Abstract
The transient receptor potential TRPM7 ion channel is required for cellular proliferation in pancreatic epithelia and adenocarcinoma. To elucidate the mechanism that mediates the function of TRPM7, we examined its role in survival of pancreatic cancer cells. RNA interference-mediated silencing of TRPM7 did not induce apoptotic cell death. TRPM7-deficient cells underwent replicative senescence with up-regulation of p16CDKN2A and WRN mRNA. The combination of anti-TRPM7 siRNA and gemcitabine produced enhanced cytotoxicity as compared to gemcitabine alone. Thus, TRPM7 is required for preventing senescence, and modulation of TRPM7 expression may help improve treatment response of pancreatic cancer by combination with apoptosis-inducing agents.
Keywords: transient receptor potential, ion channel, TRPM7, replicative senescence, pancreatic cancer
1. Introduction
The goal of this study is to elucidate the mechanism that mediates the proliferative role of the transient receptor potential melastatin-subfamily member 7, TRPM7, ion channel in pancreatic cancer by examining its requirement for cell survival. Pancreatic adenocarcinoma, the most common type of primary cancer in the pancreas, is among the most lethal human diseases [1]. A number of oncogenes and tumor suppressor genes involved in the development of pancreatic neoplasia have been revealed, but their clinical utility as therapeutic targets in pancreatic adenocarcinoma remain to be demonstrated. Ion channels including the transient receptor potential (TRP) family members have been implicated in human malignancies [2], but their roles in pancreatic cancer were mostly unknown. Identification of the roles of the TRP ion channels in pancreatic adenocarcinoma and determination of the mechanisms that mediate their functions are expected to generate new insights into pancreatic carcinogenesis and provide new biomarkers and targets for therapy.
The TRP family of trans-plasma membrane channels act as cellular sensors of physical and chemical stimuli by modulating ionic homeostasis [3]. The TRP melastatin-subfamily (TRPM) ion channels mediate diverse physiological functions through regulation of cytosolic levels of Ca2+ and Mg2+ and multiple signaling pathways [4]. The TRP ion channel, TRPM7, is a Mg2+/Ca2+-permeable channel with kinase activity and it regulates various cellular processes [5,6]. These include cell proliferation [7–10], survival [5,11–14], differentiation [15], adhesion [16], volume [17], migration [18,19], and neurotransmitter release [20]. Recent discovery of Trpm7 ion channel as a developmental regulator of exocrine pancreas in zebrafish has provided a novel link of development to human pancreatic cancer [10].
The zebrafish sweetbread (swd) mutations (swdp75fm and swdp82mf) that affect exocrine pancreas and Trpm7 were recovered from an ethylnitrosourea-induced mutagenesis screen [21,22]. Genetic and embryological analyses of the zebrafish swd, trpm7j124e1, and trpm7b508 mutations have led to identification of the developmental role of Trpm7 in exocrine pancreas through controlling cell cycle progression and epithelial growth and consequently the organ size [10]. In normal adult tissues, the human orthologue TRPM7 is ubiquitously expressed [23], but in pancreatic adenocarcinoma, expression of TRPM7 is aberrantly up-regulated and required for cellular proliferation [10]. In both zebrafish larvae and human pancreatic adenocarcinoma cells, TRPM7-controlled cellular proliferation is Mg2+-dependent and it involves modulation of p21CDKN1A and cyclin G1 [10]. In the developing zebrafish, supplementary Mg2+ or anti-sense oligos-induced repression of suppressor of cytokine signaling 3a (socs3a) is required for Trpm7-mediated epithelial proliferation in the exocrine pancreas [10]. However, the mechanisms underlying the proliferative role of TRPM7 in pancreatic cancer remain to be determined.
In this study, we examined the requirement of TRPM7 for survival of pancreatic adenocarcinoma cells. RNA interference-mediated silencing of TRPM7 induced replicative senescence, but not apoptosis, with up-regulated expression of the senescence-associated genes including the cyclin-dependent kinase inhibitor p16CDKN2A and the Werner’s syndrome gene WRN. Combination of small interfering RNA (siRNA) directed against TRPM7 and the conventionally used apoptosis-inducing drug, gemcitabine, produced enhancement of cytotoxicity. Results of these data indicate that TRPM7 is required for preventing non-apoptotic cell death through replicative senescence and suggest that modulation of TRPM7 offers new options for therapeutic targeting in pancreatic cancer.
2. Materials and methods
2.1. Cell cultures
The human pancreatic adenocarcinoma cell lines BxPC-3 and PANC-1 were obtained from the American Type Culture Collection (ATCC, Manassas, Virginia, U.S.A.), and maintained according to the ATCC instructions. The cell culture medium was supplemented with 10% heat-inactivated fetal bovine serum (FBS, Hyclone®, Thermo Fisher Scientific Inc., Pittsburgh, Pennsylvania, U.S.A.), 100 U/ml penicillin (Gibco™, Invitrogen Corporation, Carlsbad, California, U.S.A.) and 100 µg/ml streptomycin (Gibco™). The cells were incubated in a humidified atmosphere containing 5% CO2 at 37°C. All experiments were performed using culture medium. The cells were used within 20 passages of the stocks frozen in liquid nitrogen.
2.2. RNA interference-mediated gene silencing
BxPC-3 and PANC-1 cells were grown to 70–80% confluency, trypsinized, and resuspended at 106 cells in 100 µl of Nucleofector® Solution (Amaxa®/ Lonza, Cologne, Germany) containing 600 nM anti-TRPM7 siRNA (sc-42662; Santa Cruz Biotechnology, Inc., Santa Cruz, California, U.S.A.) or non-targeting control siRNA (sc-37007; Santa Cruz Biotechnology). Transfection was performed using Nucleofector II (Amaxa®/Lonza) according the manufacturer’s instructions. Forty-eight hours following transfection, total RNA was extracted and analyzed using real-time polymerase chain reaction (PCR) to verify knock down of TRPM7 [10].
2.3. Drugs and small molecules
Gemcitabine-HCl (Toronto Research Chemicals, Toronto, Canada) was dissolved in phosphate buffered saline (PBS), pH 7.4 at 10 mM. Suberoylanilide hydroxamic acid (SAHA, BioMol®, Enzo Life Sciences International, Inc., Plymouth Meeting, Pennsylvania, U.S.A.) was dissolved in dimethyl sulfoxide (DMSO, Sigma-Aldrich®, St. Louis, Missouri, U.S.A.) at 50 mM. The stock solutions of gemcitabine (10 mM) and SAHA (50 mM) were divided into aliquots and stored at −20°C, and diluted with culture medium prior to addition to the cultured cells. For controls conducted in parallel, 0.01% DMSO or no drug was added to the medium.
2.4. Flow cytometric analysis of apoptosis
BxPC-3 and PANC-1 transfected with anti-TRPM7 or non-targeting control siRNA were seeded at 2×105 cells / 3 ml in each well of a 6 well cell culture cluster (costar®, Corning Incorporated, Corning, New York, U.S.A.) and incubated at 37°C for 72 h. The cells were then washed with PBS (pH 7.4), and incubated with fluorescein isothiocyanate (FITC)-conjugated Annexin V (Invitrogen™) and propidium iodide (PI, Invitrogen™), and analyzed for apoptosis by flow cytometry as described [24].
