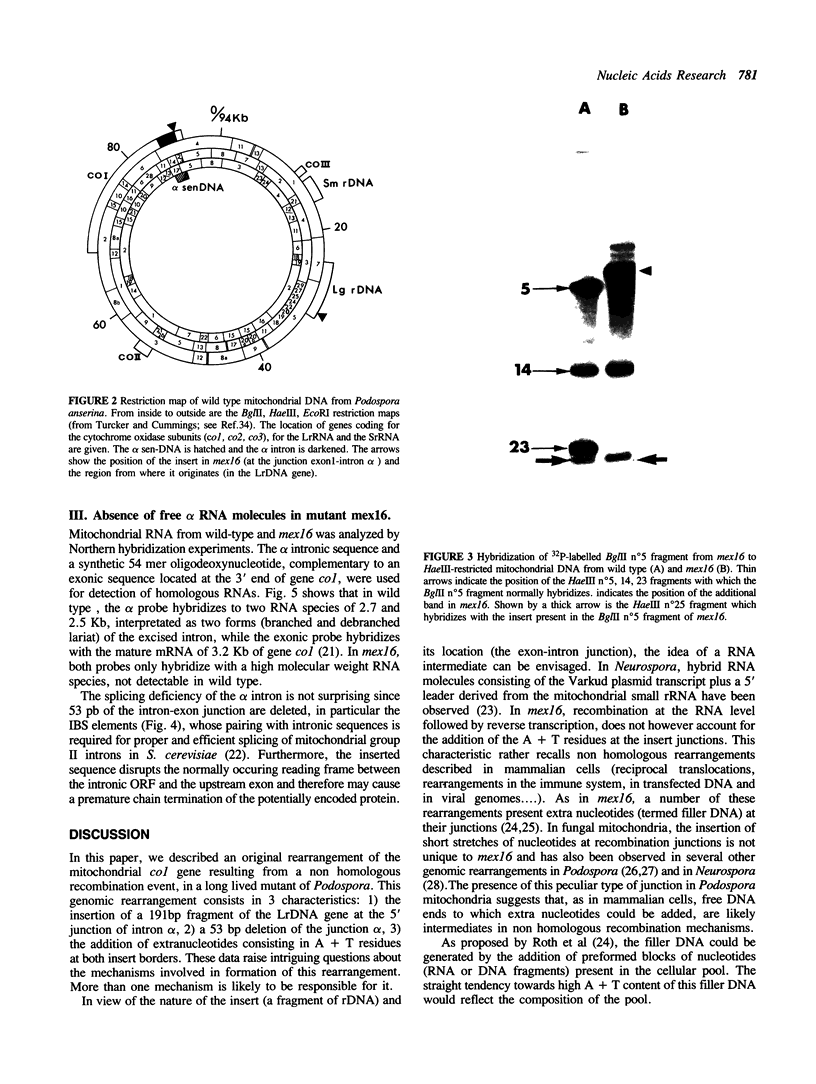
Abstract
A rearrangement of the mitochondrial genome of a long lived mutant of Podospora anserina is presented. It consists in the insertion of 191 bp of the LrDNA gene (coding for the large ribosomal RNA) at the junction between exon1 and intron alpha of gene co1 (coding for subunit 1 of cytochrome oxidase). This insertion is accompanied by a 53 bp deletion of the junction and the presence of extra A and T nucleotides at both sides of the inserted sequence. We discuss possible mechanisms of production of this rearrangement. The presence of extra nucleotides at the recombination junctions suggests that it may pass through a stage of free DNA ends originating from a DNA break at the junction between exon1 and intron alpha of gene co1. The possibility that such a DNA break plays a major role in the instability of the mitochondrial genome is envisaged.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akins R. A., Grant D. M., Stohl L. L., Bottorff D. A., Nargang F. E., Lambowitz A. M. Nucleotide sequence of the Varkud mitochondrial plasmid of Neurospora and synthesis of a hybrid transcript with a 5' leader derived from mitochondrial RNA. J Mol Biol. 1988 Nov 5;204(1):1–25. doi: 10.1016/0022-2836(88)90594-3. [DOI] [PubMed] [Google Scholar]
- Akins R. A., Kelley R. L., Lambowitz A. M. Characterization of mutant mitochondrial plasmids of Neurospora spp. that have incorporated tRNAs by reverse transcription. Mol Cell Biol. 1989 Feb;9(2):678–691. doi: 10.1128/mcb.9.2.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcour L., Begel O. Mitochondrial genes in Podospora anserina: recombination and linkage. Mol Gen Genet. 1977 May 20;153(1):11–21. doi: 10.1007/BF01035991. [DOI] [PubMed] [Google Scholar]
- Belcour L., Vierny C. Variable DNA splicing sites of a mitochondrial intron: relationship to the senescence process in Podospora. EMBO J. 1986 Mar;5(3):609–614. doi: 10.1002/j.1460-2075.1986.tb04254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colleaux L., D'Auriol L., Galibert F., Dujon B. Recognition and cleavage site of the intron-encoded omega transposase. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6022–6026. doi: 10.1073/pnas.85.16.6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings D. J., Belcour L., Grandchamp C. Mitochondrial DNA from Podospora anserina. I. Isolation and characterization. Mol Gen Genet. 1979 Mar 27;171(3):229–238. doi: 10.1007/BF00267577. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., Domenico J. M., Nelson J. DNA sequence and secondary structures of the large subunit rRNA coding regions and its two class I introns of mitochondrial DNA from Podospora anserina. J Mol Evol. 1989 Mar;28(3):242–255. doi: 10.1007/BF02102482. