Abstract
Resting naive CD8+ T cells have an astounding capacity to react to pathogens by massive expansion and differentiation into cytotoxic effector cells that migrate to all corners of the body to clear the infection. The initial interaction with antigen-presenting cells in the central lymphoid organs drives an orchestrated program of differentiation aimed at producing sufficient numbers of effectors to get the job done without resulting in clonal exhaustion. Interactions with antigen-presenting cells and other immune cells continue at the site of infection to regulate further on-site expansion and differentiation, all with the goal of protecting the host with minimal bystander tissue damage. Here we review recent advances in CD8+ T cell recognition of antigen in lymphoid as well as in nonlymphoid tissues in the periphery, and how CD8+ T cell expansion and differentiation are controlled in these contexts.
Main Text
Introduction to Cytotoxic T Cells
Much of the early work that would eventually lead to the recognition of antigen-specific cell-mediated lysis of target cells relied on allogeneic, MHC-disparate tissue and tumor transplantation models and allogeneic mixed lymphocyte cultures. In many of these systems, a subset of thymus-derived T lymphocytes with clonally distributed receptors was shown to be responsible for in vitro cell-mediated lysis of target cells (Cantor and Boyse, 1975, Cerottini et al., 1970, Golstein et al., 1972). However, it was work in a syngeneic system with lymphocytic choriomeningitis virus (LCMV)-infected mice that revealed the dual specificity of specific T lymphocytes for viral antigen plus self-MHC that explained the involvement of MHC class I molecules with CD8+ T cell recognition of antigen and introduced the notion of “altered self” (Zinkernagel and Doherty, 1974). Just how readily viruses and other infections stimulate potent cytotoxic T lymphocyte (CTL) responses is illustrated by human cases of acute infectious mononucleosis or “kissing disease” caused by exposure to the Epstein-Barr gamma herpes virus (EBV). The disease is characterized by swollen lymph nodes and a remarkable rise in the number of peripheral blood monocytes. In fact the bulk of the monocytosis turns out to be a lymphocytosis consisting mostly of activated CD8+ CTL with specificity for EBV peptides (Callan et al., 1996). The response to EBV provides a remarkable example of the magnitude of the proliferative burst of clones of antigen-specific CD8+ lymphocytes in response to an infectious agent. Similarly, it had been realized for many years that infection of mice with LCMV led to an inversion of the CD4:CD8 ratio because of a dramatic increase in CD8+ T cell numbers but it was not until tetramer staining or the adoptive transfer of small numbers of TCR transgenic CD8+ T cells was employed that it was realized that the bulk of the CD8+ expansion was due to antigen-driven proliferation (Butz and Bevan, 1998, Murali-Krishna et al., 1998). During many infections, all T lymphocytes regardless of specificity may undergo cytokine-driven phenotypic changes—so-called bystander activation—but only those T cells that recognize pathogen-encoded antigen go through multiple rounds of replication to generate enormous numbers of CTL effector progeny that are the foot soldiers of the adaptive immune response.
Recruiting: Initial CD8+ T Cell Activation
During an infection, naive CD8+ T cells are primed by antigen-presenting cells (APCs) in secondary lymphoid organs such as lymph nodes (LN) and spleen. How are the CD8+ T cells activated by the APCs? Seeing is believing. The application of multiphoton-based intravital microscopy (IVM) has greatly advanced our knowledge about immune response initiation. Previous work had shown that in the absence of antigen, naive T cells in the LNs engage in what appears to be a random walk in the T cell area, which is actually their wandering on the fibroblastic reticular network (Bajénoff et al., 2006). Subsequent to injection of peptide-loaded dendritic cells (DCs), T cells scan the HEV-associated DC forming antigen-specific contacts with the DCs, leading to T cell activation (Bousso and Robey, 2003, Mempel et al., 2004, Miller et al., 2003). However, because injected peptide-pulsed DCs supply the antigenic stimulus in these experiments, where and when APC and naive T cells interact during an infection remained undefined. More recent research has revisited this issue of CD8+ T cell recruitment in infectious settings (Chtanova et al., 2009, Hickman et al., 2008, John et al., 2009). Two groups using virus or parasite infection models have shown that naive CD8+ T cells first contact the antigen-bearing DCs in the subcapsular sinus region or the interfollicular region of the draining LN (Hickman et al., 2008, John et al., 2009). This peripheral location in the LN is in sharp contrast to the central HEV region after peptide-pulsed DC immunization. In naive mice, CD8+ T cells mainly reside in the T cell zones while the DCs form an extensive network throughout the T cell zone, B cell follicle, and some areas of the subcapsular sinus (Lindquist et al., 2004). Shortly after infection, at the same time that the infectious agents can be detected in the LNs, the CD8+ T cells and DCs are quickly enriched in the peripheral regions of the nodes (Hickman et al., 2008, John et al., 2009). Particulate antigen and pathogens arrive via the lymphatics at the subcapsular sinus of the draining LN. The first and major cell population infected by pathogen is CD169+ macrophages lining the subcapsular sinus. However, instead of these antigen-rich macrophages, naive CD8+ T cells favor the DC population to deliver the first kiss to start their differentiation to effector cells. During vaccinia virus (VV) or vesicular stomatitis virus (VSV) infection, naive CD8+ T cell relocation is largely antigen specific (Hickman et al., 2008). In contrast, during Toxoplasma gondii infection, the relocation is cognate antigen independent. Indeed, T. gondii infection induces rapid remodeling of the lymph node architecture and decreases the expression of chemokine CCL21 (John et al., 2009), similar to the changes after LCMV infection (Mueller et al., 2007). Distinct features of the VV, VSV, or T. gondii infection may contribute to the different mechanisms underlying CD8+ T cell relocation. Upon T. gondii rechallenge, memory CD8+ T cells migrate to the region close to the LN subcapsular sinus to form contact with the APCs in an antigen-specific fashion (Chtanova et al., 2009). Based on these observations, we can conclude that shortly after a peripheral infection in response to the gradient of cognate antigen and/or the dramatic changes of the lymph node structure and chemokine levels, naive CD8+ T cells quickly migrate toward the peripheral regions of the T cell zone close to the subcapsular sinus region and the infected macrophage population. Meanwhile, DCs also relocate to the same area and acquire antigens from the infected cells through direct infection or cross-presentation. At this peripheral site of the LNs, initial antigen-specific contacts between the CD8+ T cells and DCs are formed that lead to CD8+ T cell activation and expansion. Because the subcapsular region is close to the antigen-rich region early during infection, it may be a more efficient strategy to initiate an immune response in such a specialized area of the lymph nodes.
How is the migration of DCs regulated during the initiation of an immune response? Recently, the chemokine-chemokine receptor pair XCL1-XCR1 has emerged as a potentially important contributor (Dorner et al., 2009). Expression of XCR1 is highly restricted to the CD8α+ population of lymphoid tissue-resident DCs. The ligand XCL1 is produced by activated CD8+ T cells. In response to TCR stimulation, with kinetics of induction almost identical to that of the early activation markers CD69 and CD25 (Dorner et al., 2009), the XCL1-XCR1 interaction plays an early role during an immune response. Indeed, with XCL1-deficient mice and anti-DEC205-mediated delivery of foreign antigen, XCL1 is required for maximal priming and expansion of CD8+ T cells (Dorner et al., 2009). In addition, chemokines such as CCL3, CCL4, and CCL17 produced by DCs have been shown to attract cognate CCR5+ or CCR4+ naive CD8+ T cells during priming (Castellino et al., 2006, Semmling et al., 2010). Therefore, a feed forward loop between the CD8+ T cells and the DCs may be established by these chemokine-chemokine receptor interactions to maximize the recruitment of both antigen-specific naive CD8+ T cells and DCs during priming. However, the role of these chemokines during the initiation phase of a CTL response to various infectious agents awaits further investigation.
