis or rich in cannabis receptors such as the hippocampus. These results suggest that early onset substance use may affect the normal development of medial temporal structures and potentially disrupt hippocampal functions.
This study strongly suggests that heavy cannabis use has significant adverse effect on brain structures and in particular the hippocampus. It is important to note that reduced hippocampus volumes reported here and elsewhere associated with cannabis use may be evident before the start of drug use, and thus may instead represent a risk factor for drug dependence. Nevertheless, we believe that despite this possibility, there is enough evidence that cannabis use does indeed exert damage on the brain and that heavy consumption represents an especial risk during adolescence. Adolescence is a critical time for brain development and maturation and is thus a vulnerable period to engage in risky behaviors, such as cannabis or other drug use, for both physiological and psychological reasons.
Acknowledgments
Funding for this study was provided by NIMH Grant MH070612, MH073150-01A2, 7K23MH64556-06 and NIDA DA015541. The authors greatly appreciate the aid and cooperation of the residents and staff of Aurora Concepts during the course of this study, as well as the participation of the healthy control subjects. Special acknowledgements in particular go to the following individuals for their help in making this project a successful one: Dr. Joseph Rhinewine, Ms. Hana Kester, Mr. Britt Anderson, and Ms. Emily Thaden.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrous DN, Adriani W, Montaron MF, Aurousseau C, Rougon G, Le Moal M, Piazza PV. Nicotine self administration impairs hippocampal plasticity. Journal of Neuroscience. 2002;22:3656–3662. doi: 10.1523/JNEUROSCI.22-09-03656.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Kirn JR, Nottebohm F. Birth of projection neurons in adult avian brain may be related to perceptual or motor learning. Science. 1990;249:1444–1446. doi: 10.1126/science.1698312. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders 4th edition. American Psychiatric Publishing Inc.; Arlington, VA: 1994. [Google Scholar]
- Ashtari M, Barr WB, Schaul N, Bogerts B. Three-dimensional fast low-angle shot imaging and computerized volume measurement of the hippocampus in patients with chronic epilepsy of the temporal lobe. AJNR American Journal of Neuroradiology. 1991;12:941–947. [PMC free article] [PubMed] [Google Scholar]
- Ashtari M, Greenwald BS, Kramer-Ginsberg E, Hu J, Wu H, Patel M, Aupperle P, Pollack S. Hippocampal/amygdala volumes in geriatric depression. Psychological Medicine. 1999;29:629–638. doi: 10.1017/s0033291799008405. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Cervellione K, Cottone J, Ardekani BA, Sevy S, Kumra S. Diffusion abnormalities in adolescents and young adults with a history of heavy cannabis use. Journal of Psychiatric Research. 2009;43:189–204. doi: 10.1016/j.jpsychires.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer T, Barsi P, Bone B, Angyalosi A, Aradi M, Szalay C, Horvath RA, Kovacs N, Kotek G, Fogarasi A, Komoly S, Janszky I, Schwarcz A, Janszky J. History of simple febrile seizures is associated with hippocampal abnormalities in adults. Epilepsia. 2008;49:1562–1569. doi: 10.1111/j.1528-1167.2008.01679.x. [DOI] [PubMed] [Google Scholar]
- Avants B, Gee JC. Geodesic estimation for large deformation anatomical shape averaging and interpolation. NeuroImage. 2004;23:S139–50. doi: 10.1016/j.neuroimage.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Avants BB, Schoenemann PT, Gee JC. Lagrangian frame diffeomorphic image registration: Morphometric comparison of human and chimpanzee cortex. Medical Image Analysis. 2006;10:397–412. doi: 10.1016/j.media.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Avants BB, Hurt H, Giannetta JM, Epstein CL, Shera DM, Rao H, Wang J, Gee JC. Effects of heavy in utero cocaine exposure on adolescent caudate morphology. Pediatric Neurology. 