2.5. Hematoxylin and eosin staining
The cells transfected with anti-TRPM7 siRNA or non-targeting control siRNA were seeded at 104 cells / 2 ml in each well of a 2 well glass slide (Lab-Tek® Chamber Slide™, Nalge Nunc International, Rochester, New York, U.S.A.) and incubated at 37°C for 48 h. The cells were then rinsed with PBS, fixed with 2% formaldehyde / 0.2% glutaldehyde in PBS (pH 7.4), and stained with hematoxylin and eosin (Richard-Allan Scientific®, Thermo Fisher Scientific, Pittsburgh, Pennsylvania, U.S.A.). The cell morphology was examined under a compound light microscope (Olympus BX-51, Tokyo, Japan). Images were captured using a digital camera (Olympus DP71, Tokyo, Japan) and processed using Adobe® Photoshop® CS3 extended.
2.6. Nuclear staining
BxPC-3 and PANC-1 cells transfected with anti-TRPM7 siRNA or non-targeting control siRNA were seeded at 104 cells / 2 ml in each well of a 2 well glass slide (Lab-Tek® Chamber Slide™), and incubated at 37°C for 48 h. The medium were removed, rinsed with PBS, fixed with 2% formaldehyde / 0.2% glutaldehyde in PBS (pH 7.4), and then mounted with Vectashield® with 4’6 diamidino-2-phenylindole (DAPI) (Vector Laboratories, Inc., Burlingame, California, U.S.A.). The DAPI-stained nuclei were examined under a compound microscope (Olympus BX-51) using fluorescence. Images were acquired using a digital camera (Olympus DP71) and processed using Adobe® Photoshop® CS3 extended.
2.7. Senescence assay
The transfected cells seeded at 4×104 cells / 4 ml in each well of a 6 well cell culture cluster (costar®, Corning Incorporated) and incubated at 37°C for 72 h and then analyzed for SA β-gal activity using the Senescence Detection Kit (Biovision Inc., Mountain View, California, U.S.A.) according the manufacturer’s instructions. Images were acquired under an inverted light microscope with phase contrast (Nikon TE-300, Melville, New York, U.S.A.) and processed using Adobe® Photoshop® CS3 extended.
2.8. Semi-quantification of p27CDKN1B, p16CDKN2A, and WRN mRNA
PANC-1 cells transfected with anti-TRPM7 siRNA or non-targeting control siRNA were seeded at 5×105 cells / ml in each well of a 6 well cell culture cluster (costar®, Corning Incorporated) and incubated at 37°C for 48 h. Total RNA was extracted using TRIzol® (Invitrogen™) and RNeasy® Mini Kit (Qiagen Inc., Valencia, California, U.S.A.) according to the manufacturer’s instructions. First-strand cDNA was generated using SuperScript reverse transcriptase and random primers (Invitrogen™) and amplified with SYBR® Green (Applied Biosystems®, Foster City, California, U.S.A.) using ABI Prism® 7700 real-time PCR system (Applied Biosystems®) as described [25]. The sequences of the primers used were designed based on human cDNA sequences (GenBank accession numbers: p27CDKN1B, BC001971; p16CDKN2A, AH005371; WRN, AY818673; GAPDH: NM_002046) as follows:
p27CDKN1B5’-GTCCATTTATCCACAGGAAA-3’, 5’-ATGGTTTTTCCATACACAGG-3’;
p16CDKN2A 5’- GGGTCGGGTAGAGGAGGT G-3’, 5’-GCGCTACCTGATTCCAATTC-3’;
WRN 5’-GACAGCGGACTTCAACCTTC-3’, 5’-TTGGCAAACCACACAGGTAA-3’;
GAPDH 5'-GAGTCAACGGATTTGGTCGT-3', 5'-TTGATTTTGGAGGGATCTCG-3'.
The relative mRNA levels of p27CDKN1B, p16CDKN2A, and WRN were determined as compared with those of GAPDH using the Comparative CT Method (Applied Biosystems®). Samples of PCR using primers directed against each tested gene were analyzed by agarose gel electrophoresis to validate specificity of amplification products.
2.9. Proliferation assay
PANC-1 cells were transfected with anti-TRPM7 siRNA or non-targeting control siRNA were seeded at 2×104 / 100 µl in each well of a 96 well cell culture cluster (costar®, Corning Incorporated) and incubated at 37°C. About 24 h following transfection, gemcitabine (1 µM or 5 µM) or SAHA (1 µM or 5 µM) was added and incubation was continued for a total of 72 h. Cellular proliferation was quantified using the CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega Corporation, Madison, Wisconsin, U.S.A.) using 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfonylphenyl)-2H-tetrazolium (MTS) according to the manufacturer’s instructions and as described [25]. This experiment was performed with each treatment in triplicate samples.
2.10. Statistical analysis
The mean, standard deviation (s.d.), and P-values were analyzed using the Student’s t-test. Statistical significance was considered at a P-value < 0.05.
3. Results
3.1. TRPM7 is required for preventing non-apoptotic death of pancreatic adenocarcinoma cells
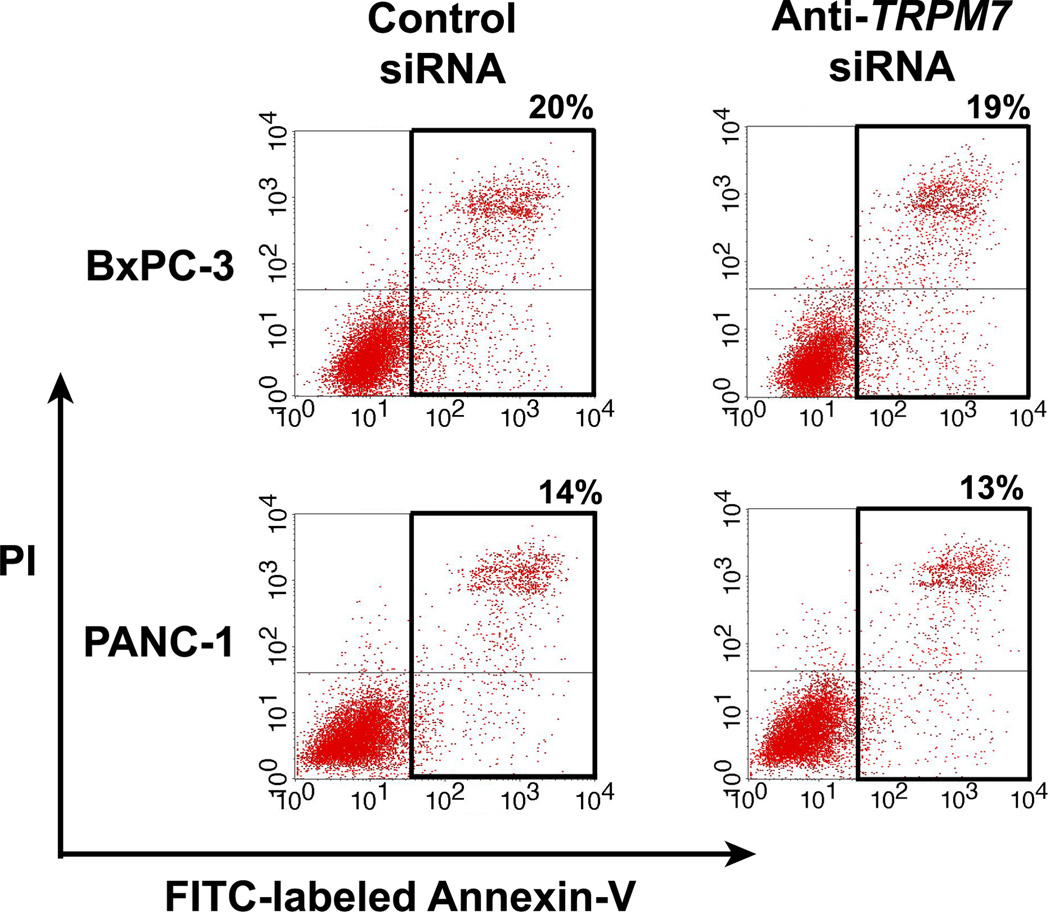
TRPM7 ion channel is necessary for cellular proliferation in pancreatic epithelia and adenocarcinoma [10]. In this study, we determined the role of TRPM7 in cell survival by using siRNA to silence its expression in the human pancreatic adenocarcinoma cell lines, BxPC-3 and PANC-1. The mRNA levels of TRPM7 in the cells transfected with anti-TRPM7 siRNA were reduced by up to 70% of that in the control siRNA-transfected cells, as determined by quantitative PCR. The protein levels of TRPM7 in the anti-TRPM7 siRNA-transfected cells were similarly reduced, as determined by immunoblotting. The TRPM7-deficient cells were analyzed by flow cytometry using annexin V as a marker of apoptotic cell death. As compared with controls, both BxPC-3 and PANC-1 cells transfected with anti-TRPM7 siRNA did not show any increase in the proportion of cells undergoing apoptosis (Fig. 1). This suggests that TRPM7 may not be required for cell survival; alternatively, the TRPM7-deficient cells might have undergone non-apoptotic cell death.
