Abstract
Fibroids are the most common gynecologic tumors. Our case discusses the outcome of a 47-year-old woman who presented to our clinic with cachexia, and a giant abdominal mass. An initial diagnostic imaging workup consisted of X-Ray, CT, and ultrasound and indicated a possible diagnosis of leiomyosarcoma. However, after surgical evaluation, she was diagnosed pathologically with an atypical presentation of a uterine leiomyoma. Our case reviews the epidemiology and presentation of both pathologies, along with the imaging workup, and the operative correlation in our patient.
Keywords: Leiomyoma, Fibroids, Pelvic Mass, cachexia, leiomyosarcoma
CASE REPORT
A 47-year-old pre-menopausal female gravida 4, para 4 was referred to the gynecologic oncology clinic at our institution for a complaint of increasing abdominal girth with a palpable abdominal mass. This mass was first worked up at another local hospital over 3 years ago, and the patient was told that it was a leiomyoma (fibroid), which did not require further management. She presented to this institution because she noticed that over the past year the mass began growing more rapidly and she had unintentional weight loss. She appeared cachetic and complained of abdominal discomfort from mass effect as well as shortness of breath. On physical exam, she had a grossly visible large fixed abdominal mass, which extended up to the sternum. She was non tender and non-tympanic. On pelvic exam, she had a posteriorly displaced cervix with no lesions or inflammation.
Her uterus and adnexae could not be assessed due to the mass. Tumor markers and results are as follows: Alpha Feto Protein: 3.0 (n= <8.7); CA-125: 48 (n=<35); CA-19–9: <0.6 (n=<35); and CEA: 1.4 (n= <3.4). Imaging studies were subsequently ordered.
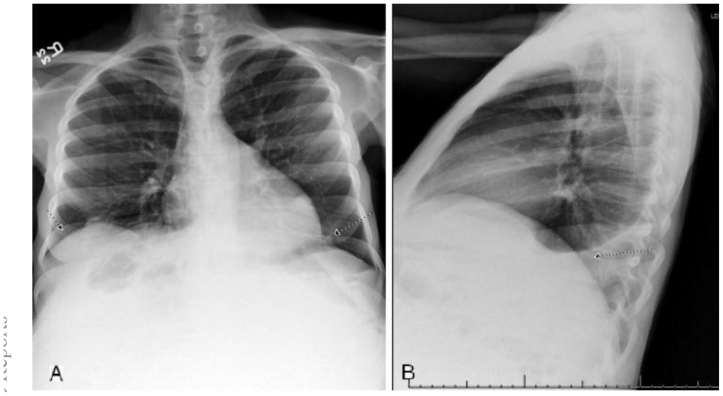
Initially, a posterior-anterior and lateral chest x-ray was performed to evaluate her shortness of breath, as well as any possible metastatic disease. (Fig. 1a,b) The films showed suboptimal lung volumes secondary to elevation of the diaphragm from the mass. There was also bi-basilar sub-segmental atelectasis with a mild to moderate left sided pleural effusion. There was no sign of metastatic parynchemal or pleural nodules, and her shortness of breath was likely due to the combination of the elevated diaphragm and pleural effusion.
Figure 1.
47 year old woman with a chief complaint of giant abdominal mass and a final diagnosis of uterine leiomyoma. (a) PA Chest X-ray with insufficient inspiration likely due to elevation of the diaphragm. There is also sub-segmental atelectasis. (b) Lateral Chest X-ray showing mild to moderate left sided pleural effusion.