2006;37:275–279. doi: 10.1016/j.pediatrneurol.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Medical Image Analysis. 2008;12:26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Yushkevich P, Pluta J, Minkoff D, Korczykowski M, Detre J, Gee JC. The optimal template effect in hippocampus studies of diseased populations. NeuroImage. 2010;49:2457–2466. doi: 10.1016/j.neuroimage.2009.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod BN, Ryan JJ, Ward LC. Evaluation of seven-subtest short forms of the Wechsler Adult Intelligence Scale-III in a referred sample. Archives of Clinical Neuropsychology. 2001;16:1–8. [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, Dant CC, Reiss AL. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereberal Cortex. 2005;15:1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Battisti RA, Roodenrys S, Johnstone SJ, Respondek C, Hermens DF, Solowij N. Chronic use of cannabis and poor neural efficiency in verbal memory ability. Psychopharmacology (Berl) 2010;209:319–330. doi: 10.1007/s00213-010-1800-4. [DOI] [PubMed] [Google Scholar]
- Bava S, Tapert SF. Adolescent brain development and the risk for alcohol and other drug problems. Neuropsychology Reviews. 2010;20:398–413. doi: 10.1007/s11065-010-9146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belue RC, Howlett AC, Westlake TM, Hutchings DE. The ontogeny of cannabinoid receptors in the brain of postnatal and aging rats. Neurotoxicology and Teratology. 1995;17:25–30. doi: 10.1016/0892-0362(94)00053-g. [DOI] [PubMed] [Google Scholar]
- Benes FM, Turtle M, Khan Y, Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Archives of General Psychiatry. 1994;51:477–484. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- Bogerts B, Meertz F, Schonfeldt-Bausch R. Basal ganglia and limbic system pathology in schizophrenia: a morphometric study of brain volume and shrinkage. Archives of General Psychiatry. 1985;42:784–791. doi: 10.1001/archpsyc.1985.01790310046006. [DOI] [PubMed] [Google Scholar]
- Bogerts B, Ashtari M, Degreef G, Alvir JMJ, Bilder RM, Lieberman JA. Reduced temporal limbic structure volumes on magnetic resonance images in first-episode schizophrenia. Psychiatry Research Neuroimaging. 1990a;35:1–13. doi: 10.1016/0925-4927(90)90004-p. [DOI] [PubMed] [Google Scholar]
- Bogerts B, Falkai P, Haupts M, Greve B, Ernst S, Tapernon-Franz U, Heinzmann U. Post-mortem volume measurements of limbic system and basal ganglia structures in chronic schizophrenics. Initial results from a new brain collection. Schizophrenia Research. 1990b;3:295–301. doi: 10.1016/0920-9964(90)90013-w. [DOI] [PubMed] [Google Scholar]
- Bogerts B, Lieberman JA, Ashtari M, Bilder RM, Degreef G, Lerner G, Johns C, Masiar S. Hippocampus-amygdala volumes and psychopathology in chronic schizophrenia. Biological Psychiatry. 1993;33:236–246. doi: 10.1016/0006-3223(93)90289-p. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–43. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Carlson G, Wang Y, Alger BE. Endocannabinoids facilitate the induction of LTP in the hippocampus. Nature Neuroscience. 2002;5:723–4. doi: 10.1038/nn879. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Getz S, Galvan A. The adolescent brain. Developmental Review. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha YM, White AM, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of delta9-THC on learning in adolescent and adult rats. Pharmacology Biochemistry and Behavior. 2006;83:448–55. doi: 10.1016/j.pbb.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Chan GC, Hinds TR, Impey S, Storm DR. Hippocampal neurotoxicity of Delta 9-tetrahydrocannabinol. Journal of Neuroscience. 1998;18:5322–5332. doi: 10.1523/JNEUROSCI.18-14-05322.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaroni S, Cuppini R, Cecchini T, Ferri P, Ambrogini P, Cuppini C, Del Grande P. Neurogenesis in the adult rat dentate gyrus is enhanced by vitamin E deficiency. Journal of Comparative Neurology. 1999;411:495–502. [PubMed] [Google Scholar]