Fig. 1.
RNA interference-mediated silencing of TRPM7 did not induce apoptotic cell death. BxPC-3 and PANC-1 cells transfected with anti-TRPM7 siRNA or non-targeting control siRNA were incubated with FITC-conjugated annexin V and PI, and then analyzed for apoptosis by flow cytometry. The cells undergoing early apoptosis (lower right) and late apoptosis (upper right) are enclosed within the black border, with the proportion of apoptotic cells as indicated. The baseline level of apoptotic cells in the control siRNA-transfected cells may be attributed to electrical pulsation during Nucleofection®, detachment of cells by trypsinization, and disaggregation of cells by filtration prior to flow cytometric analysis. This experiment was repeated twice with similar results.
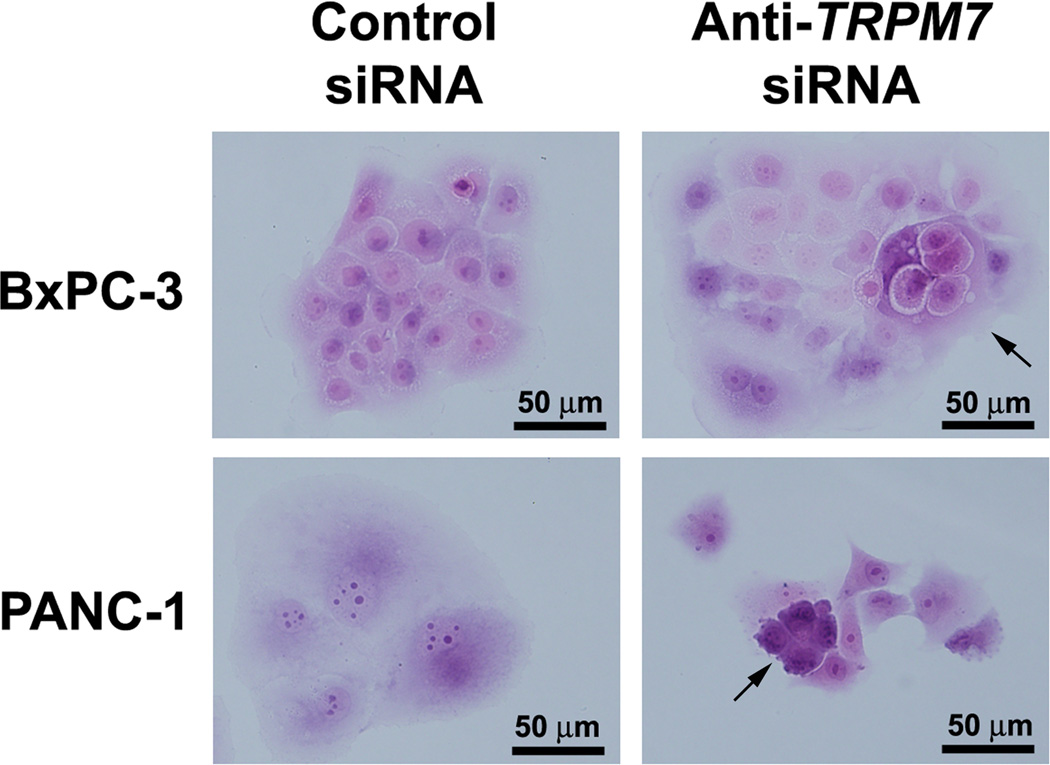
Previously, we have shown that anti-TRPM7 siRNA induced formation of abnormally appearing nuclei and cytoplasmic vacuoles in BxPC-3 and PANC-1 as seen under phase-contrast microscopy [10]. Here, we further examined the TRPM7-deficient cells following staining of the nuclei and cytoplasm with hematoxylin and eosin, respectively. Consistent with the observations in the micrographs with phase contrast, bright field examination reveals that both BxPC-3 and PANC-1 cells with targeted knock down of TRPM7 expression exhibit morphological features indicative of a senescent phenotype (Fig. 2). The cells are enlarged and multi-nucleated with formation of cytoplasmic vacuoles. These features are suggestive of non-apoptotic cell death through replicative senescence [26].
Fig. 2.
SiRNA-mediated repression of TRPM7 induced cellular morphology indicative of a senescent phenotype. BxPC-3 and PANC-1 cells transfected with anti-TRPM7 siRNA or non-targeting control siRNA were stained with hematoxylin and eosin, and examined under a compound light microscope. Arrows point at the enlarged cells containing multiple nuclei in both BxPC-3 and PANC-1 and cytoplasmic vacuoles in BxPC-3. Similar results were obtained in duplicate culture dishes and the experiment was repeated twice.