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., MacNeil I. A., Domenico J., Matsuura E. T. Excision-amplification of mitochondrial DNA during senescence in Podospora anserina. DNA sequence analysis of three unique "plasmids". J Mol Biol. 1985 Oct 20;185(4):659–680. doi: 10.1016/0022-2836(85)90052-x. [DOI] [PubMed] [Google Scholar]
- Delahodde A., Goguel V., Becam A. M., Creusot F., Perea J., Banroques J., Jacq C. Site-specific DNA endonuclease and RNA maturase activities of two homologous intron-encoded proteins from yeast mitochondria. Cell. 1989 Feb 10;56(3):431–441. doi: 10.1016/0092-8674(89)90246-8. [DOI] [PubMed] [Google Scholar]
- Herbert C. J., Labouesse M., Dujardin G., Slonimski P. P. The NAM2 proteins from S. cerevisiae and S. douglasii are mitochondrial leucyl-tRNA synthetases, and are involved in mRNA splicing. EMBO J. 1988 Feb;7(2):473–483. doi: 10.1002/j.1460-2075.1988.tb02835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquier A., Michel F. Multiple exon-binding sites in class II self-splicing introns. Cell. 1987 Jul 3;50(1):17–29. doi: 10.1016/0092-8674(87)90658-1. [DOI] [PubMed] [Google Scholar]
- Jamet-Vierny C., Begel O., Belcour L. Senescence in Podospora anserina: amplification of a mitochondrial DNA sequence. Cell. 1980 Aug;21(1):189–194. doi: 10.1016/0092-8674(80)90126-9. [DOI] [PubMed] [Google Scholar]
- Koll F, Begel O, Belcour L. Insertion of short poly d(A) d(T) sequences at recombination junctions in mitochondrial DNA of Podospora. Mol Gen Genet. 1987 Oct;209(3):630–632. doi: 10.1007/BF00331176. [DOI] [PubMed] [Google Scholar]
- Koll F, Begel O, Belcour L. Insertion of short poly d(A) d(T) sequences at recombination junctions in mitochondrial DNA of Podospora. Mol Gen Genet. 1987 Oct;209(3):630–632. doi: 10.1007/BF00331176. [DOI] [PubMed] [Google Scholar]
- Landau N. R., Schatz D. G., Rosa M., Baltimore D. Increased frequency of N-region insertion in a murine pre-B-cell line infected with a terminal deoxynucleotidyl transferase retroviral expression vector. Mol Cell Biol. 1987 Sep;7(9):3237–3243. doi: 10.1128/mcb.7.9.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levings C. S., 3rd, Brown G. G. Molecular biology of plant mitochondria. Cell. 1989 Jan 27;56(2):171–179. doi: 10.1016/0092-8674(89)90890-8. [DOI] [PubMed] [Google Scholar]
- Pritchard M. A., Dura J. M., Pélisson A., Bucheton A., Finnegan D. J. A cloned I-factor is fully functional in Drosophila melanogaster. Mol Gen Genet. 1988 Nov;214(3):533–540. doi: 10.1007/BF00330491. [DOI] [PubMed] [Google Scholar]