- Cole J, Toga AW, Hojatkashani C, Thompson P, Costafreda SG, Cleare AJ, Williams SC, Bullmore ET, Scott JL, Mitterschiffthaler MT, Walsh ND, Donaldson C, Mirza M, Marquand A, Nosarti C, McGuffin P, Fu CH. Subregional hippocampal deformations in major depressive disorder. Journal of Affective Disorders. 2010 April 13; doi: 10.1016/j.jad.2010.03.004. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft RJ, Mackay AJ, Mills AT, Gruzelier JG. The relative contributions of ecstasy and cannabis to cognitive impairment. Psychopharmacology (Berl) 2001;153:373–9. doi: 10.1007/s002130000591. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, Noll J, Boring AM. Sex differences in brain maturation during childhood and adolescence. Cereberal Cortex. 2001;11:552–57. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test: Adult Version Manual. The Psychological Corporation; San Antonio: 1987. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test: Children's Version Manual. The Psychological Corporation; San Antonio: 1994. [Google Scholar]
- Durston S, Hulshoff Pol HE, Casey BJ, Giedd JN, Buitelaar JK, van Engeland H. Anatomical MRI of the developing human brain: what have we learned? J Am Acad Child Adolesc Psychiatry. 2001;40:1012–20. doi: 10.1097/00004583-200109000-00009. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Barrot M, Schad CA, Self DW, Nestler EJ. Opiates inhibit neurogenesis in the adult rat hippocampus. Proceedings of the National Academy of Sciences U.S.A. 2000;97:7579–7584. doi: 10.1073/pnas.120552597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ, Mandyam CD. Drug dependence and addiction, II. Images in Neuroscience. 2004;161:426. doi: 10.1176/appi.ajp.161.3.426. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Cameron HA, Encinas JM, Meltzer LA, Ming GL, Overstreet-Wadiche LS. Adult neurogenesis, mental health, and mental illness: hope or hype? Journal of Neuroscience. 2008;28:11785–11791. doi: 10.1523/JNEUROSCI.3798-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FG. Neurogenesis in the adult hippocampus. Nature Medicine. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkai P, Bogerts B. Cell loss in the hippocampus of schizophrenics. European Archives of Psychiatry and Neurological Sciences. 1986;236:154–61. doi: 10.1007/BF00380943. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Boden JM, Horwood LJ. Cannabis use and other illicit drug use: testing the cannabis gateway hypothesis. Addiction. 2006;101:556–569. doi: 10.1111/j.1360-0443.2005.01322.x. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Blaha CD, Yang CR, Philips AG. Dopamine D1 and NMDA receptors mediate potentiation of basolateral amygdala-evoked firing of nucleus accumbens neurons. Journal of Neuroscience. 2001;21:6370–6376. doi: 10.1523/JNEUROSCI.21-16-06370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedland CS, Whitlow CT, Miller MD, Porrino LJ. Dose-dependent effects of Delta9-tetrahydrocannabinol on rates of local cerebral glucose utilization in rat. Synapse. 2002;45:134–42. doi: 10.1002/syn.10089. [DOI] [PubMed] [Google Scholar]
- Fried P, Watkinson B, James D, Gray R. Current and former marijuana use: preliminary findings of a longitudinal study of effects on IQ in young adults. Canadian Medical Association Journal. 2002;166:887–891. [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4-18. Cerebral Cortex. 1996;6:551–60. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P, McEwen BS, Flügge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proceedings of the National Academy of Sciences U.S.A. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant I, Gonzalez R, Carey CL, Natarajan L, Wolfson T. Non-acute (residual) neurocognitive effects of cannabis use: A meta-analytic study. Journal of the International Neuropsychological Society. 2003;9:679–689. doi: 10.1017/S1355617703950016. [DOI] [PubMed] [Google Scholar]