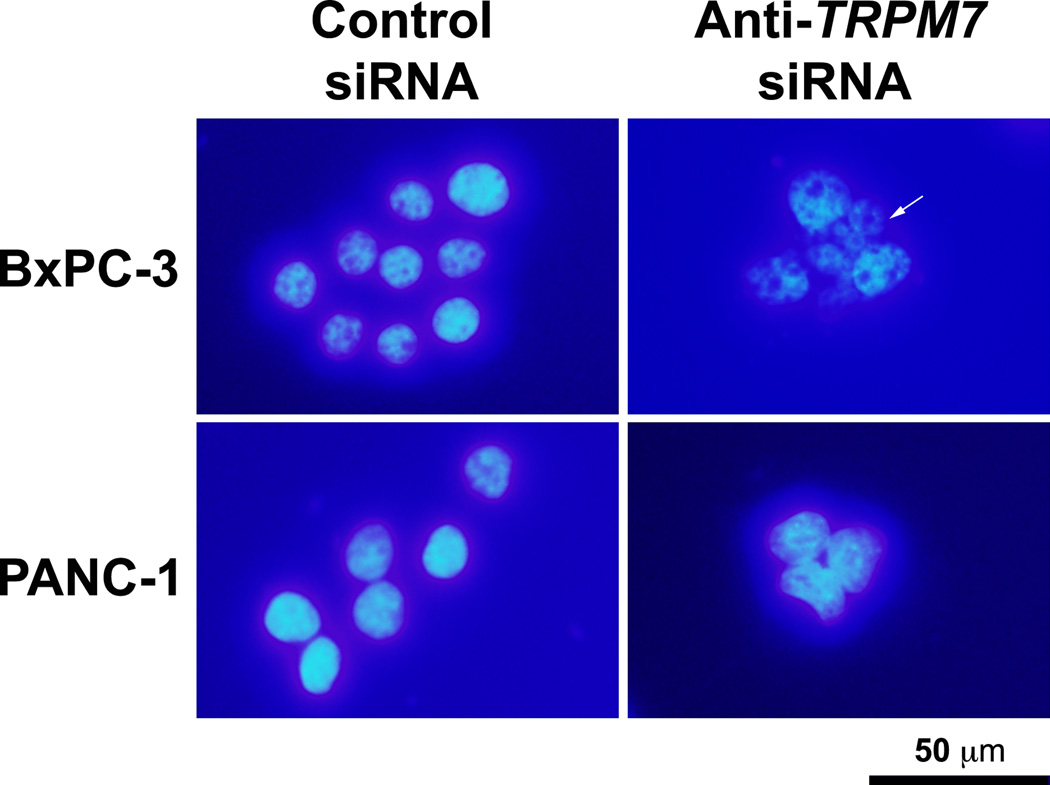
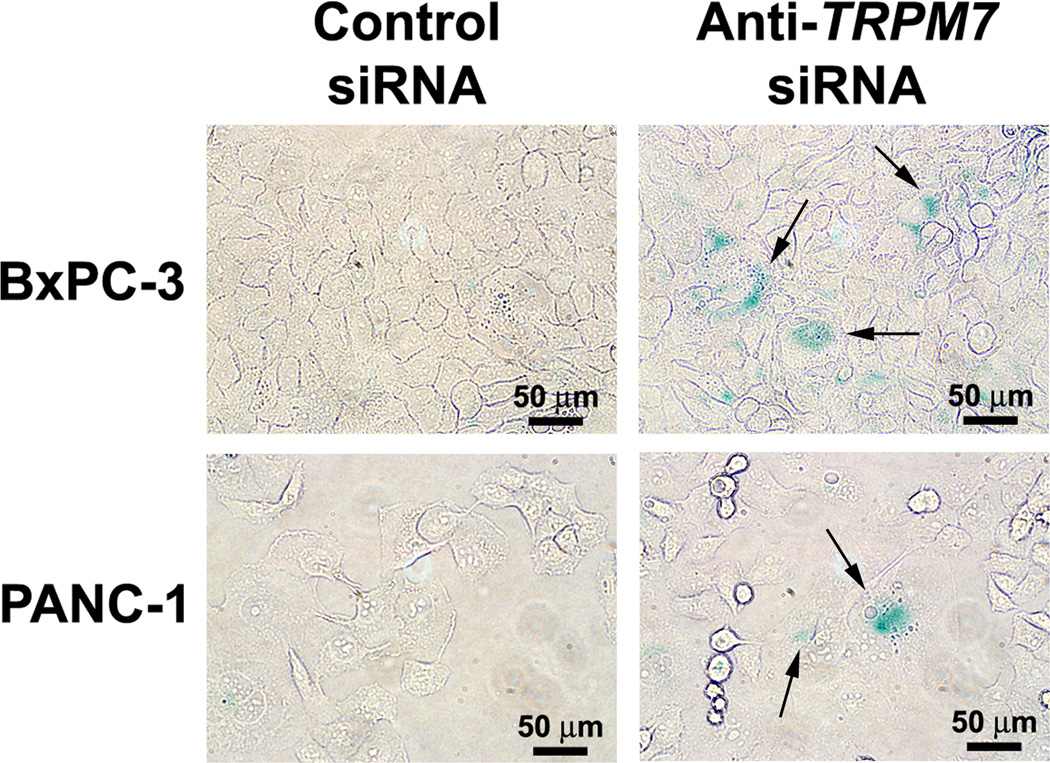
To further characterize the multi-nucleated cells, BxPC-3 and PANC-1 transfected with anti-TRPM7 siRNA or non-targeting control siRNA were stained with DAPI that binds to genomic DNA. In both BxPC-3 and PANC-1, silencing of TRPM7 induced aberrant nuclear figures suggesting mitotic arrest (Fig. 3). To determine the effect of targeted inhibition of TRPM7 on cellular senescence, the TRPM7-deficient cells were assayed for SA β-gal activity, which is an established indication of cellular senescence. In the BxPC-3 and PANC-1 cells transfected with anti-TRPM7 siRNA but not in the controls, SA β-gal activity was detected (Fig. 4). Taken together, these data suggest that TRPM7 is required for preventing replicative senescence.
Fig. 3.
TRPM7-deficient cells contained multiple nuclei suggesting mitotic arrest. BxPC-3 and PANC-1 cells transfected with anti-TRPM7 siRNA or non-targeting control siRNA were stained with DAPI, and examined under a compound light microscope with fluorescence. Arrows point at multinucleated cells. This experiment was repeated twice with duplicate culture dishes in each experiment, and similar results were observed.
Fig. 4.
TRPM7-deficient cells underwent replicative senescence. BxPC-3 and PANC-1 cells transfected with anti-TRPM7 siRNA or non-targeting control siRNA were analyzed for SA β-gal activity. Images were acquired under an inverted light microscope with phase contrast. Arrows point at some of the cells displaying SA β-gal activity (blue color). The images shown are representative of three independent experiments with duplicate culture wells in each experiment.