- Roth D. B., Chang X. B., Wilson J. H. Comparison of filler DNA at immune, nonimmune, and oncogenic rearrangements suggests multiple mechanisms of formation. Mol Cell Biol. 1989 Jul;9(7):3049–3057. doi: 10.1128/mcb.9.7.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotig A., Colonna M., Bonnefont J. P., Blanche S., Fischer A., Saudubray J. M., Munnich A. Mitochondrial DNA deletion in Pearson's marrow/pancreas syndrome. Lancet. 1989 Apr 22;1(8643):902–903. doi: 10.1016/s0140-6736(89)92897-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz-Sommer Z., Leclercq L., Göbel E., Saedler H. Cin4, an insert altering the structure of the A1 gene in Zea mays, exhibits properties of nonviral retrotransposons. EMBO J. 1987 Dec 20;6(13):3873–3880. doi: 10.1002/j.1460-2075.1987.tb02727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turker M. S., Cummings D. J. Podospora anserina does not senesce when serially passaged in liquid culture. J Bacteriol. 1987 Feb;169(2):454–460. doi: 10.1128/jb.169.2.454-460.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turker M. S., Domenico J. M., Cummings D. J. A novel family of mitochondrial plasmids associated with longevity mutants of Podospora anserina. J Biol Chem. 1987 Feb 15;262(5):2250–2255. [PubMed] [Google Scholar]
- Turker M. S., Domenico J. M., Cummings D. J. Excision-amplification of mitochondrial DNA during senescence in Podospora anserina. A potential role for an 11 base-pair consensus sequence in the excision process. J Mol Biol. 1987 Nov 20;198(2):171–185. doi: 10.1016/0022-2836(87)90304-4. [DOI] [PubMed] [Google Scholar]
- Vierny C., Keller A. M., Begel O., Belcour L. A sequence of mitochondrial DNA is associated with the onset of senescence in a fungus. Nature. 1982 May 13;297(5862):157–159. doi: 10.1038/297157a0. [DOI] [PubMed] [Google Scholar]
- Wallace D. C. Mitochondrial DNA mutations and neuromuscular disease. Trends Genet. 1989 Jan;5(1):9–13. doi: 10.1016/0168-9525(89)90005-x. [DOI] [PubMed] [Google Scholar]
- Wenzlau J. M., Saldanha R. J., Butow R. A., Perlman P. S. A latent intron-encoded maturase is also an endonuclease needed for intron mobility. Cell. 1989 Feb 10;56(3):421–430. doi: 10.1016/0092-8674(89)90245-6. [DOI] [PubMed] [Google Scholar]
- Wolf K., Del Giudice L. The variable mitochondrial genome of ascomycetes: organization, mutational alterations, and expression. Adv Genet. 1988;25:185–308. doi: 10.1016/s0065-2660(08)60460-5. [DOI] [PubMed] [Google Scholar]
- Wright R. M., Horrum M. A., Cummings D. J. Are mitochondrial structural genes selectively amplified during senescence in Podospora anserina? Cell. 1982 Jun;29(2):505–515. doi: 10.1016/0092-8674(82)90167-2. [DOI] [PubMed] [Google Scholar]