- Gray KM, Watson NL, Christie DK. Challenges in Quantifying Marijuana Use. American Journal of Addiction. 2009;18:178–179. doi: 10.1080/10550490902772579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Chung MK, Avants BB, Shirtcliff EA, Gee JC, Davidson RJ, Pollak SD. Early stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk. Journal of Neuroscience. 2010a;30:7466–7472. doi: 10.1523/JNEUROSCI.0859-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KL, Medina KL, Nagel BJ, Spadoni AD, Gorlick A, Tapert SF. Hippocampal volumes in adolescents with and without a family history of alcoholism. American Journal of Drug and Alcohol Abuse. 2010b;36:161–167. doi: 10.3109/00952991003736397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey MA, Sellman JD, Porter RJ, Frampton CM. The relationship between non-acute adolescent cannabis use and cognition. Drug and Alcohol Review. 2007;26:309–19. doi: 10.1080/09595230701247772. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Hampson RE, Deadwyler SA. Effects of delta-9-tetrahydrocannabinol on delayed match to sample performance in rats: alterations in short-term memory associated with changes in task specific firing of hippocampal cells. Journal of Pharmacology and Experimental Therapeutics. 1993;264:294–307. [PubMed] [Google Scholar]
- Hoffman AF, Lupica CR. Mechanisms of cannabinoid inhibition of GABA(A) synaptic transmission in the hippocampus. Journal of Neuroscience. 2000;20:2470–9. doi: 10.1523/JNEUROSCI.20-07-02470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen L. Cannabis and the brain. Brain. 2003;126:1252–70. doi: 10.1093/brain/awg143. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Mencl WE, Westerveld M, Pugh KR. Impact of cannabis use on brain function in adolescents. Annals of New York Academy of Sciences. 2004;1021:384–90. doi: 10.1196/annals.1308.053. [DOI] [PubMed] [Google Scholar]
- Jager G, Van Hell HH, De Win MM, Kahn RS, Van Den Brink W, Van Ree JM, Ramsey NF. Effects of frequent cannabis use on hippocampal activity during an associative memory task. European Neuropsychopharmacology. 2007;17:289–97. doi: 10.1016/j.euroneuro.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Jager G, Block RI, Luijten M, Ramsey NF. Cannabis Use and Memory Brain Function in Adolescent Boys: A Cross-Sectional Multicenter Functional Magnetic Resonance Imaging Study. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:561–572.e3. doi: 10.1016/j.jaac.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Zhang Y, Xiao L, Van Cleemput J, Ji SP, Bai G, Zhang X. Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects. Journal of Clinical Investigations. 2005;115:3104–3116. doi: 10.1172/JCI25509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Saykin AJ, Flashman LA, McAllister TW, Sparling MB. Brain activation on fMRI and verbal memory ability: functional neuroanatomic correlates of CVLT performance. Journal of the International Neuropsychology Society. 2001;7:55–62. doi: 10.1017/s135561770171106x. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent druge use: Overview of key findings, 2007. National Institute on Drug Abuse; Bethesda, MD: 20008. (NIH Publication No. 08-6418) [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kim D, Thayer SA. Cannabinoids inhibit the formation of new synapses between hippocampal neurons in culture. Journal of Neuroscience. 2001;21:RC146. doi: 10.1523/JNEUROSCI.21-10-j0004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, Christensen GE, Collins DL, Gee J, Hellier P, Song JH, Jenkinson M, Lepage C, Rueckert D, Thompson P, Vercauteren T, Woods RP, Mann JJ, Parsey RV. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. NeuroImage. 2009;46:786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A, Ghosh SS, Avants B, Yeo BT, Fischl B, Ardekani B, Gee JC, Mann JJ, Parsey RV. Evaluation of volume-based and surface-based brain image registration methods. Neuroimage. 2010;51:214–220. doi: 10.1016/j.neuroimage.2010.01.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick C, Murrie V, Cook M, Andrews D, Desmond P, Hopper J. Degree of left hippocampal atrophy correlates with severity of neuropsychological deficits. Seizure. 1997;6:213–218. doi: 10.1016/s1059-1311(97)80008-8. [DOI] [PubMed] [Google Scholar]