3.2. RNA interference-mediated silencing of TRPM7 up-regulates expression of senescence-associated genes
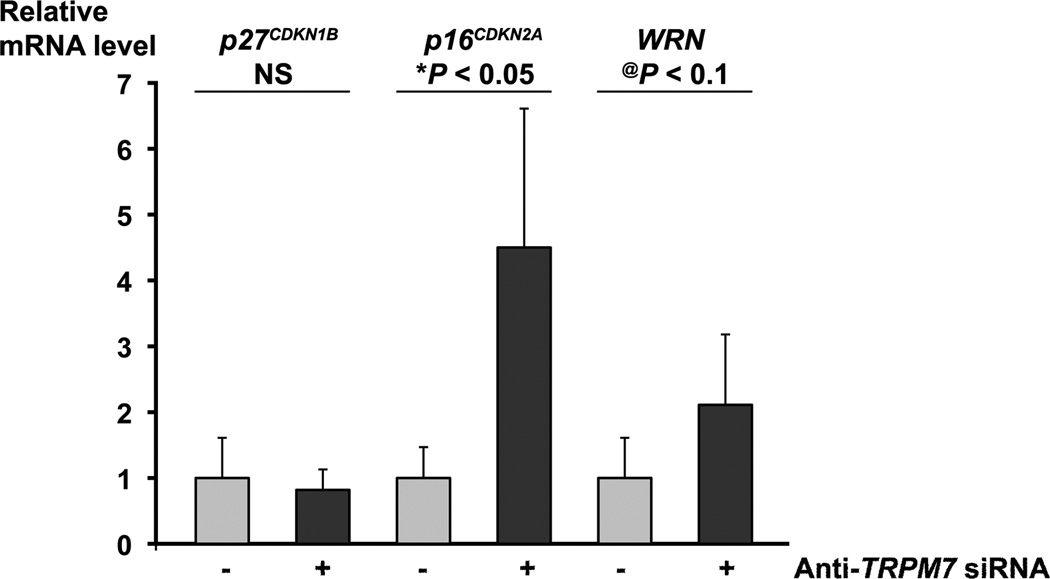
To gain insights into the mechanism underlying the requirement of TRPM7 for preventing replicative senescence, we analyzed the expression of senescence-associated genes by real-time PCR. We have previously shown that, in BxPC-3 and PANC-1 cells transfected with anti-TRPM7 siRNA, the mRNA level of the cell cycle inhibitor p21CDKN1A was increased whereas those of cyclin G1 and cyclin B1 were decreased [10]. In this study, we examined the mRNA levels of several markers of cellular senescence including the cyclin-dependent kinase inhibitors p27CDKN1B and p16CDKN2A, and the DNA helicase/exonuclease-encoding gene WRN. Up-regulated expression of p27CDKN1B and p16CDKN2A as well as deregulation of WRN have been associated with cell cycle arrest and replicative senescence [27,28]. In TRPM7-deficient PANC-1 cells, the mRNA levels of p16INK4A and WRN were elevated whereas that of p27CDKN1B remained unaltered (Fig. 5). Similarly, in TRPM7-deficient BxPC-3 cells, the mRNA levels of p16INK4A and WRN were elevated by 1.4-fold and 26%, respectively.
Fig. 5.
Up-regulated levels of p16CDKN2A and WRN mRNA in TRPM7-deficient cells. PANC-1 cells transfected with anti-TRPM7 or non-targeting control siRNAs were analyzed for the relative mRNA levels of p27CDKN1B, p16CDKN2A, and WRN by real-time PCR. Each column represents the mean mRNA level + s.d. (n = 3) relative to GAPDH. The experiment was repeated twice with similar results. *P < 0.05 indicates statistically significant difference. @P < 0.1 suggests a statistical trend. NS, not statistically significant.
3.3. Combination of anti-TRPM7 siRNA and gemcitabine produces enhanced cytotoxicity
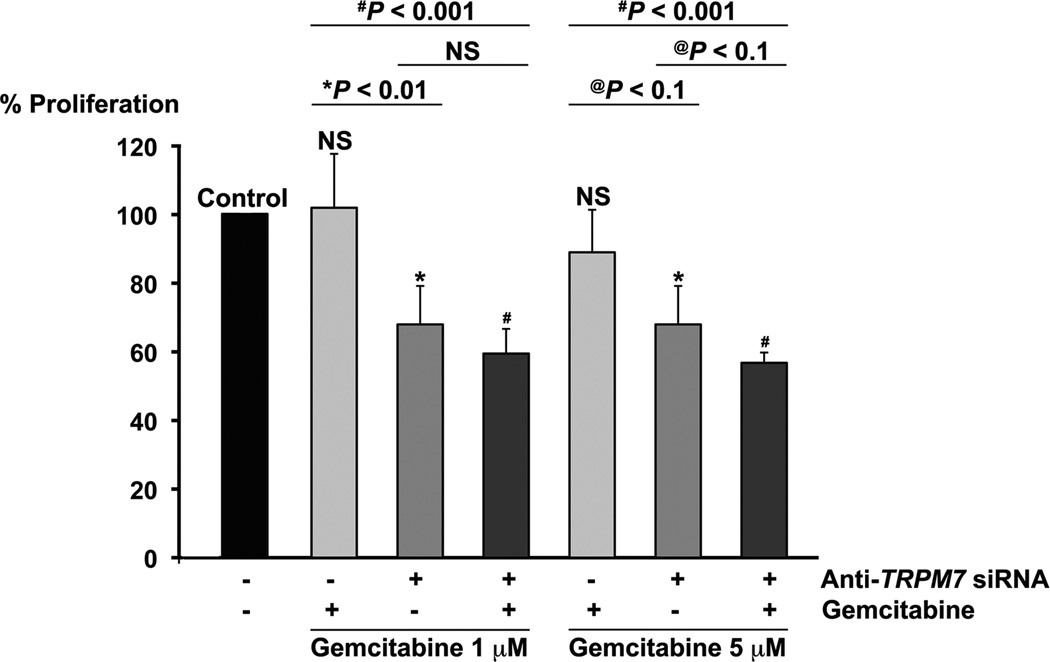
The induction of non-apoptotic cell death by anti-TRPM7 siRNA might be exploited for enhancing cytotoxicity by combination with apoptosis-producing drugs, particularly in cancer cells with defects in the apoptotic pathways. The pancreatic adenocarcinoma cell line PANC-1 is relatively resistant to the conventionally used chemotherapeutic agent gemcitabine [29]. As a nucleoside analog, gemcitabine interferes with DNA metabolism and induces apoptotic cell death with modulation of the apoptosis-regulating genes, such as p53, BCL-2, BCL-xL, and BAX [30] and activation of caspases that control apoptotic pathways [31]. We hypothesize that the combination of anti-TRPM7 siRNA, which induces non-apoptotic cell death, with gemcitabine produces enhanced cytotoxicity in pancreatic cancer cells. To test this hypothesis, we assayed for proliferation in PANC-1 cells transfected with either anti-TRPM7 siRNA or non-targeting control siRNA, and incubated in the presence or absence of gemcitabine. At clinically active concentrations of 1 µM or 5 µM [32,33], gemcitabine did not produce any significant effect on cellular proliferation in PANC-1 (Fig. 6). Comparing with gemcitabine alone, the combination of gemcitabine at 1 µM or 5 µM and anti-TRPM7 siRNA significantly reduced proliferation by 43% and 32%, respectively (Fig. 6).
Fig. 6.
Combination of anti-TRPM7 siRNA with gemcitabine produced enhanced cytotoxicity. PANC-1 cells transfected with anti-TRPM7 siRNA or non-targeting control siRNA were incubated in the presence or absence of gemcitabine at 1 µM or 5 µM and then analyzed for proliferation using the MTS assay. Each column represents the mean (+ s.d.) proliferation as indicated by MTS absorbance relative to that of control (cells transfected with non-targeting control siRNA and incubated in the absence of gemcitabine). Statistical analysis was performed by comparing each experimental group with control except where indicated. *P < 0.01 and #P < 0.001 indicates statistically significant difference; @P < 0.1, a statistical trend; NS, not statistically significant. This experiment was repeated twice with similar results.