At the peak of the primary response to pathogen, the population of antigen-induced CD8+ effector T cells is phenotypically and functionally heterogeneous. So-called short-lived effector cells (SLEC) form the bulk of the population but will mostly die off when infection is cleared, whereas memory precursor effector cells (MPEC) that may have received less stimulation survive and contribute preferentially to the memory population (Mescher et al., 2006, Parish and Kaech, 2009). Elegant experiments involving single-cell transfer and CD8+ T cell barcoding demonstrate that a single naive CD8+ T cell can differentiate into a diverse population of effector and memory cells (Gerlach et al., 2010, Stemberger et al., 2007). In apparent contrast to this notion of functional diversity arising from a single CD8+ T cell is the suggestion that CD8+ T cell fate is fixed to some degree even before the first cell division. Use of a bicistronic reporter mouse strain Ifng-YFP to visualize activation of the gene encoding interferon (IFN)-γ reveals that the YFP signal is turned on within 24 hr of DC-peptide immunization (Beuneu et al., 2010). Importantly, even before the first cell division, a high degree of variation in the amount of YFP signal is observed in the responding T cells. Interestingly, isolated single CD8+ T cells maintain their high versus low YFP signal intensity even after a few rounds of cell division in vitro, i.e., the YFP signal intensity is maintained stably throughout expansion. Higher YFP signal intensity correlates with higher TNF-α and IL-2 production (Beuneu et al., 2010). The variation of Ifng gene induction reported by the YFP signal is controlled by the MHC-peptide epitope density on the DC. Whether this early heterogeneity contributes to the different effector and memory CD8+ T cell differentiation in the long term is not resolved at present. Because we know that a single CD8+ cell can give rise to heterogenous progeny, i.e., SLEC and MPEC, then later encounters with DCs as well as other extrinsic factors must be able to modulate the differentiation. During bacterial, parasitic, or viral infection, factors such as inflammatory signals, costimulation, and encounters with different APCs add another layer of complexity during CD8+ effector T cell differentiation.
Other work suggests that CD8+ T cell clonal heterogeneity may be imposed at the first cell division. First division CD8+ T cells segregate their protein degradation machinery in an asymmetric manner, resulting in different amounts of the critical transcriptional factor T-bet distributed to the two daughter cells (Chang et al., 2011). Notably, even 2-fold differences in T-bet expression can affect CD8+ T cell effector differentiation with higher amounts promoting SLEC differentiation (Joshi et al., 2007). The CD8hi daughter cell (considered to be proximal to the APC) contains fewer proteasomes and more T-bet whereas the CD8lo daughter cell (distal) has more proteasomes and less T-bet. Genetic models of Itk deficiency and SLP-76 mutation that fail to phosphorylate T-bet do not show asymmetric distribution of T-bet. However, even though SLP-76 mutant CD8+ T cells cannot efficiently degrade T-bet during the first cell division, SLP-76 mutant CD8+ T cells still develop into MPEC and memory cells efficiently after LCMV infection (Smith-Garvin et al., 2010). This latter finding is inconsistent with the notion that the asymmetric distribution of T-bet at the first division controls cell fate. Although asymmetric cell division may play a fundamental role in development, asymmetric distribution of the protein degradation machinery during mitosis is an evolutionarily conserved strategy without any fate-determining consequence. Even HeLa cells dividing in vitro show asymmetric segregation of the protein-degradation machinery (Fuentealba et al., 2008). Proteasomes and proteins targeted for degradation are concentrated in the pericentrosomal region. During mitosis, whereas the centrioles and associated centrosomal MTOC (microtubule organizing center) are divided equally, the pericentrosomal materials remain largely in one daughter cell. Whether asymmetric first cell division during the CD8+ immune response is just another example of this conserved phenomenon or has specific functional consequences for effector versus memory cell differentiation awaits discrimination.
Thus, we see that specialized mechanisms of antigen presentation efficiently recruit naive antigen-specific T cells into an immune response. During rapid cell division, daughter cells become heterogeneous by as yet undefined signals to provide both short-lived effectors as well as memory precursors.
Preparing and Expanding the Troops
Calculations from data such as in Badovinac et al. (2007) imply that a naive CD8+ T cell may go through as many as 19 cell divisions in the week after pathogen stimulation, representing a potential 500,000-fold expansion! The rate of maximal CD8+ cell division is usually quoted as 4–6 hr though one paper estimates a 2 hr cycle (Yoon et al., 2010). Upon antigen priming, resting naive T cells must undergo dramatic changes in metabolism, such as enhanced uptake of glucose, amino acids, and iron (Michalek and Rathmell, 2010). Another critical change is the metabolic switch from oxidative phosphorylation to aerobic glycolysis, presumably to meet the sudden demand for the building blocks of nucleic acids, lipids, and protein to make new cells (Michalek and Rathmell, 2010, Vander Heiden et al., 2009). How is this striking switch from rest to rapid expansion regulated in T cells? Recently, it has been demonstrated that in addition to costimulatory signals, such as CD28-induced PI-3K-Akt-mTOR signaling (Michalek and Rathmell, 2010), TCR-ERK signaling is required for enhanced glycolysis and glucose plus glutamine uptake (Carr et al., 2010, Marko et al., 2010). Surprisingly, phosphoinositide-dependent kinase 1 (PDK1), but not Akt, is required for CD8+ T cell proliferation and glucose metabolism in vitro (Macintyre et al., 2011). Consistent with these data, PTEN deficiency or constitutively active Akt does not improve antigen-specific CD8+ T cell expansion during LCMV infection (Hand et al., 2010). Indeed, constitutive Akt signaling induces more dramatic contraction and defective memory cell formation. However, PDK1 is not involved in amino acid uptake in activated CD8+ T cells (Macintyre et al., 2011). Therefore, other pathways may exist to regulate different aspects of activated CD8+ T cell metabolism. Indeed, TCR engagement alone leads to the activation of multiple molecular pathways, including MAPK and mTOR to activate ribosomal protein S6 and the protein translation machinery (Salmond et al., 2009). mTOR is a conserved environmental sensor that controls cell growth, proliferation, and metabolism (Powell and Delgoffe, 2010). According to dogma, TCR plus CD28 and cytokine receptor stimulation activates the PI-3K-PDK1-Akt pathway. In turn, activated Akt induces mTOR activity, which leads to the activation of multiple downstream targets, including 4E-BP1 and S6K1 (Powell and Delgoffe, 2010). However, because Akt activity is not required for IL-2-induced activated CD8+ T cell proliferation and metabolism in vitro (Macintyre et al., 2011), this classic pathway may need to be modified. Nevertheless, the metabolic changes are critical for effector CD8+ T cell differentiation.