- Kretschmann HJ, Kammradt G, Krauthausen I, Sauer B, Wingert F. Brain growth in man. Bibliotheca anatomica. 1986;28:1–26. [PubMed] [Google Scholar]
- Kronmüller KT, Schröder J, Köhler S, Götz B, Victor D, Unger J, Giesel F, Magnotta V, Mundt C, Essig M, Pantel J. Hippocampal volume in first episode and recurrent depression. Psychiatry Research. 2009;174:62–6. doi: 10.1016/j.pscychresns.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Lawston J, Borella A, Robinson JK, Whitaker-Azmitia PM. Changes in hippocampal morphology following chronic treatment with the synthetic cannabinoid WIN 55,212-2. Brain Research. 2000;877:407–410. doi: 10.1016/s0006-8993(00)02739-6. [DOI] [PubMed] [Google Scholar]
- Landfield PW, Cadwallader LB, Vinsant S. Quantitative changes in hippocampal structure following long-term exposure to delta 9-tetrahydrocannabinol: possible mediation by glucocorticoid systems. Brain Research. 1998;443:47–62. doi: 10.1016/0006-8993(88)91597-1. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. The laterodorsal tegmentum is essential for burst firing of ventral tegmental area dopamine neurons. Proceedings of the National Academy of Sciences U.S.A. 2006;103:5167–5172. doi: 10.1073/pnas.0510715103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luengo MA, Kulis S, Marsiglia FF, Romero E, Gómez-Fraguela JA, Villar P, Nieri T. A cross-national study of preadolescent substance use: exploring differences between youth in Spain and Arizona. Substance Use and Misuse. 2008;43:1571–93. doi: 10.1080/10826080802241078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons MJ, Bar JL, Panizzons MS, Toomey R, Eisen S, Xian H, Tsuang MT. Neuropsychological consequences of regular marijuana use: a twin study. Psychological Medicine. 2004;34:L1239–1250. doi: 10.1017/s0033291704002260. [DOI] [PubMed] [Google Scholar]
- Martin BR, Compton DR, Prescott WR, Barrett RL, Razdan RK. Pharmacological evaluation of dimethylheptyl analogs of delta 9-THC: reassessment of the putative three-point cannabinoid-receptor interaction. Drug and Alcohol Dependence. 1995;37:231–40. doi: 10.1016/0376-8716(94)01081-u. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- McDaniel MA. Big-brained people are smarter: A meta-analysis of the relationship between in vivo brain volume and intelligence. Intelligence. 2005;33:337–346. [Google Scholar]
- Meador K. Memory loss after left anterior temporal lobectomy in patients with mesial temporal lobe sclerosis. Epilepsy Currents. 2006;6:44–45. doi: 10.1111/j.1535-7511.2006.00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Shear PK, Schafer J. Memory functioning in polysubstance dependent women. Drug and Alcohol Dependence. 2006;84:248–55. doi: 10.1016/j.drugalcdep.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Medina KL, Nagel BJ, Park A, McQueeny T, Tapert SF. Depressive symptoms in adolescents: associations with white matter volume and marijuana use. Journal of Child Psychology and Psychiatry. 2007a;48:592–600. doi: 10.1111/j.1469-7610.2007.01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicology and Teratology. 2007b;29:141–52. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–5. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. Journal of Cognitive Neuroscience. 2004;16:1227–33. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- Nicolson R, DeVito TJ, Vidal CN, Sui Y, Hayashi KM, Drost DJ, Williamson PC, Rajakumar N, Toga AW, Thompson PM. Detection and mapping of hippocampal abnormalities in autism. Psychiatry Research. 2006;148:11–21. doi: 10.1016/j.pscychresns.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. Journal of Neurochemistry. 2002;83:1087–1093. doi: 10.1046/j.1471-4159.2002.01214.x. [DOI] [PubMed] [Google Scholar]