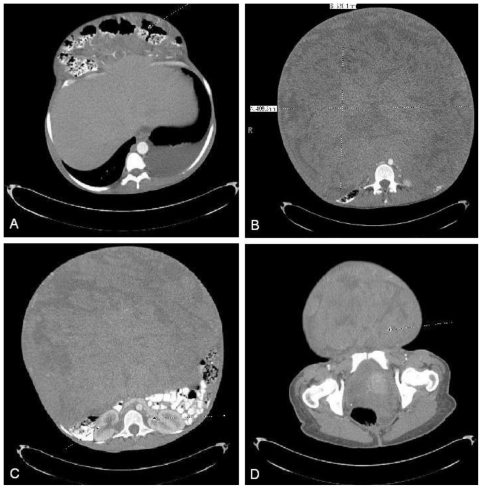
She then underwent an abdominal and pelvic computed tomography (CT) scan with intravenous contrast to evaluate the abdominal mass. (Fig. 2 and Fig. 3)
Figure 2.
47 year old woman with a chief complaint of abdominal mass and a final diagnosis of uterine leiomyoma. A CT scan with 5mm contiguous axial images of abdomen/pelvis was performed with oral and IV contrast. (90ml Optiray) (a) Transverse colon herniated out via abdominal wall defect. (b) Measurement of the mass. It measured 33cm by 40.63 cm. It has heterogeneous attenuation. (c) Abdominal mass can be seen compressing retroperitoneal contents. (d) Mass extends into the pelvis.
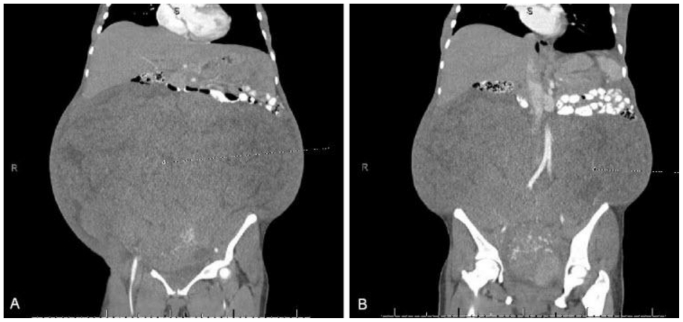
Figure 3.
47 year old woman with a chief complaint of abdominal mass and a final diagnosis of uterine leiomyoma. A CT scan with 5mm contiguous axial images of abdomen/pelvis was performed with oral and IV contrast. (90ml Optiray) The above images are coronal reconstructions. (a) Anterior coronal reconstruction with tumor visible and compressing abdominal contents superiorly. It appears inseparable from the colon. (b) Posterior coronal with tumor visible and compressing abdominal contents superiorly. It appears inseparable from the colon.
There was displacement of the transverse colon anteriorly through a superior abdominal hernia. (Fig. 2a) There was a mass measuring 33×33×41cm with heterogeneous attenuation. (Fig. 2b) The mass caused displacement of the retroperitoneal organs, and compression of the great vessels. (Fig. 2c) There were multiple feeding vessels originating from the celiac axis, superior mesenteric artery, and uterine artery. There was a small amount of abdominal fluid. The mass appeared inseparable from both the uterus and the transverse colon. It extended into the pelvis. (Fig. 2d) There was no clear fat plane or capsule distinguishing the mass from the adjacent bowel, vessels or muscles on axial or sagittal section. (Fig. 2 and Fig. 3) Given the lack of clear tissue separation and the clinical history, the above radiologic findings appeared to be most consistent with a leiomyosarcoma.
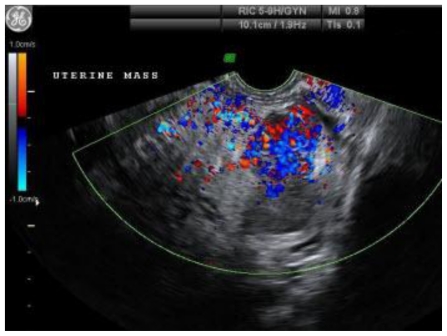
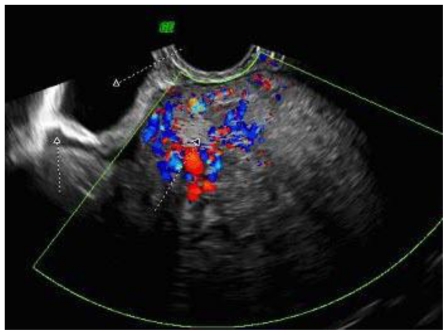
To further evaluate the origin and the vascularity of the mass, she underwent a trans-vaginal ultrasound. (Fig. 4 and Fig. 5) This was an indeterminate study due to the size of the mass. The mass was heterogeneous and mostly solid. Doppler studies showed that it was highly vascular with low resistance to flow. (Fig. 4) The mass appeared to originate from the posterior portion of the uterus. (Fig. 5) However, neither ovary could be visualized so an ovarian origin could not be ruled out. The combination of size, vascularity, lack of fat planes delineating the mass from the surrounding abdominal contents, as well as possible colonic invasion raised our suspicion of a malignant process, of which leiomyosarcoma was the most probable diagnosis.