To achieve maximal expansion, CD8+ T cells need to integrate multiple signals, including the TCR, costimulatory signals, and inflammatory cytokines, such as IL-12 and type I IFN (Mescher et al., 2006, Parish and Kaech, 2009). Many members of the tumor necrosis factor receptor (TNFR) family such as 4-1BB, CD27, and OX-40 deliver important costimulatory signals to CD8+ T cells (Watts, 2005). Another TNFR family member, glucocorticoid-induced TNFR-related protein (GITR), delivers a prosurvival signal to effector CD8+ T cells to promote CD8+ T cell expansion and protect mice from severe influenza virus infection (Snell et al., 2010). In addition, MyD88, a well-studied adaptor molecule in innate immunity that is essential for most TLR and IL-1 and IL-18 signaling, has an intrinsic role in CD8+ T cells. With adoptive transfer of T cells, mixed bone marrow chimeric mice, or T cell-specific deletion of MyD88, several groups have demonstrated an intrinsic role for MyD88 in effector T cell expansion and survival subsequent to a variety of infectious agents (Bartholdy et al., 2009, Quigley et al., 2009, Rahman et al., 2008, Zhao et al., 2009). TLR2 signaling in CD8+ T cells induces mammalian target of rapamycin (mTOR) activation, which in turn promotes T-bet synthesis and cytolytic effector gene transcription in vitro (Geng et al., 2010). However, inconsistent results were obtained regarding the in vivo role of TLR2 during infections (Quigley et al., 2009, Zhao et al., 2009). Therefore, a resolution of which upstream signals activate MyD88 in CD8+ T cells awaits further studies. Interestingly, although MyD88 is critical for CD8+ T cell primary expansion, it does not appear to be required for the CD8+ memory T cell recall response to LCMV infection (Rahman et al., 2011).
From these studies it is clear that the dramatic rate of expansion of CD8+ T cells responding to pathogen-encoded antigen depends on signals from multiple receptors that impinge upon cell division and cell survival.
Weaponry: Terminal Effector Differentiation
Proinflammatory cytokines, such as IL-12, play a key role in terminal differentiation of CD8+ effector T cells (Curtsinger et al., 2003, Pearce and Shen, 2007). Mechanistically, IL-12 promotes effector CD8+ T cell differentiation through the induction of T-bet (Joshi et al., 2007, Takemoto et al., 2006) via an mTOR-dependent pathway (Rao et al., 2010).
IL-2 was originally defined as a T cell growth factor in vitro. It is predominately produced by activated CD4+ T cells and at lower amounts by activated CD8+ T cells (Malek and Castro, 2010). The in vivo role of IL-2 signaling in CD8+ T cells during infections has been elucidated with CD25-deficient (Il2ra −/−) and wild-type (WT) mixed bone marrow chimeric mice. Clonal expansion is only slightly decreased in cells lacking CD25 but they show phenotypic and functional alterations (Bachmann et al., 2007, Obar et al., 2010, Williams et al., 2006). Compared to control cells, the induced and expanded Il2ra −/− effector CD8+ T cells exhibit a trend toward a more MPEC or central memory (Tcm) cell phenotype during primary responses, including increased CD62L and CD127 and decreased KLRG1 expression. Recent papers have established an important function for IL-2 in CD8+ effector T cell terminal differentiation. Consistent with the previous results with a similar in vitro culture system (Carrio et al., 2004), Pipkin et al. (2010) show that activated CD8+ T cells cultured in a high concentration of IL-2 gradually acquire superior effector functions compared with cells cultured in low concentrations of IL-2. After adoptive transfer in vivo, CD8+ T cells from high IL-2 cultures fail to maintain their number over the long term and are delayed in upregulating CD62L expression. Il2ra −/− CD8+ effector T cells are defective in ex vivo killing, correlated with the decreased expression of granzymeB and perforin (Pipkin et al., 2010). Another study reports that whereas CD25 expression is uniformly high on antigen-specific CD8+ T cells early during LCMV infection, a biphasic expression pattern is evident by day 3.5–5 postinfection (Kalia et al., 2010). After isolation at this stage and transfer into infection-matched recipient mice, CD25hi cells go on to express higher amounts of KLRG1 and granzymeB and lower amounts of CD62L and IL-2 compared with transferred CD25lo cells. CD25hi cells are defective in their long-term homeostasis and their recall response. Detailed analysis reveals that the KLRG1hiCD127lo SLEC population drops to less than 10% of control cells in the absence of CD25 during the contraction phase (Mitchell et al., 2010). Taken together, these results demonstrate that IL-2 signaling, delivered early after infection, promotes SLEC differentiation. In the absence or decreased amount of IL-2 signaling, CD8+ T cells exhibit defective effector function and preferentially become CD62Lhi memory cells. This conclusion has been further confirmed with γc-deficient CD8+ T cells (Decaluwe et al., 2010). IL-2 is therefore better viewed as a differentiation factor than as a growth factor during the in vivo response to pathogens.
What are the downstream targets of IL-2 signaling that promote effector CD8+ T cell development? IL-2 signaling activates multiple molecular pathways, including STAT5, PI-3K, and MAPK (Malek and Castro, 2010). Therefore, distinct molecules may contribute to different aspects of effector CD8+ T cell differentiation.
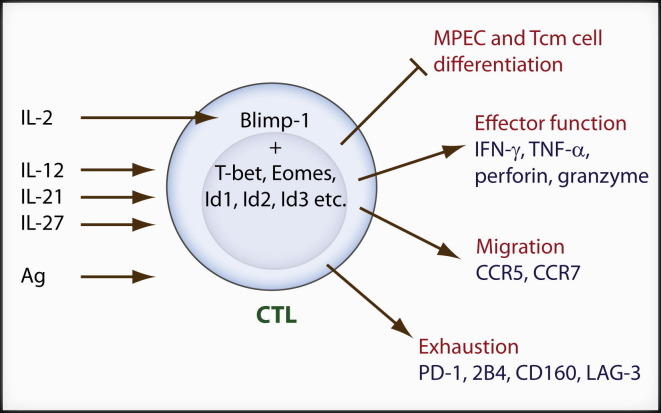
IL-2 signals activate the transcriptional repressor Blimp-1 (encoded by the Prdm1 gene) and Blimp-1 inhibits IL-2 production (Malek and Castro, 2010). Three groups have studied the requirement for Blimp-1 during CD8+ effector T cell development (Kallies et al., 2009, Rutishauser et al., 2009, Shin et al., 2009). The expression of Blimp-1 in CD8+ T cells is largely confined to the KLRG1hiCD127lo SLEC population during acute infections, decreasing as memory cells are formed (Kallies et al., 2009, Rutishauser et al., 2009). During chronic viral infection, Blimp-1 is expressed at a much higher level than during acute infection (Shin et al., 2009). In the absence of Blimp-1, clonal expansion of CD8+ T cells is apparently normal during primary infection with LCMV whereas the contraction is delayed (Rutishauser et al., 2009). Interestingly, during influenza virus respiratory infection, Prdm1 −/− effector CD8+ T cells are defective in accumulation in the lung presumably because of slightly decreased CCR5 expression (Kallies et al., 2009). However, a migratory defect is not obvious during LCMV infection (Rutishauser et al., 2009). One striking phenotype of Prdm1-deficient CD8+ T cells is that they exhibit severe defects in effector functions and SLEC differentiation. Instead, Prdm1 −/− CD8+ T cells more readily upregulate MPEC markers. For example, Prdm1 −/− CD8+ T cells express lower amounts of granzymeB, perforin, and KLRG1 but have higher amounts of CD127, CCR7, CD62L, CD27, and IL-2. During chronic infection, Blimp-1 deficiency in CD8+ T cells leads to decreased expression of multiple inhibitory receptors associated with CD8+ T cell exhaustion, such as PD-1, LAG-3, CD160, and 2B4 (Shin et al., 2009). Interestingly, Blimp-1 haploinsufficiency in antigen-specific CD8+ T cells leads to enhanced virus clearance because of balancing decreased expression of inhibitory receptors with maintenance of sufficient effector function (Shin et al., 2009). Because clonally exhausted CD8+ T cells may be considered an extreme state of effector cells, all these results demonstrate that Blimp-1 is an important regulator of SLEC differentiation. Several transcriptional regulators are differentially expressed between Prdm1 −/− and WT CD8+ effector cells. Those factors promoting CD8+ memory cell differentiation such as Tcf-1, Bcl6, and Eomes are inhibited by Blimp-1 whereas those promoting CD8+ effector cell differentiation such as Tbx21 and Id2 are enhanced by Blimp-1 (Kallies et al., 2009, Rutishauser et al., 2009). Among these transcriptional regulators, Bcl-6 is another transcription repressor that functions as a reciprocal antagonist of Blimp-1 in B cells, CD4+ T cells, and probably also in CD8+ T cells (Crotty et al., 2010). Blimp-1 may be a critical factor pushing SLEC differentiation downstream of IL-2 signals (Figure 1 ).