- Noonan MA, Choi KH, Self DW, Eisch AJ. Withdrawal from cocaine self-administration normalizes deficits in proliferation and enhances maturity of adult-generated hippocampal neurons. Journal of Neuroscience. 2008;28:2516–2526. doi: 10.1523/JNEUROSCI.4661-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EA, Collins PF, Hooper CJ, Muetzel R, Lim KO, Luciana M. White matter integrity predicts delay discounting behavior in 9- to 23-year-olds: a diffusion tensor imaging study. Journal of Cognitive Neuroscience. 2009;21:1406–21. doi: 10.1162/jocn.2009.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluta J, Avants BB, Glynn S, Awate S, Gee JC, Detre JA. Appearance and incomplete label matching for diffeomorphic template based hippocampus segmentation. Hippocampus. 2009;19:565–571. doi: 10.1002/hipo.20619. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Conti G, Zocchi A, Fleschi C, Orzi F. Metabolic mapping of the effects of WIN 555212-2 intravenous administration in the rat. Neuropsychopharmacology. 1999;21:773–6. doi: 10.1016/S0893-133X(99)00064-0. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Jacobs A, Mialet JP, Yurgelun-Todd D, Gruber S. Evidence for a sex-specific residual effect of cannabis on visuospatial memory. Psychother Psychosom. 1997;66:179–84. doi: 10.1159/000289132. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Yurgelun-Todd D. The residual cognitive effects of heavy marijuana use in college students. JAMA. 1996;275:521–7. [PubMed] [Google Scholar]
- Pope HG, Jr., Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug and Alcohol Dependence. 2003;9:303–10. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- Posthuma D, De Geus EJC, Baaré WFC, Hulshoff Pol HE, Kahn RS, Boomsma DI. The association between brain volume and intelligence is of genetic origin. Nature Neuroscience. 2002;5:83–84. doi: 10.1038/nn0202-83. [DOI] [PubMed] [Google Scholar]
- Puighermanal E, Marsicano G, Busquets-Garcia A, Lutz B, Maldonado R, Ozaita A. Cannabinoid modulation of hippocampal long-term memory is mediated by mTOR signaling. Nature Neuroscience. 2009;12:1152–8. doi: 10.1038/nn.2369. [DOI] [PubMed] [Google Scholar]
- Quickfall J, Crockford D. Brain neuroimaging in cannabis use: a review. The Journal of Neuropsychiatry and Clinical Neurosciences. 18:318–32. doi: 10.1176/jnp.2006.18.3.318. [DOI] [PubMed] [Google Scholar]
- Rao H, Betancourt L, Giannetta JM, Brodsky NL, Korczykowski M, Avants BB, Gee JC, Wang J, Hurt H, Detre JA, Farah MJ. Early parental care is important for hippocampal maturation: evidence from brain morphology in humans. Neuroimage. 2010;49:1144–1150. doi: 10.1016/j.neuroimage.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch R, Babb TL. Hippocampal Neuron Loss and Memory Scores Before and After Temporal Lobe Surgery for Epilepsy. Archives of Neurology. 1993;50:812–817. doi: 10.1001/archneur.1993.00540080023008. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children: a volumetric imaging study. Brain. 1996;119:1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Reznikov K, Hauser KF, Nazarevskaja G, Trunoa Y, Derjabin V, Bakalkin G. Opioids modulate cell division in the germinal zone of the late embryonic neocortex. European Journal of Neuroscience. 1999;11:2711–2719. doi: 10.1046/j.1460-9568.1999.00680.x. [DOI] [PubMed] [Google Scholar]
- Rhinewine JP, Lencz T, Thaden EP, Cervellione KL, Burdick KE, Henderson I, Bhaskar S, Keehlisen L, Kane J, Kohn N, Fisch GS, Bilder RM, Kumra S. Neurocognitive profile in adolescents with early-onset schizophrenia: clinical correlates. Biological Psychiatry. 2005;58:705–12. doi: 10.1016/j.biopsych.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Robbe D, Montgomery SM, Thome A, Rueda-Orozco PE, McNaughton BL, Buzsaki G. Cannabinoids reveal importance of spike timing coordination in hippocampal function. Nature Neuroscience. 2006;9:1526–33. doi: 10.1038/nn1801. [DOI] [PubMed] [Google Scholar]