Figure 4.
47 year old woman with a chief complaint of giant abdominal mass and a final diagnosis of uterine leiomyoma. A trans-vaginal ultrasound with Doppler flow demonstrates vascularity within the uterine mass. The transducer was set at RIC 3D 5–9Hz.
Figure 5.
47 year old woman with a chief complaint of giant abdominal mass and a final diagnosis of uterine leiomyoma. A trans-vaginal ultrasound with Doppler flow was performed. The above cut illustrates the anatomy of our patient. The bladder, cervix, and mass are all visualized. The tumor appears to be originating from the posterior portion of the uterus. The transducer was set at RIC 3D 5–9Hz.
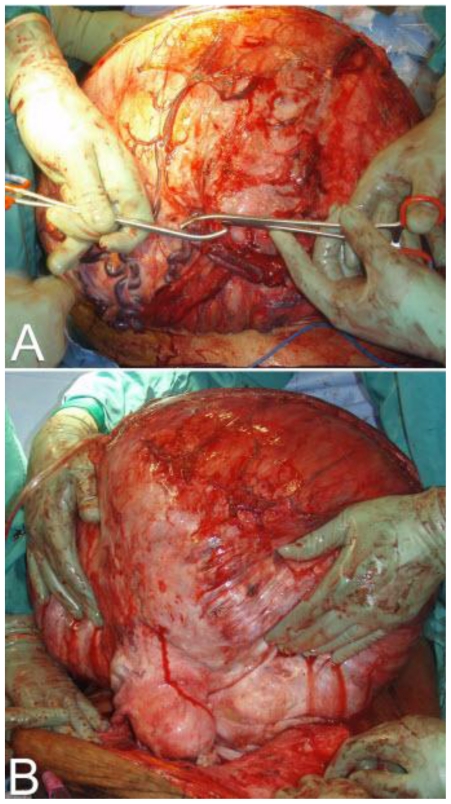
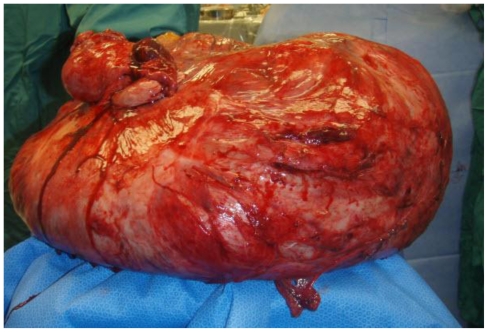
On the basis of the above clinical and radiologic findings, our patient was taken to surgery. The patient underwent a total abdominal hysterectomy with bilateral salpingo-oopherectomy, appendectomy, and partial omentectomy. The operation began with a mid-line vertical skin incision extending from the pubic symphysis to 15cm above the umbilicus. The mass was then mobilized and the venous sinuses were clamped off. The dissection continued until the posterior wall of the abdomen was reached. The mass was adherent to the transverse colon and the omentum. It was subsequently separated from these structures. The mass appeared to originate from the fundus of the uterus. A total abdominal hysterectomy with bilateral salpingo-oopherectomy was performed to allow the mass to be removed en bloc. The specimen was sent for frozen section. Grossly it measured 74cm and weighed 26.94kg. (Fig. 6, Fig. 7 and Fig. 8) Pathological analysis revealed cells with no mitotic activity and no atypia. There was hyalinization along with areas of infarction. These findings were consistent with a pathological diagnosis of leiomyoma. The patient was then closed. During the course of the operation, there was an estimated 1.8L of blood loss which required 5 units of packed red blood cells. Post operatively, the patient had a stable hemoglobin and hematocrit of 11 and 33 respectively. However, the next day her hemoglobin and hematocrit dropped to 8.1 and 26.4. She became hypotensive and tachycardic, and she was subsequently re-operated on for hemorrhagic shock from a hemoperitoneum. There was approximately 2 liters of blood in her abdomen without any distinct areas of hemorrhage. The remainder of her postoperative course was uneventful and she was discharged home on post-operative day 3 from the re-exploration.