Figure 1.
IL-2- and Blimp-1-Dependent Effector CD8+ T Cell Differentiation
After antigen priming, activated CD8+ T cells receive signals from multiple cytokines such as IL-2, IL-21, Il-12, and Il-27. IL-2 signaling induces Blimp-1 expression which cooperates with other transcription factors to promote CD8+ (CTL) T cell effector differentiation, migration to the peripheral sites during acute infection, and exhaustion during chronic infection while inhibiting MPEC and Tcm cell differentiation.
There are several differences between Il2ra −/− and Prdm1 −/− CD8+ T cells. Il2ra −/− antigen-specific CD8+ T cells disappear rapidly during chronic LCMV infection whereas Prdm1 −/− cells are maintained at a normal to slightly higher numbers than control CD8+ T cells (Bachmann et al., 2007, Shin et al., 2009). These differences are due to two main reasons. First, IL-2 signaling modulates other intracellular pathways in CD8+ T cells. For example, IL-2 inhibits the activity of Foxo through a PDK1-Akt-dependent mechanism (Macintyre et al., 2011). Foxo induces another transcription factor, KLF2, that activates several key molecules related to effector T cell migration including CD62L, S1P1, and CCR7 (Takada et al., 2011). Second, in addition to IL-2, other cytokines, such as IL-12, IL-27, and IL-21, might induce Blimp-1 expression in activated CD8+ T cells (Gong and Malek, 2007, Kwon et al., 2009, Sun et al., 2011). Like Blimp-1, IL-21 signaling plays an important role in CD8+ T cells during chronic viral infection (Elsaesser et al., 2009, Fröhlich et al., 2009, Yi et al., 2009).
Besides the differences between IL-2 signaling-induced and Blimp-1-controlled molecular pathways, Blimp-1 may not lie in the center of effector CD8+ T cell differentiation program. The transcriptional regulators that are differentially expressed between Prdm1 −/− and WT cells may not lie downstream of Blimp-1. A large group of genes differentially expressed between Prdm1 −/− and WT cells do not contain Blimp-1 binding sites and may not be direct targets of Blimp-1 (Rutishauser et al., 2009). In addition, other factors may affect CD8+ effector cell fate decision. Runx3, Notch2, and the canonical Wnt pathway effector, Tcf-1, are all involved in CD8+ effector T cell differentiation (Cruz-Guilloty et al., 2009, Jeannet et al., 2010, Maekawa et al., 2008, Zhou et al., 2010). Interestingly, the expression of Tcf-1 is substantially perturbed in the absence of Blimp-1, PDK1, or active Akt (Macintyre et al., 2011, Rutishauser et al., 2009). In summary, we have just begun to elucidate the molecular pathways controlling CD8+ effector T cell differentiation. The interactions and relationships between different signaling pathways and transcriptional regulators will hold the key toward better understanding of the molecular control of CD8+ effector T cell development.
Getting There: Migration of Effector CTL
Shortly after recognition of antigen on DCs in the central lymphoid organs, activated T cells upregulate the inflammatory cytokine receptor, CXCR3, allowing them to enter peripheral tissues (Groom and Luster, 2011). CD4+ T cell help in activating the APC is required for the generation of the CD8+ primary response to noninflammatory antigens and certain viral infections such as herpes simplex virus (HSV), whereas the primary CD8+ response to influenza, Listeria, and LCMV infection is CD4+ T cell independent (Bevan, 2004). An additional function of CD4+ T cell help is apparent in peripheral infected tissues where it is required for the enhanced recruitment of CTL into the infected site (Nakanishi et al., 2009). After vaginal infection with HSV-2, CD4+ effector T cells enter the infected vaginal tissues earlier than CD8+ effector T cells. CD4+ cells secrete IFN-γ, presumably in response to seeing antigen at the site, promoting the vaginal epithelium to secrete CXCL9 and CXCL10, which guide the migration of CD8+ effector cells into the infected tissues through a CXCR3-dependent mechanism (Nakanishi et al., 2009). Other peripheral tissues such as lung and intestine show no requirement for CD4+ help for the recruitment of effector CD8+ T cells in this model but these sites do not express viral antigen. Infected skin may be another site that requires CD4+ T cell help to more efficiently attract effector CD8+ T cells (Gebhardt and Carbone, 2009).
Even after leaving the central lymphoid organs and trafficking via the blood to the peripheral site of infection, CD8+ effector CTL continue to engage in antigen-specific interactions that (apart from resulting in cytolysis of infected targets) drive further proliferation and cytokine release. A combination of local CFSE and BrdU administration reveals extensive continued proliferation of newly arrived CTL in the lung contributing substantially to the overall magnitude of the response to influenza infection (Bedoui and Gebhardt, 2011, McGill and Legge, 2009). The greatest on-site proliferation coincides with the appearance in the lung on monocyte-derived inflammatory DCs. Another recent study, also with sublethal influenza infection, demonstrates that although infected lung epithelial cells can trigger CTL degranulation and killing, inflammatory cytokine secretion requires antigen presentation by CD11c+ hematopoietic cells expressing costimulatory ligands (Hufford et al., 2011). Whether recruited monocyte-derived inflammatory APCs present antigen by becoming infected themselves or by cross-presentation or cross-dressing is not known (Wakim and Bevan, 2011). Thus we see that even after migration from the central lymphoid organs where the response initiates, CD8+ T cell interactions with helper T cells and with specialized APCs continue at peripheral sites of infection.