- Robbe D, Buzsáki G. Alteration of theta timescale dynamics of hippocampal place cells by a cannabinoid is associated with memory impairment. Journal of Neuroscience. 2009;29:12597–605. doi: 10.1523/JNEUROSCI.2407-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roofeh D, Cottone J, Burdick KE, Lencz T, Gyato K, Cervellione KL, Napolitano B, Kester H, Anderson B, Kumra S. Deficits in memory strategy use are related to verbal memory impairments in adolescents with schizophrenia-spectrum disorders. Schizophrenia Research. 2006;851:201–12. doi: 10.1016/j.schres.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Rubino T, Realini N, Braida D, Guidi S, Capurro V, Vigano D, Guidali C, Pinter M, Sala M, Bartesaghi R, Parolaro D. Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus. 2009;19:763–772. doi: 10.1002/hipo.20554. [DOI] [PubMed] [Google Scholar]
- Sass JK, Westerveld M, Buchanan CP, Spencer SS, Kim JH, Spencer DD. Degree of Hippocampal Neuron Loss Determines Severity of Verbal Memory Decrease After Left Anteromesiotemporal Lobectomy. Epilepsia. 2005;35:1179–1186. doi: 10.1111/j.1528-1157.1994.tb01786.x. [DOI] [PubMed] [Google Scholar]
- Scallet AC, Uemura E, Andrews A, Ali SF, McMillan DE, Paule MG, Brown RM, Slikker W., Jr Morphometric studies of the rat hippocampus following chronic delta-9-tetrahydrocannabinol (THC). Brain Research. 1987;436:193–8. doi: 10.1016/0006-8993(87)91576-9. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: a cross-sectional diffusion-tensor MR imaging study. Radiology. 2002;222:212–18. doi: 10.1148/radiol.2221010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK, Dardzinski BJ. Developmental differences in white matter architecture between boys and girls. Human Brain Mapping. 2008;29:696–710. doi: 10.1002/hbm.20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Koch M. Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology. 2003;28:1760–9. doi: 10.1038/sj.npp.1300225. [DOI] [PubMed] [Google Scholar]
- Schwartz RH, Gruenewald PJ, Klitzner M, Fedio P. Short-term memory impairment in cannabis-dependent adolescents. American Journal of Diseases of Children. 1989;143:1214–9. doi: 10.1001/archpedi.1989.02150220110030. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Cheung EH, Brown GG, Brown SA, Tapert SF. fMRI response to spatial working memory in adolescents with comorbid marijuana and alcohol use disorders. Drug and Alcohol Dependence. 2005;79:201–10. doi: 10.1016/j.drugalcdep.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Brown SA, Tapert SF. The influence of marijuana use on neurocognitive functioning in adolescents. Current Drug Abuse Reviews. 2008a;1:99–111. doi: 10.2174/1874473710801010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Nagel BJ, Schweinsburg BC, Park A, Theilmann RJ, Tapert SF. Abstinent adolescent marijuana users show altered fMRI response during spatial working memory. Psychiatry Research: Neuroimaging. 2008b;163:40–51. doi: 10.1016/j.pscychresns.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Medina KL, McQueeny T, Brown SA, Tapert SF. The influence of recency of use on fMRI response during spatial working memory in adolescent marijuana users. Journal of Psychoactive Drugs. 2010;28:3586–3594. doi: 10.1080/02791072.2010.10400703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott RC, King MD, Gadian DG, Neville BG, Connelly A. Hippocampal abnormalities after prolonged febrile convulsion: a longitudinal MRI study. Brain. 2003;126:2551–7. doi: 10.1093/brain/awg262. Epub 2003 Aug 22. [DOI] [PubMed] [Google Scholar]
- Shin LM, Shin PS, Heckers S, Krangel TS, Macklin ML, Orr SP, Lasko N, Segal E, Makris N, Richert K, Levering J, Schacter DL, Alpert NM, Fischman AJ, Pitman RK, Rauch SL. Hippocampal function in posttraumatic stress disorder. Hippocampus. 2004;14:292–300. doi: 10.1002/hipo.10183. [DOI] [PubMed] [Google Scholar]
- Smith SM. BET: Brain Extraction Tool, FMRIB technical Report TR00SMS2b. 2002 http://www.fmrib.ox.ac.uk/fsl/bet2/ Oxford Centre for Functional Magnetic Resonance Imaging of the Brain.