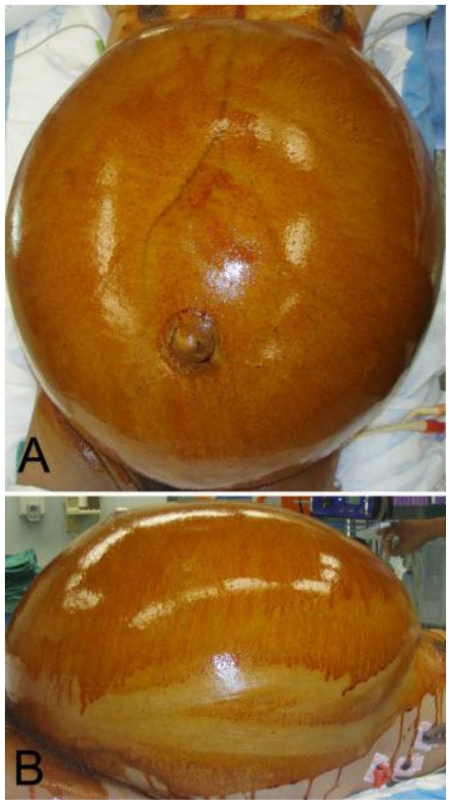
Figure 6.
47 year old woman with a chief complaint of giant abdominal mass and a final diagnosis of uterine leiomyoma. Patient is being prepped for surgery. (a) Lateral View. (b) Overhead View.
Figure 7.
47 year old woman with a chief complaint of giant abdominal mass and a final diagnosis of uterine leiomyoma. (a) Initial dissection. (b) Mass removal en bloc (74cm/27 kg).
Figure 8.
47 year old woman with a chief complaint of giant abdominal mass and a final diagnosis of uterine leiomyoma. This is the mass after it had been removed. Grossly it measured 74cm and weighed 27kg.
DISCUSSION
Uterine leiomyomas are a frequently encountered benign gynecologic tumor. They are diagnosed clinically in approximately 25% of women (1). However, the actual incidence is higher as the majority of patients are asymptomatic (2, 3). The most common presenting symptoms are abnormal bleeding, pain, and pelvic pressure. An atypical fibroid presentation is particularly challenging to diagnose because it may share many characteristics with a leiomyosarcoma. Rapidity of growth should alert the clinician to the possibility of uterine sarcoma. Fibroids are thought to derive from a clonal proliferation of a single cell within the uterine wall (1).