Discipline: Self-Control by CD8+ Effectors
In addition to killing infected cells and releasing cytokines such as IFN-γ at the peripheral site of infection, recent reports have assigned another duty to effector CD8+ T cells, namely to serve a regulatory role in preventing excessive tissue injury by secretion of the immunosuppressive cytokine IL-10 (Palmer et al., 2010, Sun et al., 2009, Trandem et al., 2011). Originally defined as a Th2 cytokine, IL-10 is secreted by multiple cell types, including B cells, NK cells, NKT cells, macrophages, DCs, monocytes, and different effector CD4+ T cell subsets (Th1, Th2, Th17, and Treg cells) (Saraiva and O'Garra, 2010). Fully differentiated effector CD8+ T cells produce large amounts of IL-10 at local infection sites in different viral infection models in the lung and brain (Palmer et al., 2010, Sun et al., 2009, Trandem et al., 2011). At the peak of the response, CD8+ T cells are the major producers of IL-10 at these peripheral sites whereas in secondary lymphoid organs, CD4+ T cell-derived IL-10 dominates (Palmer et al., 2010, Sun et al., 2009, Trandem et al., 2011). During the later phase of the response when the infection has been controlled, IL-10+CD8+ T cells rapidly disappear while IL-10+ CD4+ cells persist, suggesting that different mechanisms regulate IL-10 production in CD8+ versus CD4+ T cells. Compared with their IL-10− colleagues at the site of infection, IL-10+CD8+ T cells are superior killers and produce normal to higher amounts of granzymeB, IFN-γ, and TNF-α. Importantly, IL-10 produced by effector CD8+ T cells is critical to prevent immunopathology during viral infection without affecting the kinetics of viral clearance. It is important to note that the IL-10-producing subset of CD8+ effector T cells does not represent a divergent effector lineage; rather, it is a transient and reversible state of CD8+ effector T cell differentiation to better balance clearance of the infection with bystander tissue damage. Thus, after transfer into infected hosts, both IL-10+ and IL-10− effector CD8+ T cells differentiate into a population with a similar percentage of IL-10-producing cells in a coronavirus-induced encephalitis model (Trandem et al., 2011).
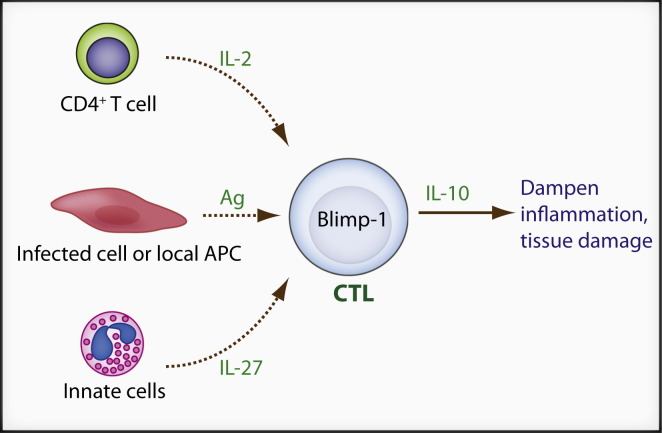
What extracellular stimuli and intracellular signaling pathways control IL-10 production in CD8+ T cells at peripheral sites of acute viral infection? In CD4+ T cells, IL-10 production is regulated by a variety of mechanisms. For instance, GATA3 and STAT6 are required for IL-10 production in Th2 cells (Saraiva and O'Garra, 2010). IRF4 and Blimp-1 regulate IL-10 in a subset of Treg cells (Cretney et al., 2011). IL-12-STAT4 and maybe c-maf control IL-10 in Th1 cells (Saraiva et al., 2009). c-maf and Ahr (acryl hydrocarbon receptor) are essential for IL-10 induction in Tr1 cells (Apetoh et al., 2010). In addition, strong TCR stimulation and ERK activation promote IL-10 production in Th1 cells (Saraiva et al., 2009). For CD8+ T cells, during intranasal influenza virus infection, IL-10 production requires the synergistic cooperation between CD4+ T cell-derived IL-2 and innate (mainly neutrophil) derived IL-27 (Sun et al., 2011). Blimp-1 expression in CD8+ T cells is essential for IL-10 production and the level of Blimp-1, but not T-bet or c-maf, correlates with the degree of IL-10 induction (Sun et al., 2011). Considering the aforementioned function of IL-2 and Blimp-1 in controlling CD8+ effector functions, these data suggest that IL-10 production may be a part of the full CD8+ T cell effector differentiation program. Strong or continued TCR-MAPK signaling may also promote IL-10 production because the majority of IL-10+CD8+ T cells are CD69+ during coronavirus infection, suggesting recent TCR stimulation (Trandem et al., 2011). Consistent with this notion, potent TCR stimulation may be a common signal to induce IL-10 in peripheral effector CD8+ T cells because IL-10 production drops quickly after virus clearance in both influenza virus and coronavirus models (Sun et al., 2009, Trandem et al., 2011). The requirement for Blimp-1, IL-2, and IL-27 in IL-10 secretion has been confirmed in a well-controlled in vitro culture system (Sun et al., 2011) and these factors plus antigen stimulation may be the general requirements for IL-10 production in effector CD8+ T cells (Figure 2 ).
Figure 2.
Self-Control by Effector CD8+ T Cells
In infected peripheral tissues, some effector CD8+ (CTL) T cells receive additional local signals including antigen, CD4+ T cell-derived IL-2, and innate cell-derived IL-27, and transiently acquire the ability to secrete IL-10 in a Blimp-1-dependent manner. CTL-derived IL-10 is critical to control local inflammation and tissue damage.
A dynamic picture emerges from these important recent discoveries. Upon acute infection, naive CD8+ T cells receive cognate antigen stimulation and differentiate into effector T cells in a highly regulated way including IL-2- and Blimp-1-dependent mechanisms. After migrating to the sites of infection, to protect the normal physiologic functions of these critical organs, such as the brain and the lung, some effector CD8+ T cells receive additional local signals including antigen, IL-2, and IL-27 and transiently acquire the ability to secrete IL-10 in a Blimp-1-dependent manner. Future experiments will determine whether IL-10+CD8+ T cells arise at other sites of infection with other pathogens.
More than 400 differentially expressed genes are identified between IL-10+ and IL-10−CD8+ effector T cells in the coronavirus model (Trandem et al., 2011), raising the possibility that there are other functions associated with IL-10 production by CD8+ effector T cells. Answers to these questions will broaden and deepen our knowledge about the role of CD8+ T cells in protecting against immunopathology at sites of infection.
Concluding Remarks
After infection and MHC class I antigen processing and presentation, antigen-specific CD8+ T cells receive multiple extracellular signals to initiate rapid proliferation and a sophisticated CTL differentiation program. A large number of signaling pathways cooperate to regulate CD8+ T cell proliferation, survival, migration, metabolism, and acquisition of effector functions. Even after effector T cells reach peripheral sites of infection, interactions with other professional hematopoietic cells continue to guide the terminal differentiation of the cells. A better understanding of the process of CD8+ effector T cell differentiation is key to aid future vaccine design and to better manipulate CD8+ effector function in order to maximize pathogen clearance while minimizing any associated immunopathology.
Contributor Information
Nu Zhang, Email: nuz@uw.edu.
Michael J. Bevan, Email: mbevan@uw.edu.