- Smith GN, Lang DJ, Kopala LC, Lapointe JS, Falkai P, Honer WG. Developmental abnormalities of the hippocampus in first-episode schizophrenia. Biological Psychiatry. 2003;53:555–61. doi: 10.1016/s0006-3223(02)01977-7. [DOI] [PubMed] [Google Scholar]
- Solowij N, Grenyer BF. Are the adverse consequences of cannabis use age-dependent? Addiction. 2002;97:1083–6. doi: 10.1046/j.1360-0443.2002.00243.x. [DOI] [PubMed] [Google Scholar]
- Solowij N, Battisti R. The chronic effects of cannabis on memory in humans: a review. Current Drug Abuse Reviews. 2008;1:81–98. doi: 10.2174/1874473710801010081. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Hare T, Casey BJ. Frontostriatal Maturation Predicts Cognitive Control Failure to Appetitive Cues in Adolescents. Journal of Cognitive Neuroscience. 2010 Sep 1; doi: 10.1162/jocn.2010.21572. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A Social Neuroscience Perspective on Adolescent Risk-Taking. Developmental Review. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiglick A, Kalant H. Learning impairment in the radial-arm maze following prolonged cannabis treatment in rats. Psychopharmacology (Berl) 1982;77:117–23. doi: 10.1007/BF00431932. [DOI] [PubMed] [Google Scholar]
- Stiglick A, Kalant H. Residual effects of chronic cannabis treatment on behavior in mature rats. Psychopharmacology (Berl) 1985;85:436–9. doi: 10.1007/BF00429660. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychological Reviews. 1992;9:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Matsuzawa H, Keww IL, Nakada T. Absolute eigenvalue diffusion tensor analysis for human brain maturation. NMR in Biomedicine. 2003;16:257–60. doi: 10.1002/nbm.848. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Hagino H, Nohara S, Zhou SY, Kawasaki Y, Takahashi T, Matsui M, Seto H, Ono T, Kurachi M. Male-specific volume expansion of the human hippocampus during adolescence. Cerebral Cortex. 2005;15:187–93. doi: 10.1093/cercor/bhh121. [DOI] [PubMed] [Google Scholar]
- Taepavarapruk P, Floresco SB, Phillips AG. Hyperlocomotion and increased dopamine efflux in the rat nucleus accumbens evoked by electrical stimulation of the ventral subiculum: role of ionotropic glutamate and dopamine D1 receptors. Psychopharmacology. 2000;151:242–251. doi: 10.1007/s002130000376. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Drummond SP, Paulus MP, Brown SA, Yang TT, Frank LR. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology (Berl) 2007;194:173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry-McElrath YM, Johnston LD, O'Malley PM, Yamaguchi R. Substance abuse counseling services in secondary schools: a national study of schools and students, 1999-2003. Journal of School Health. 2005;75:334–341. doi: 10.1111/j.1746-1561.2005.00047.x. [DOI] [PubMed] [Google Scholar]
- Tzilos GK, Cintron CB, Wood JB, Simpson NS, Young AD, Pope HG, Jr., Yurgelun-Todd DA. Lack of hippocampal volume change in long-term heavy cannabis users. American Journal of Addiction. 2005;14:64–72. doi: 10.1080/10550490590899862. [DOI] [PubMed] [Google Scholar]
- United States Department of Health and Human Services. Substance Abuse and Mental Health Services Administration. Office of Applied Studies . National Survey on Drug Use and Health. Inter-university Consortium for Political and Social Research [distributor]; Ann Arbor, MI: 2007. [Computer file]. ICPSR23782-v2. 2009-08-12. doi:10.3886/ICPSR23782. [Google Scholar]
- Varma VK, Malhotra AK, Dang R, Das K, Nehra R. Cannabis and cognitive functions: a prospective study. Drug Alcohol Depend. 1988;21:147–52. doi: 10.1016/0376-8716(88)90061-0. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WAIS-III: Wechsler Adult Intelligence Scale–Third Edition: administration and scoring manual. The Psychological Corporation; San Antonio: 1997. [Google Scholar]
- Wickett JC, Vernon PA, Lee Donald H. Relationships between factors of intelligence and brain volume. Personality and Individual Differences. 2000;29:1095–1122. [Google Scholar]
- Wilkinson G. Wide Range Acheivement Test-3 (WRAT-3) Jastak Associates; Wilmington, DE: 1993. [Google Scholar]
- Wise LE, Thorpe AJ, Lichtman AH. Hippocampal CB1 receptors mediate the memory impairing effects of Δ9-tetrahydrocannabinol. Neuropsychopharmacology. 2009;34:2072–2080. doi: 10.1038/npp.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yücel M, Solowij N, Respondek C, Whittle S, Fornito A, Pantelis C, Lubman DI. Regional brain abnormalities associated with long-term heavy cannabis use. Archives of General Psychiatry. 2008;65:694–701. doi: 10.1001/archpsyc.65.6.694. [DOI] [PubMed] [Google Scholar]
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. NeuroImage. 2006;31:1116–28. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]