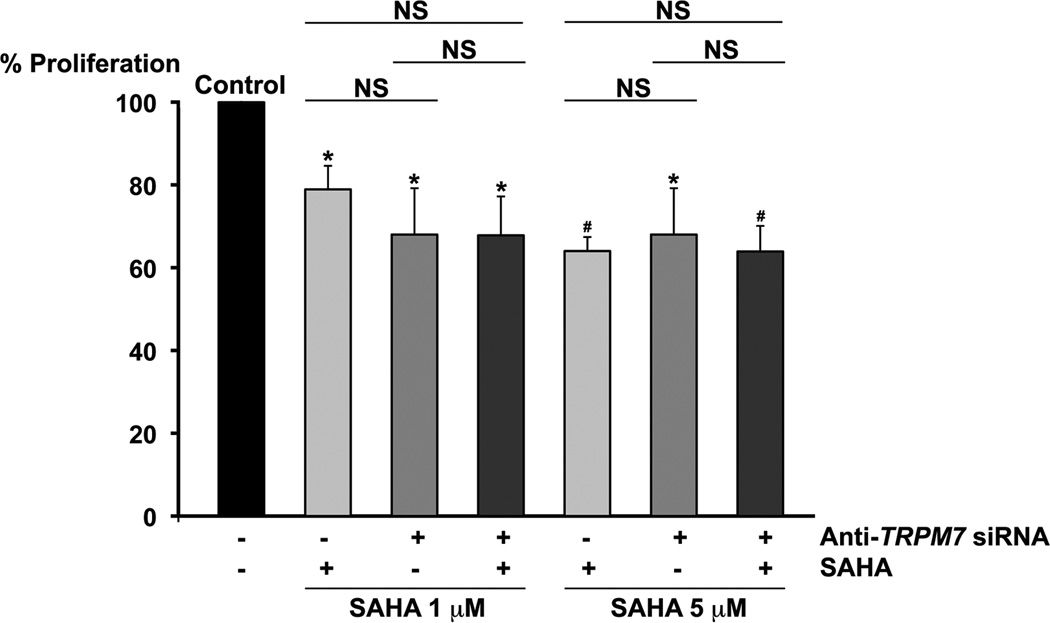
Besides, we examined the anti-proliferative effect of the combination of anti-TRPM7 siRNA and the clinically used inhibitor of histone deacetylases (HDACs), SAHA, which exerts anti-tumor effect on pancreatic adenocarcinoma cell lines. SAHA induces apoptosis with up-regulation of BAX expression and activation of caspases [24] as well as cellular senescence. Comparing with SAHA alone, the combination of anti-TRPM7 siRNA and SAHA at either 1 µM or 5 µM produced no enhanced cytotoxicity (Fig. 7), and this might be partly related to their overlapping mechanisms of cell death through senescence.
Fig. 7.
No enhanced cytotoxicity by anti-TRPM7 siRNA in combination with SAHA. PANC-1 cells transfected with anti-TRPM7 siRNA or non-targeting control siRNA were incubated in the presence or absence of SAHA at 1 µM or 5 µM and then analyzed for proliferation using the MTS assay. Each column represents the mean (+ s.d.) proliferation as indicated by MTS absorbance relative to that of control (cells transfected with non-targeting control siRNA and incubated in the absence of SAHA). Statistical analysis was performed by comparing each experimental group with control except where indicated. *P < 0.01 and #P < 0.001 indicate statistically significant differences between each treatment group and the control. NS, not statistically significant. These results are representative of three independent experiments.
4. Discussion
TRP channels have been implicated in cellular proliferation and survival, and this study suggests a novel link of an ion channel to replicative senescence. Various lines of evidence have provided strong support for the cellular effects of TRPM7 through controlling intracellular homeostasis of Mg2+ and Ca2+. We have recently shown that supplementary Mg2+ rescues the proliferative defect of the exocrine pancreatic epithelia of the zebrafish larvae with mutations in trpm7 and in the TRPM7-silenced human pancreatic adenocarcinoma cells [10]. These findings are in agreement with the previous study showing that TRPM7-deficient malignant B-lymphocytes or embryonic stem cells lacking TRPM7 kinase domain undergo proliferative arrest, and they can be activated to re-enter cell cycle by supplementary Mg2+ in the culture medium [7,34,35]. Moreover, TRPM7-mediated elevation of cytosolic Mg2+ or Ca2+ triggered by intracellular depletion of Mg2+ controls migration of osteoblasts and fibroblasts, respectively [18,19]. We hypothesize that metabolic sensing by TRPM7 ion channel controls cellular influx of Mg2+ and/or Ca2+ that interacts with the epidermal growth factor (EGF)-induced signaling pathways, resulting in the pro-survival and other mitogenic effects on pancreatic epithelia and cancer cells.
Mg2+ and Ca2+ are essential for normal cellular and physiological functions, and perturbed homeostasis of these ions contributes to various pathological states [36]. Chronic inadequacy of Mg2+ is associated with increased risk of developing aging-related diseases, possibly related to oxidative stress and inflammation [37]. In human endothelia and fibroblasts, Mg2+ deficiency accelerates cellular senescence as evidenced by reduced replicative lifespan and increased SA β-gal activity [38,39]. Although Ca2+ plays a contributory role in the regulation of apoptotic cell death by activating various pro-apoptotic factors [40], a rise in cytosolic Ca2+ level by stimulation with vitamin D3 and ATP can induce non-apoptotic cell death through autophagy [41]. Whether changes in cytosolic Ca2+ can induce other forms of non-apoptotic cell death, such as cellular senescence and mitotic catastrophe, is unclear. By using electrophysiological assay and live cell imaging analysis, we aim to determine the role of Mg2+ and Ca2+ in mediating the proliferative and pro-survival roles of TRPM7 in pancreatic epithelia and cancer cells.
Genetic silencing of TRPM7-mediated cellular senescence in pancreatic cancer cells is associated with up-regulated expression of cell cycle regulators and tumor suppressor genes. Consistent with the role of TRPM7 in Mg2+ transport, Mg2+ deficiency-induced senescence in endothelial cells is associated with up-regulated expression of p16CDKN2A and p21CDKN1A as well as telomeric attrition [38,39]. In agreement with these findings, we show that expression of p21CDKN1A and p16CDKN2A is up-regulated in TRPM7-deficient pancreatic cancer cells [10] (this study). Genetic alterations in p21CDKN1A and p16CDKN2A have been implicated in the multi-step pancreatic carcinogenesis [42]. Recent evidence suggests cooperative roles of p16CDKN2A and p21CDKN1A for mediating cellular senescence and tumor suppression [43]. Thus, the concerted up-regulation of p16CDKN2A and p21CDKN1A in the TRPM7-deficient cells suggest a contributory role of TRPM7 for proliferation and survival in the pathogenesis of pancreatic neoplasia through regulation of cyclin-dependent kinase inhibitors. It is notable that targeted knockdown of TRPM7 increases the mRNA level of WRN, which is a tumor suppressive gene being mutated in patients with Werner’s syndrome that is characterized by adult-onset progeria and predisposition to various malignancies including pancreatic adenocarcinoma [28]. Ongoing studies are designed to directly determine the role of the senescence-associated genes in mediating the proliferative and pro-survival functions of TRPM7.