Fibroids are encapsulated and have increased amounts of collagen and elastin (1). Histologically, they consist of spindle shaped cells in a whorl shaped pattern (1). The presence of hyalinization indicates that the tumor has been growing for some length of time. The presence of calcifications indicates dystrophy due to perfusion insufficiency. A rare variant of this presentation is benign metastasizing leiomyoma. These are histologically benign cells, as evidenced by lack of invasion, and low level of mitosis; however, they have been transported to new locations (1,4). The most common extra-uterine site is the lung (1). Currently, there is debate as to the classification of these lesions as leiomyomas of uncertain malignant potential. Fibroids, if symptomatic, are associated with cyclical pain that correlates to the menstrual cycle. The actual mechanism responsible for the increased pain and growth is a combination of hyper-responsiveness to estrogen and progesterone, as well as a variety of secondary paracrine and autocrine mediators (5). The reliance on estrogen and progesterone explains why fibroids often improve post-menopausally. The only FDA approved medical therapy for fibroids are GnRH agonists (5). However, as GnRH agonists can only be used for a limited time, fibroids will re-grow if not removed surgically (5). The traditional surgical option was a hysterectomy. Recently, there has been an increase in uterine sparing procedures. Of these, the most common is uterine artery embolization. However, because this can lead to uterine necrosis, it should not be used if the patient is not willing to accept the risk of infertility (5). Recently, this technique has been studied as a prehysterectomy treatment to improve the outcome of the hysterectomy. It has been shown to increase the likelihood of a more conservative surgical procedure (6). This would not have been an option for our patient, because of the uncertainty of diagnosis.
Uterine leiomyosarcomas are malignant smooth muscle tumors. They are differentiated from fibroids by the Stanford criteria: mitotic index (>10), cellular atypia, and coagulative necrosis. If two of the above criteria are met, there is a higher incidence of concurrent metastasis. Other histologic features associated with leiomyosarcomas are infiltrative borders and hypercellularity (7). The clinical picture upon initial presentation is very similar to that of fibroids. While they typically grow rapidly, like a traditional malignancy, it is important to note that, due to their low incidence, the most likely diagnosis of a rapidly expanding uterine mass is still a fibroid (7). Unlike fibroids, leiomyosarcomas are aggressive and metastasize early to the lungs and liver (8).
It is important to differentiate between the two because their natural histories are markedly different. Uterine leiomyomas can typically be observed without harm to the patient. Uterine leiomyosarcomas require aggressive surgical management with adjuvant chemotherapy, irradiation or both. These treatments are often palliative in nature, as the tumors do not typically respond well. Before proceeding on this course, it is imperative to obtain histologic confirmation, the only definitive diagnostic modality, in order to guide the clinician’s management of the patient and to aid in determining the urgency of surgical intervention.
In our patient, the presence of cachexia increased the suspicion for malignancy. The mass appeared to be attached to both the uterus and colon and lacked clear fat planes on CT. These findings were all consistent with a malignant process. However, many findings were inconsistent with this diagnosis. The tumor had been present for 3 years. While it increased in size over the past year, only a rare group of fibroids with a deletion on chromosome 1 dedifferentiate into a malignant process (4). Leiomyosarcomas are aggressive, and would more than likely spread over the time course of her symptoms. Furthermore, there was a high level of vascularity on both CT and Ultrasound. This was an inconclusive finding as fibroids are vascular tumors at times with large parasitic vessels, commonly to the omentum and would likely show the same level of vascularity as a leiomyosarcoma. Finally, our patient did not undergo a full metastatic workup prior to surgery, but on the imaging obtained there was no evidence of metastatic disease.
An atypical presentation of fibroids is often confusing to clinicians. Atypical presentations often have signs that would be unequivocally indicative of a malignant process with other tumors. One example would be lung metastasis in the abovementioned case of benign metastasizing leiomyoma. In our patient, despite the size of the mass, the constellation of equivocal findings made the benign and more common fibroid a more likely diagnosis.
For our patient, surgical management was needed regardless of the diagnosis. An exploratory laparotomy was the only method available due to the size of the mass. While the radiologic findings were not conclusive, the management would not have changed due to the patient’s unique presentation. However, in a more traditional presentation of fibroids or smaller masses presenting with inconclusive imaging findings, it is important to note that the correct diagnosis is fibroids in the vast majority of patients. This affects the management of the patient because she may be observed for a time if she does not want or is a poor surgical candidate. While it is necessary for the overwhelming majority of patients to rule out a malignant process, it is important to instruct clinicians to counsel the patients on the epidemiology of these findings for the psychological well being of the patient. This is needed because patients undergo an extreme amount of stress, anxiety, and depression when faced with the potential diagnosis of cancer. Discussing the low likelihood of malignancy will help to quell these above feelings as well as assist in obtaining informed consent.