References
- Apetoh L., Quintana F.J., Pot C., Joller N., Xiao S., Kumar D., Burns E.J., Sherr D.H., Weiner H.L., Kuchroo V.K. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat. Immunol. 2010;11:854–861. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann M.F., Wolint P., Walton S., Schwarz K., Oxenius A. Differential role of IL-2R signaling for CD8+ T cell responses in acute and chronic viral infections. Eur. J. Immunol. 2007;37:1502–1512. doi: 10.1002/eji.200637023. [DOI] [PubMed] [Google Scholar]
- Badovinac V.P., Haring J.S., Harty J.T. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8(+) T cell response to infection. Immunity. 2007;26:827–841. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajénoff M., Egen J.G., Koo L.Y., Laugier J.P., Brau F., Glaichenhaus N., Germain R.N. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. 2006;25:989–1001. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholdy C., Christensen J.E., Grujic M., Christensen J.P., Thomsen A.R. T-cell intrinsic expression of MyD88 is required for sustained expansion of the virus-specific CD8+ T-cell population in LCMV-infected mice. J. Gen. Virol. 2009;90:423–431. doi: 10.1099/vir.0.004960-0. [DOI] [PubMed] [Google Scholar]
- Bedoui S., Gebhardt T. Interaction between dendritic cells and T cells during peripheral virus infections: A role for antigen presentation beyond lymphoid organs? Curr. Opin. Immunol. 2011;23:124–130. doi: 10.1016/j.coi.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Beuneu H., Lemaître F., Deguine J., Moreau H.D., Bouvier I., Garcia Z., Albert M.L., Bousso P. Visualizing the functional diversification of CD8+ T cell responses in lymph nodes. Immunity. 2010;33:412–423. doi: 10.1016/j.immuni.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Bevan M.J. Helping the CD8(+) T-cell response. Nat. Rev. Immunol. 2004;4:595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- Bousso P., Robey E. Dynamics of CD8+ T cell priming by dendritic cells in intact lymph nodes. Nat. Immunol. 2003;4:579–585. doi: 10.1038/ni928. [DOI] [PubMed] [Google Scholar]
- Butz E.A., Bevan M.J. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity. 1998;8:167–175. doi: 10.1016/s1074-7613(00)80469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callan M.F., Steven N., Krausa P., Wilson J.D., Moss P.A., Gillespie G.M., Bell J.I., Rickinson A.B., McMichael A.J. Large clonal expansions of CD8+ T cells in acute infectious mononucleosis. Nat. Med. 1996;2:906–911. doi: 10.1038/nm0896-906. [DOI] [PubMed] [Google Scholar]
- Cantor H., Boyse E.A. Functional subclasses of T-lymphocytes bearing different Ly antigens. I. The generation of functionally distinct T-cell subclasses is a differentiative process independent of antigen. J. Exp. Med. 1975;141:1376–1389. doi: 10.1084/jem.141.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr E.L., Kelman A., Wu G.S., Gopaul R., Senkevitch E., Aghvanyan A., Turay A.M., Frauwirth K.A. Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J. Immunol. 2010;185:1037–1044. doi: 10.4049/jimmunol.0903586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrio R., Bathe O.F., Malek T.R. Initial antigen encounter programs CD8+ T cells competent to develop into memory cells that are activated in an antigen-free, IL-7- and IL-15-rich environment. J. Immunol. 2004;172:7315–7323. doi: 10.4049/jimmunol.172.12.7315. [DOI] [PubMed] [Google Scholar]
- Castellino F., Huang A.Y., Altan-Bonnet G., Stoll S., Scheinecker C., Germain R.N. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 2006;440:890–895. doi: 10.1038/nature04651. [DOI] [PubMed] [Google Scholar]
- Cerottini J.C., Nordin A.A., Brunner K.T. Specific in vitro cytotoxicity of thymus-derived lymphocytes sensitized to alloantigens. Nature. 1970;228:1308–1309. doi: 10.1038/2281308a0. [DOI] [PubMed] [Google Scholar]
- Chang J.T., Ciocca M.L., Kinjyo I., Palanivel V.R., McClurkin C.E., Dejong C.S., Mooney E.C., Kim J.S., Steinel N.C., Oliaro J. Asymmetric proteasome segregation as a mechanism for unequal partitioning of the transcription factor T-bet during T lymphocyte division. Immunity. 2011;34:492–504. doi: 10.1016/j.immuni.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chtanova T., Han S.J., Schaeffer M., van Dooren G.G., Herzmark P., Striepen B., Robey E.A. Dynamics of T cell, antigen-presenting cell, and pathogen interactions during recall responses in the lymph node. Immunity. 2009;31:342–355. doi: 10.1016/j.immuni.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cretney E., Xin A., Shi W., Minnich M., Masson F., Miasari M., Belz G.T., Smyth G.K., Busslinger M., Nutt S.L., Kallies A. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat. Immunol. 2011;12:304–311. doi: 10.1038/ni.2006. [DOI] [PubMed] [Google Scholar]
- Crotty S., Johnston R.J., Schoenberger S.P. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat. Immunol. 2010;11:114–120. doi: 10.1038/ni.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Guilloty F., Pipkin M.E., Djuretic I.M., Levanon D., Lotem J., Lichtenheld M.G., Groner Y., Rao A. Runx3 and T-box proteins cooperate to establish the transcriptional program of effector CTLs. J. Exp. Med. 2009;206:51–59. doi: 10.1084/jem.20081242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtsinger J.M., Lins D.C., Mescher M.F. Signal 3 determines tolerance versus full activation of naive CD8 T cells: Dissociating proliferation and development of effector function. J. Exp. Med. 2003;197:1141–1151. doi: 10.1084/jem.20021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaluwe H., Taillardet M., Corcuff E., Munitic I., Law H.K., Rocha B., Rivière Y., Di Santo J.P. Gamma(c) deficiency precludes CD8+ T cell memory despite formation of potent T cell effectors. Proc. Natl. Acad. Sci. USA. 2010;107:9311–9316. doi: 10.1073/pnas.0913729107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner B.G., Dorner M.B., Zhou X., Opitz C., Mora A., Güttler S., Hutloff A., Mages H.W., Ranke K., Schaefer M. Selective expression of the chemokine receptor XCR1 on cross-presenting dendritic cells determines cooperation with CD8+ T cells. Immunity. 2009;31:823–833. doi: 10.1016/j.immuni.2009.08.027. [DOI] [PubMed] [Google Scholar]
- Elsaesser H., Sauer K., Brooks D.G. IL-21 is required to control chronic viral infection. Science. 2009;324:1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhlich A., Kisielow J., Schmitz I., Freigang S., Shamshiev A.T., Weber J., Marsland B.J., Oxenius A., Kopf M. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 2009;324:1576–1580. doi: 10.1126/science.1172815. [DOI] [PubMed] [Google Scholar]
- Fuentealba L.C., Eivers E., Geissert D., Taelman V., De Robertis E.M. Asymmetric mitosis: Unequal segregation of proteins destined for degradation. Proc. Natl. Acad. Sci. USA. 2008;105:7732–7737. doi: 10.1073/pnas.0803027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt T., Carbone F.R. Immunology: A helpers' guide to infection. Nature. 2009;462:418–419. doi: 10.1038/462418a. [DOI] [PubMed] [Google Scholar]