The finding that induction of non-apoptotic cell death by inhibiting the expression of TRPM7 suggests a unique opportunity for improving therapeutic response in pancreatic adenocarcinoma. These tumor cells possess various combinations of mutations in K-RAS, TP53, p16CDKN2A, and DPC4/SMAD4[44]. As a result of mutations in the components of the apoptotic pathways such as TP53, the pancreatic cancer cells are generally resistant to the apoptosis-inducing agents such as the clinically used chemotherapeutic drugs, gemcitabine and 5-fluorouracil. As demonstrated in this study, targeted knockdown of TRPM7 in combination with gemcitabine produces enhanced cytotoxicity in PANC-1, which contains mutations in K-RAS, TP53, and p16CDKN2A [44]. We hypothesize that anti-TRPM7 siRNA and gemcitabine cause cell death by non-apoptotic and apoptotic pathways, respectively, such that the combination produces enhanced cytotoxicity; however, the precise mechanisms remain to be determined. In future studies, we attempt to further investigate the combined use of modulators of TRPM7 with apoptotic-inducing agents and the underlying molecular basis, with the goal of overcoming therapeutic resistance in pancreatic adenocarcinoma.
In summary, we present evidence that RNA interference-mediated silencing of TRPM7 induces replicative senescence in pancreatic adenocarcinoma. We propose that TRPM7 contributes to uncontrolled cellular proliferation at least in part by preventing non-apoptotic cell death during the multi-step pancreatic carcinogenesis. Future studies aimed to understand the signaling mechanisms that mediate the proliferative and pro-survival roles of TRPM7 are expected to help develop therapeutic interventions by targeted modulation of this ion channel and its associated signaling in pancreatic cancer and other malignancies.
Acknowledgements
N.S.Y. is supported by the Physician Scientist Stimulus Package from The Pennsylvania State University College of Medicine. This work was supported by the research start-up fund from the Penn State Hershey Cancer Institute (N.S.Y.), American Cancer Society (Grant IRG-77-004-31) administered through The Holden Comprehensive Cancer Center at The University of Iowa (N.S.Y.), and Cancer Center Support Grant (P30 CA 086862) by the National Cancer Institute to the Holden Comprehensive Cancer Center at the University of Iowa (N.S.Y.).
Abbreviations
- DAPI
4’6 diamidino-2-phenylindole
- DMSO
dimethyl sulfoxide
- FITC
fluorescein isothiocyanate
- MTS
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfonylphenyl)-2H-tetrazolium
- PCR
polymerase chain reaction
- PBS
phosphate buffered saline
- PI
propidium iodide
- siRNA
small interfering RNA
- SA β-gal
senescence-associated β-galactosidase
- SAHA
suberoylanilide hydroxamic acid; socs3a, suppressor of cytokine signaling 3a
- swd
sweetbread
- TRP
transient receptor potential
- TRPM7
transient receptor potential melastatin-subfamily member 7
- WRN
Werner
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
None declared.
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer Statistics 2011. CA Cancer J. Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Lehen’kyi V, Prevarskaya N. Oncogenic TRP channels. Adv. Exp. Med. Biol. 2011;1772:929–945. doi: 10.1007/978-94-007-0265-3_48. [DOI] [PubMed] [Google Scholar]
- 3.Nilius B, Owsianik G. The transient receptor potential family of ion channels. Genome Biol. 2011;12:218. doi: 10.1186/gb-2011-12-3-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fleig A, Penner R. The TRPM ion channel subfamily: molecular, biophysical and functional features. Trends Pharm. Sci. 2004;25:633–639. doi: 10.1016/j.tips.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Nadler MJ, Hermosura MC, Inabe K, Perraud AL, Zhu Q, Stokes AJ, Kurosaki T, Kinet JP, Penner R, Scharenberg AM, Fleig A. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature. 2001;411:590–595. doi: 10.1038/35079092. [DOI] [PubMed] [Google Scholar]
- 6.Runnels LW, Yue L, Clapham DE. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science. 2001;291:1043–1047. doi: 10.1126/science.1058519. [DOI] [PubMed] [Google Scholar]
- 7.Schmitz C, Perraud A-L, Johnson CO, Inabe K, Smith MK, Penner R, Kurosaki T, Fleig A, Scharenberg AM. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell. 2003;114:191–200. doi: 10.1016/s0092-8674(03)00556-7. [DOI] [PubMed] [Google Scholar]
- 8.Abed E, Moreau R. Importance of melastatin-like transient receptor potential 7 and cations (magnesium, calcium) in human osteoblast-like cell proliferation. Cell Prolif. 2007;40:849–865. doi: 10.1111/j.1365-2184.2007.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inoue K, Xiong Z-G. Silencing TRPM7 promotes growth/proliferation and nitric oxide production of vascular endothelial cells via the ERK pathway. Cardiovas. Res. 2009;83:547–557. doi: 10.1093/cvr/cvp153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yee NS, Zhou W, Liang I-C. Transient receptor potential ion channel Trpm7 regulates exocrine pancreatic epithelial proliferation by Mg2+-sensitive Socs3a signaling in development and cancer. Dis. Model Mech. 2011;4:240–254. doi: 10.1242/dmm.004564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aarts M, Ilihara K, Wei W-L, Xiong Z-G, Arundine M, Cerwinski W, MacDonald JF, Tymianski M. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115:863–877. doi: 10.1016/s0092-8674(03)01017-1. [DOI] [PubMed] [Google Scholar]
- 12.McNeill MS, Paulsen J, Bonde G, Burnight E, Hsu M-Y, Cornell RA. Cell death of melanophores in zebrafish trpm7 mutant embryos depends on melanin synthesis. J. Invest. Derm. 2007;127:2020–2030. doi: 10.1038/sj.jid.5700710. [DOI] [PubMed] [Google Scholar]
- 13.Wykes RC, Lee M, Duffy SM, Yang W, Seward EP, Bradding P. Functional transient receptor potential melastatin 7 channels are critical for human mast cell survival. J. Immunol. 2007;179:4045–4052. doi: 10.4049/jimmunol.179.6.4045. [DOI] [PubMed] [Google Scholar]
- 14.Cheng H, Feng JM, Figueiredo ML, Zhang H, Nelson PL, Marigo V, Beck A. Transient receptor potential melastatin type 7 channel is critical for the survival of bone marrow derived mesenchymal stem cells. Stem Cells Dev. 2010;19:1393–1403. doi: 10.1089/scd.2009.0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin J, Desai BN, Navarro B, Donovan A, Andrews NC, Clapham DE. Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science. 2008;322:756–759. doi: 10.1126/science.1163493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark K, Langeslag M, van Leeuwen B, Ran L, Ryazanov AG, Figdor CG, Moolenaar WH, Jalink K, van Leeuwen FN. TRPM7, a novel regulator of actomyosin contractility and cell adhesion. EMBO J. 2006;25:290–301. doi: 10.1038/sj.emboj.7600931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Numata T, Shimizu T, Okada Y. TRPM7 is a stretch- and swelling-activated cation channel involved in volume regulation in human epithelial cells. Am. J. Physiol. Cell. Physiol. 2007;292:C460–C467. doi: 10.1152/ajpcell.00367.2006. [DOI] [PubMed] [Google Scholar]