TEACHING POINT
An atypical presentation of a leiomyoma is challenging to differentiate from a leiomyosarcoma. However, due to its high incidence, even in the presence of signs and symptoms that raise the suspicion of malignancy, it is still the most common diagnosis.
ACKNOWLEDGEMENTS
Dr. Paul Norris of the University of Miami Miller School of Medicine, Department of Obstetrics and Gynecology.
ABBREVIATIONS
- CT
Computed Tomography
- FDA
Food and Drug Administration
- FDG
Fluorodeoxyglucose
- GnRH
Gonadotropin-releasing hormone
- mCi
milli Curie
- PET
Positron emission tomography
REFERENCES
- 1.Drinville J, Memarzadeh S. Chapter 39 Benign Disorders of the Uterine Corpus. In: DeCherney A, Nathan L, editors. CURRENT Diagnosis & Treatment Obstetrics & Gynecology. 10th edition. Columbus, OH: The McGraw-Hill Companies, Inc.; 2007. [Google Scholar]
- 2.Stewart E. Epidemiology, clinical manifestations, diagnosis, and natural history of uterine leiomyomas [serial online] In: Basow DS, editor. UpToDate. UpToDate; Waltham, MA: 2009. [Google Scholar]
- 3.Cramer S, Patel A. The frequency of uterine leiomyomas. Am J Clin Pathol. 1990 Oct;94(4):435–8. doi: 10.1093/ajcp/94.4.435. [DOI] [PubMed] [Google Scholar]
- 4.Esteban J, Allen W, Schaerf R. Benign Metastasizing Leiomyoma of the Uterus: Histologic and Immunohistochemical Characterization of Primary and Metastatic Lesions. Arch Pathol Lab Med. 1999 Oct;123(10):960–2. doi: 10.5858/1999-123-0960-BMLOTU. [DOI] [PubMed] [Google Scholar]
- 5.Walker C, Stewart E. Uterine Fibroids: The Elephant in the Room. 2005 Jun 10;308(5728):1589–92. doi: 10.1126/science.1112063. [DOI] [PubMed] [Google Scholar]
- 6.Dumousset E, Chabrot P, Rabischong B, et al. Preoperative Uterine Artery Embolization (PUAE) Before Uterine Fibroid Myomectomy. Cardiovasc Intervent Radiol. 2008 May-Jun;31(3):514–2.0. doi: 10.1007/s00270-005-0342-3. [DOI] [PubMed] [Google Scholar]
- 7.Memarzadeh S, Mundt A, Berek J. Uterine Sarcoma: Classification, clinical manifestations, and diagnosis. [serial online] In: Basow DS, editor. UpToDate. UpToDate; Waltham, MA: 2009. [Google Scholar]
- 8.Dorigo O, Goodman A. Chapter 51 Premalignant & Malignant Disorders of the Uterine Corpus. In: DeCherney A, Nathan L, editors. CURRENT Diagnosis & Treatment Obstetrics & Gynecology. 10th edition. Columbus, OH: The McGraw-Hill Companies, Inc.; 2007. [Google Scholar]
- 9.Buttram V, Reiter R. Uterine leiomyomata: etiology, symptomatology, and management. Fertil Steril. 1981 Oct;36(4):433–45. doi: 10.1016/s0015-0282(16)45789-4. [DOI] [PubMed] [Google Scholar]
- 10.Ellenson L, Pirog E. Chapter 22. The Female Genital Tract. In: Kumar V, Abbas A, Fausto N, Aster J, editors. Kumar: Robbins and Cotran: Pathologic Basis of Disease, Professional Edition. 8th edition. Philadelphia, PA: Saunders Elsevier; 2010. [Google Scholar]