- Geng D., Zheng L., Srivastava R., Asprodites N., Velasco-Gonzalez C., Davila E. When Toll-like receptor and T-cell receptor signals collide: a mechanism for enhanced CD8 T-cell effector function. Blood. 2010;116:3494–3504. doi: 10.1182/blood-2010-02-268169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach C., van Heijst J.W., Swart E., Sie D., Armstrong N., Kerkhoven R.M., Zehn D., Bevan M.J., Schepers K., Schumacher T.N. One naive T cell, multiple fates in CD8+ T cell differentiation. J. Exp. Med. 2010;207:1235–1246. doi: 10.1084/jem.20091175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golstein P., Wigzell H., Blomgren H., Svedmyr E.A. Cells mediating specific in vitro cytotoxicity. II. Probable autonomy of thymus-processed lymphocytes (T cells) for the killing of allogeneic target cells. J. Exp. Med. 1972;135:890–906. doi: 10.1084/jem.135.4.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong D., Malek T.R. Cytokine-dependent Blimp-1 expression in activated T cells inhibits IL-2 production. J. Immunol. 2007;178:242–252. doi: 10.4049/jimmunol.178.1.242. [DOI] [PubMed] [Google Scholar]
- Groom J.R., Luster A.D. CXCR3 ligands: Redundant, collaborative and antagonistic functions. Immunol. Cell Biol. 2011;89:207–215. doi: 10.1038/icb.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand T.W., Cui W., Jung Y.W., Sefik E., Joshi N.S., Chandele A., Liu Y., Kaech S.M. Differential effects of STAT5 and PI3K/AKT signaling on effector and memory CD8 T-cell survival. Proc. Natl. Acad. Sci. USA. 2010;107:16601–16606. doi: 10.1073/pnas.1003457107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman H.D., Takeda K., Skon C.N., Murray F.R., Hensley S.E., Loomis J., Barber G.N., Bennink J.R., Yewdell J.W. Direct priming of antiviral CD8+ T cells in the peripheral interfollicular region of lymph nodes. Nat. Immunol. 2008;9:155–165. doi: 10.1038/ni1557. [DOI] [PubMed] [Google Scholar]
- Hufford M.M., Kim T.S., Sun J., Braciale T.J. Antiviral CD8+ T cell effector activities in situ are regulated by target cell type. J. Exp. Med. 2011;208:167–180. doi: 10.1084/jem.20101850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannet G., Boudousquié C., Gardiol N., Kang J., Huelsken J., Held W. Essential role of the Wnt pathway effector Tcf-1 for the establishment of functional CD8 T cell memory. Proc. Natl. Acad. Sci. USA. 2010;107:9777–9782. doi: 10.1073/pnas.0914127107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John B., Harris T.H., Tait E.D., Wilson E.H., Gregg B., Ng L.G., Mrass P., Roos D.S., Dzierszinski F., Weninger W., Hunter C.A. Dynamic imaging of CD8(+) T cells and dendritic cells during infection with Toxoplasma gondii. PLoS Pathog. 2009;5:e1000505. doi: 10.1371/journal.ppat.1000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi N.S., Cui W., Chandele A., Lee H.K., Urso D.R., Hagman J., Gapin L., Kaech S.M. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia V., Sarkar S., Subramaniam S., Haining W.N., Smith K.A., Ahmed R. Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity. 2010;32:91–103. doi: 10.1016/j.immuni.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Kallies A., Xin A., Belz G.T., Nutt S.L. Blimp-1 transcription factor is required for the differentiation of effector CD8(+) T cells and memory responses. Immunity. 2009;31:283–295. doi: 10.1016/j.immuni.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Kwon H., Thierry-Mieg D., Thierry-Mieg J., Kim H.P., Oh J., Tunyaplin C., Carotta S., Donovan C.E., Goldman M.L., Tailor P. Analysis of interleukin-21-induced Prdm1 gene regulation reveals functional cooperation of STAT3 and IRF4 transcription factors. Immunity. 2009;31:941–952. doi: 10.1016/j.immuni.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist R.L., Shakhar G., Dudziak D., Wardemann H., Eisenreich T., Dustin M.L., Nussenzweig M.C. Visualizing dendritic cell networks in vivo. Nat. Immunol. 2004;5:1243–1250. doi: 10.1038/ni1139. [DOI] [PubMed] [Google Scholar]
- Macintyre A.N., Finlay D., Preston G., Sinclair L.V., Waugh C.M., Tamas P., Feijoo C., Okkenhaug K., Cantrell D.A. Protein kinase B controls transcriptional programs that direct cytotoxic T cell fate but is dispensable for T cell metabolism. Immunity. 2011;34:224–236. doi: 10.1016/j.immuni.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa Y., Minato Y., Ishifune C., Kurihara T., Kitamura A., Kojima H., Yagita H., Sakata-Yanagimoto M., Saito T., Taniuchi I. Notch2 integrates signaling by the transcription factors RBP-J and CREB1 to promote T cell cytotoxicity. Nat. Immunol. 2008;9:1140–1147. doi: 10.1038/ni.1649. [DOI] [PubMed] [Google Scholar]
- Malek T.R., Castro I. Interleukin-2 receptor signaling: At the interface between tolerance and immunity. Immunity. 2010;33:153–165. doi: 10.1016/j.immuni.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marko A.J., Miller R.A., Kelman A., Frauwirth K.A. Induction of glucose metabolism in stimulated T lymphocytes is regulated by mitogen-activated protein kinase signaling. PLoS ONE. 2010;5:e15425. doi: 10.1371/journal.pone.0015425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill J., Legge K.L. Cutting edge: Contribution of lung-resident T cell proliferation to the overall magnitude of the antigen-specific CD8 T cell response in the lungs following murine influenza virus infection. J. Immunol. 2009;183:4177–4181. doi: 10.4049/jimmunol.0901109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mempel T.R., Henrickson S.E., Von Andrian U.H. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- Mescher M.F., Curtsinger J.M., Agarwal P., Casey K.A., Gerner M., Hammerbeck C.D., Popescu F., Xiao Z. Signals required for programming effector and memory development by CD8+ T cells. Immunol. Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- Michalek R.D., Rathmell J.C. The metabolic life and times of a T-cell. Immunol. Rev. 2010;236:190–202. doi: 10.1111/j.1600-065X.2010.00911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M.J., Wei S.H., Cahalan M.D., Parker I. Autonomous T cell trafficking examined in vivo with intravital two-photon microscopy. Proc. Natl. Acad. Sci. USA. 2003;100:2604–2609. doi: 10.1073/pnas.2628040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D.M., Ravkov E.V., Williams M.A. Distinct roles for IL-2 and IL-15 in the differentiation and survival of CD8+ effector and memory T cells. J. Immunol. 2010;184:6719–6730. doi: 10.4049/jimmunol.0904089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S.N., Hosiawa-Meagher K.A., Konieczny B.T., Sullivan B.M., Bachmann M.F., Locksley R.M., Ahmed R., Matloubian M. Regulation of homeostatic chemokine expression and cell trafficking during immune responses. Science. 2007;317:670–674. doi: 10.1126/science.1144830. [DOI] [PubMed] [Google Scholar]
- Murali-Krishna K., Altman J.D., Suresh M., Sourdive D.J., Zajac A.J., Miller J.D., Slansky J., Ahmed R. Counting antigen-specific CD8 T cells: A reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- Nakanishi Y., Lu B., Gerard C., Iwasaki A. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. 2009;462:510–513. doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obar J.J., Molloy M.J., Jellison E.R., Stoklasek T.A., Zhang W., Usherwood E.J., Lefrançois L. CD4+ T cell regulation of CD25 expression controls development of short-lived effector CD8+ T cells in primary and secondary responses. Proc. Natl. Acad. Sci. USA. 2010;107:193–198. doi: 10.1073/pnas.0909945107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer E.M., Holbrook B.C., Arimilli S., Parks G.D., Alexander-Miller M.A. IFNgamma-producing, virus-specific CD8+ effector cells acquire the ability to produce IL-10 as a result of entry into the infected lung environment. Virology. 2010;404:225–230. doi: 10.1016/j.virol.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish I.A., Kaech S.M. Diversity in CD8(+) T cell differentiation. Curr. Opin. Immunol. 2009;21:291–297. doi: 10.1016/j.coi.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce E.L., Shen H. Generation of CD8 T cell memory is regulated by IL-12. J. Immunol. 2007;179:2074–2081. doi: 10.4049/jimmunol.179.4.2074. [DOI] [PubMed] [Google Scholar]