- 18.Wei C, Wang X, Chen M, Ouyang K, Song L-S, Chen H. Calcium flickers steer cell migration. Nature. 2009;457:901–905. doi: 10.1038/nature07577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abed E, Moreau R. Importance of melastatin-like transient receptor potential 7 and magnesium in the stimulation of osteoblast proliferation and migration by platelet-derived growth factor. Am. J. Physiol. Cell. Physiol. 2009;297:C360–C368. doi: 10.1152/ajpcell.00614.2008. [DOI] [PubMed] [Google Scholar]
- 20.Low SE, Amburgey K, Horstick E, Linsley J, Sprague SM, Cui WW, Zhou W, Hirata H, Saint-Amant L, Hume RI, Kuwada JY. TRPM7 is required within zebrafish sensory neurons for the activation of touch-evoked escape behaviors. J. Neurosci. 2011;31:11633–11644. doi: 10.1523/JNEUROSCI.4950-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yee NS, Lorent K, Pack M. Exocrine pancreas development in zebrafish. Dev. Biol. 2005;284:84–101. doi: 10.1016/j.ydbio.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 22.Yee NS. Zebrafish as a biological system for identifying and evaluating therapeutic targets and compounds. In: Han H, Grippo PJ, editors. Drug discovery in pancreatic cancer: models and techniques. New York: Springer; 2010. pp. 95–112. [Google Scholar]
- 23.Fonfria E, Murdock PR, Cusdin FS, Benham CD, Kelsell RE, McNulty S, Tissue S. distribution profiles of the human TRPM cation channel family. J. Recept. Signal Transduc. Res. 2006;26:159–178. doi: 10.1080/10799890600637506. [DOI] [PubMed] [Google Scholar]
- 24.Chun SG, Zhou W, Yee NS. Combined targeting of histone deacetylases and hedgehog signaling enhances cytotoxicity in pancreatic cancer. Cancer Biol. Ther. 2009;8:1328–1339. doi: 10.4161/cbt.8.14.8633. [DOI] [PubMed] [Google Scholar]
- 25.Yee NS, Zhou W, Lee M. Transient receptor potential channel TRPM8 is over-expressed and required for cellular proliferation of pancreatic adenocarcinoma. Cancer Lett. 2010;297:49–55. doi: 10.1016/j.canlet.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okada H, Mak TW. Pathways of apoptotic and non-apoptotic death in tumour cells. Nat. Rev. Cancer. 2004;4:592–603. doi: 10.1038/nrc1412. [DOI] [PubMed] [Google Scholar]
- 27.Collado M, Serrano M. Senescence in tumours: evidence from mice and humans. Nature Rev. Cancer. 2010;10:51–57. doi: 10.1038/nrc2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chun SG, Yee NS. Werner’s syndrome as a hereditary risk factor for exocrine pancreatic cancer: Potential role of WRN in pancreatic tumorigenesis and patient-tailored therapy. Cancer Biol. Ther. 2010;10:430–437. doi: 10.4161/cbt.10.5.12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang P-H, Motoo Y, Sawabu N, Minamoto T. Effect of gemcitabine on the expression of apoptosis-related genes in human pancreatic cancer cells. World J. Gastroenterol. 2006;12:1597–1602. doi: 10.3748/wjg.v12.i10.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi X, Liu S, Kleeff J, Friess, Buchler MW. Acquired resistance of pancreatic cancer cells towards 5-Fluorouracil and gemcitabine is associated with altered expression of apoptosis-regulating genes. Oncology. 2002;62:354–362. doi: 10.1159/000065068. [DOI] [PubMed] [Google Scholar]
- 31.Chandler NM, Canete JJ, Callery MP. Caspase-3 drives apoptosis in pancreatic cancer cells after treatment with gemcitabine. J. Gastrointest. Surg. 2004;8:1072–1078. doi: 10.1016/j.gassur.2004.09.054. [DOI] [PubMed] [Google Scholar]
- 32.Grunewald R, Kantarjian H, Keating MJ, Abbruzzese J, Tarassoff P, Plunkett W. Pharmacologically directed design of the dose rate and schedule of 2’,2’-difluorodeoxycytidine (gemcitabine) administration in leukemia. Cancer Res. 1990;50:6823–6826. [PubMed] [Google Scholar]
- 33.Abbruzzese JL, Grunewald R, Weeks EA, Gravel D, Adams T, Nowak B, Mineishi S, Tarassoff P, Satterlee W, Raber MN, Plunkett W. A phase I clinical, plasma, and cellular pharmacology study of gemcitabine. J. Clin. Oncol. 1991;9:491–498. doi: 10.1200/JCO.1991.9.3.491. [DOI] [PubMed] [Google Scholar]
- 34.Sahni J, Tamura R, Sweet IR, Scharenberg AM. TRPM7 regulates quiescent/proliferative metabolic transitions in lymphocytes. Cell Cycle. 2010;9:3565–3574. doi: 10.4161/cc.9.17.12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryazanova L, Rondon LJ, Zierler S, Hu Z, Galli J, Yamaguchi TP, Mazur A, Fleig A, Ryazanov AG. TRPM7 is essential for Mg2+ homeostasis in mammals. Nature Commun. 2010;1:109. doi: 10.1038/ncomms1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolf FI, Trapani V. Cell pathophysiology of magnesium. Clin. Sci. 2008;114:27–35. doi: 10.1042/CS20070129. [DOI] [PubMed] [Google Scholar]
- 37.Barbagallo L, Dominguez LJ. Magnesium and aging. Curr. Pharm. Design. 2010;16:832–839. doi: 10.2174/138161210790883679. [DOI] [PubMed] [Google Scholar]
- 38.Ferre S, Mazur A, Maier JAM. Low magnesium induces senescent features in cultured human endothelial cells. Magnes. Res. 2007;20:66–71. [PubMed] [Google Scholar]
- 39.Killilea DW, Ames BN. Magnesium deficiency accelerates cellular senescence in cultured human fibroblasts. Proc. Natl. Acad. Sci. U.S.A. 2008;105:5768–5773. doi: 10.1073/pnas.0712401105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rong Y, Distelhorst CW. Bcl-2 protein family members: versatile regulators of calcium signaling in cell survival and apoptosis. Annu. Rev. Physiol. 2008;70:73–91. doi: 10.1146/annurev.physiol.70.021507.105852. [DOI] [PubMed] [Google Scholar]
- 41.Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene. 2008;27:6407–6418. doi: 10.1038/onc.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hidalgo M. Pancreatic cancer. N. Engl. J. Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 43.Takeuchi S, Takahashi A, Motoi N, Yoshimoto S, Tajima T, Yamakoshi K, Hirao A, Yanagi S, Fukami K, Ishikawa Y, Sone S, Hara E, Ohtani N. Intrinsic cooperation between p16INK4a and p21 Waf1Cip1 in the onset of cellular senescence and tumor suppression in vivo. Cancer Res. 2010;70:9381–9390. doi: 10.1158/0008-5472.CAN-10-0801. [DOI] [PubMed] [Google Scholar]
- 44.Deer EL, Gonzalez-Hernandez J, Coursen JD, Shea JE, Ngatia J, Scaife CL, Firpo MA, Mulvihill SJ. Phenotype and genotype of pancreatic cancer cell lines. Pancreas. 2010;39:425–435. doi: 10.1097/MPA.0b013e3181c15963. [DOI] [PMC free article] [PubMed] [Google Scholar]