- Pipkin M.E., Sacks J.A., Cruz-Guilloty F., Lichtenheld M.G., Bevan M.J., Rao A. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32:79–90. doi: 10.1016/j.immuni.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J.D., Delgoffe G.M. The mammalian target of rapamycin: Linking T cell differentiation, function, and metabolism. Immunity. 2010;33:301–311. doi: 10.1016/j.immuni.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley M., Martinez J., Huang X., Yang Y. A critical role for direct TLR2-MyD88 signaling in CD8 T-cell clonal expansion and memory formation following vaccinia viral infection. Blood. 2009;113:2256–2264. doi: 10.1182/blood-2008-03-148809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A.H., Cui W., Larosa D.F., Taylor D.K., Zhang J., Goldstein D.R., Wherry E.J., Kaech S.M., Turka L.A. MyD88 plays a critical T cell-intrinsic role in supporting CD8 T cell expansion during acute lymphocytic choriomeningitis virus infection. J. Immunol. 2008;181:3804–3810. doi: 10.4049/jimmunol.181.6.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A.H., Zhang R., Blosser C.D., Hou B., Defranco A.L., Maltzman J.S., Wherry E.J., Turka L.A. Antiviral memory CD8 T-cell differentiation, maintenance, and secondary expansion occur independently of MyD88. Blood. 2011;117:3123–3130. doi: 10.1182/blood-2010-11-318485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R.R., Li Q., Odunsi K., Shrikant P.A. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 2010;32:67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser R.L., Martins G.A., Kalachikov S., Chandele A., Parish I.A., Meffre E., Jacob J., Calame K., Kaech S.M. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31:296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmond R.J., Emery J., Okkenhaug K., Zamoyska R. MAPK, phosphatidylinositol 3-kinase, and mammalian target of rapamycin pathways converge at the level of ribosomal protein S6 phosphorylation to control metabolic signaling in CD8 T cells. J. Immunol. 2009;183:7388–7397. doi: 10.4049/jimmunol.0902294. [DOI] [PubMed] [Google Scholar]
- Saraiva M., O'Garra A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- Saraiva M., Christensen J.R., Veldhoen M., Murphy T.L., Murphy K.M., O'Garra A. Interleukin-10 production by Th1 cells requires interleukin-12-induced STAT4 transcription factor and ERK MAP kinase activation by high antigen dose. Immunity. 2009;31:209–219. doi: 10.1016/j.immuni.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semmling V., Lukacs-Kornek V., Thaiss C.A., Quast T., Hochheiser K., Panzer U., Rossjohn J., Perlmutter P., Cao J., Godfrey D.I. Alternative cross-priming through CCL17-CCR4-mediated attraction of CTLs toward NKT cell-licensed DCs. Nat. Immunol. 2010;11:313–320. doi: 10.1038/ni.1848. [DOI] [PubMed] [Google Scholar]
- Shin H., Blackburn S.D., Intlekofer A.M., Kao C., Angelosanto J.M., Reiner S.L., Wherry E.J. A role for the transcriptional repressor Blimp-1 in CD8(+) T cell exhaustion during chronic viral infection. Immunity. 2009;31:309–320. doi: 10.1016/j.immuni.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Garvin J.E., Burns J.C., Gohil M., Zou T., Kim J.S., Maltzman J.S., Wherry E.J., Koretzky G.A., Jordan M.S. T-cell receptor signals direct the composition and function of the memory CD8+ T-cell pool. Blood. 2010;116:5548–5559. doi: 10.1182/blood-2010-06-292748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell L.M., McPherson A.J., Lin G.H., Sakaguchi S., Pandolfi P.P., Riccardi C., Watts T.H. CD8 T cell-intrinsic GITR is required for T cell clonal expansion and mouse survival following severe influenza infection. J. Immunol. 2010;185:7223–7234. doi: 10.4049/jimmunol.1001912. [DOI] [PubMed] [Google Scholar]
- Stemberger C., Huster K.M., Koffler M., Anderl F., Schiemann M., Wagner H., Busch D.H. A single naive CD8+ T cell precursor can develop into diverse effector and memory subsets. Immunity. 2007;27:985–997. doi: 10.1016/j.immuni.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Sun J., Madan R., Karp C.L., Braciale T.J. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat. Med. 2009;15:277–284. doi: 10.1038/nm.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Dodd H., Moser E.K., Sharma R., Braciale T.J. CD4+ T cell help and innate-derived IL-27 induce Blimp-1-dependent IL-10 production by antiviral CTLs. Nat. Immunol. 2011;12:327–334. doi: 10.1038/ni.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada K., Wang X., Hart G.T., Odumade O.A., Weinreich M.A., Hogquist K.A., Jameson S.C. Kruppel-like factor 2 is required for trafficking but not quiescence in postactivated T cells. J. Immunol. 2011;186:775–783. doi: 10.4049/jimmunol.1000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto N., Intlekofer A.M., Northrup J.T., Wherry E.J., Reiner S.L. Cutting edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J. Immunol. 2006;177:7515–7519. doi: 10.4049/jimmunol.177.11.7515. [DOI] [PubMed] [Google Scholar]
- Trandem K., Zhao J., Fleming E., Perlman S. Highly activated cytotoxic CD8 T cells express protective IL-10 at the peak of coronavirus-induced encephalitis. J. Immunol. 2011;186:3642–3652. doi: 10.4049/jimmunol.1003292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakim L.M., Bevan M.J. Cross-dressed dendritic cells drive memory CD8+ T-cell activation after viral infection. Nature. 2011;471:629–632. doi: 10.1038/nature09863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts T.H. TNF/TNFR family members in costimulation of T cell responses. Annu. Rev. Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- Williams M.A., Tyznik A.J., Bevan M.J. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi J.S., Du M., Zajac A.J. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009;324:1572–1576. doi: 10.1126/science.1175194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon H., Kim T.S., Braciale T.J. The cell cycle time of CD8+ T cells responding in vivo is controlled by the type of antigenic stimulus. PLoS ONE. 2010;5:e15423. doi: 10.1371/journal.pone.0015423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., De Trez C., Flynn R., Ware C.F., Croft M., Salek-Ardakani S. The adaptor molecule MyD88 directly promotes CD8 T cell responses to vaccinia virus. J. Immunol. 2009;182:6278–6286. doi: 10.4049/jimmunol.0803682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Yu S., Zhao D.M., Harty J.T., Badovinac V.P., Xue H.H. Differentiation and persistence of memory CD8(+) T cells depend on T cell factor 1. Immunity. 2010;33:229–240. doi: 10.1016/j.immuni.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R.M., Doherty P.C. Immunological surveillance against altered self components by sensitised T lymphocytes in lymphocytic choriomeningitis. Nature. 1974;251:547–548. doi: 10.1038/251547a0. [DOI] [PubMed] [Google Scholar]