Abstract
We present a case of glioblastoma multiforme of the optic pathways in a 68 year old lady. Glioblastomas of the optic pathways are rare tumors; the predominant non enhancing component and the vast extent of involvement makes this a unique case. This case report further increases the database of knowledge available on the MRI characteristics of malignant optic glioma of adulthood.
Keywords: MRI, Glioblastoma, Optic pathways, malignant glioma
CASE REPORT
A 68 years old lady presented with complaints of loss of vision in the left eye since past 1 to 2 months. It was not associated with any pain or discharge. There was also a history of irritability and involuntary movements of the body since two to three months. Her general physical examination was normal. Best corrected visual acuity in the right eye was 6/24 and counting fingers at four meters in the left eye. Anterior segment examination revealed sluggish pupillary reaction in both the eyes. On fundus examination, bilateral papilloedema was detected. The color vision on Ishiara’s chart was 5/14 in the right eye and 2/14 in the left eye. On confrontation test, temporal fields of both right and left eyes were decreased.
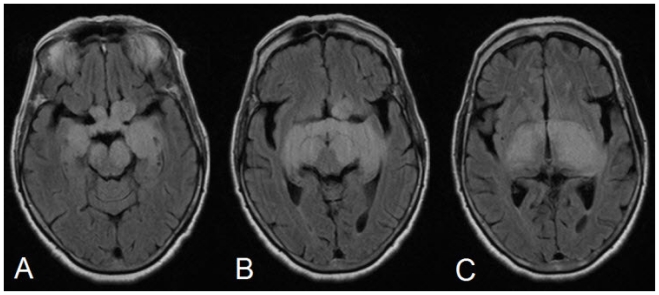
With this background, an MRI of the brain was done on 1.5 Tesla Siemens Essenza system. We found that there was thickening of the prechiasmatic intracranial optic nerves, the optic chiasm and post chiasmatic optic tracts with lobulated contours and diffuse signal alteration showing hyperintense signal on T2W/FLAIR images (Fig. 1).
Figure 1.
68 yrs old female with glioblastoma of the optic pathways. FLAIR (TR -9000ms, TE = 87, TI = 2500) showing thickening of the prechiasmatic intracranial optic nerves, chiasm and post chiasmatic optic tracts with hyperintense signal alteration.
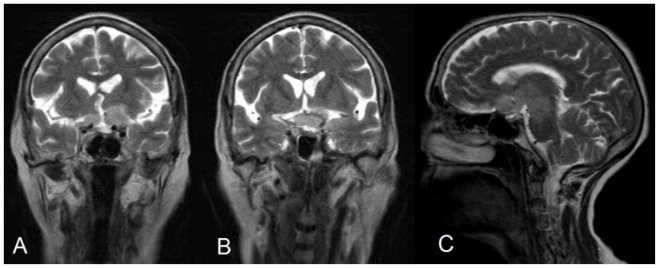
Contiguous large well marginated near symmetrical signal alteration was also seen in the hypothalamus, bilateral thalamocapsular (reaching upto paraventricular region on left side) regions, bilateral mesial temporal lobes, crus cerebri and the upper midbrain, showing hyperintense signal on T2W/FLAIR images (Fig. 2) The lesion appeared hypointense to isointense on T1W images and showed no restriction on diffusion images. The involved structures appear expanded. The intracanalicular and intraorbital optic nerves appeared normal.
Figure 2.
68 yrs old female with glioblastoma of the optic pathways. T2W Coronal (TR -4100ms, TE = 91.0 SL -5mm, FOV 173 × 230) and T2W sag images showing symmetrical hyperintense signal alteration in the hypothalamus, bilateral thalamocapsular regions, bilateral mesial temporal lobes, crus cerebri and the upper midbrain. Bulky chiasm and pre chiasmatic nerves are also noted.
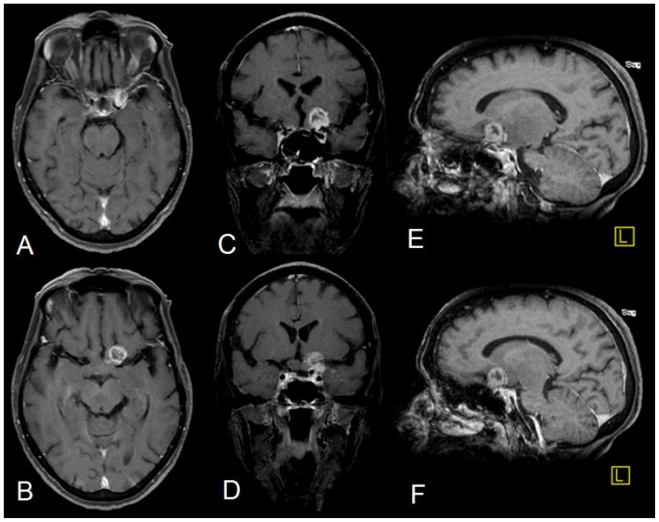
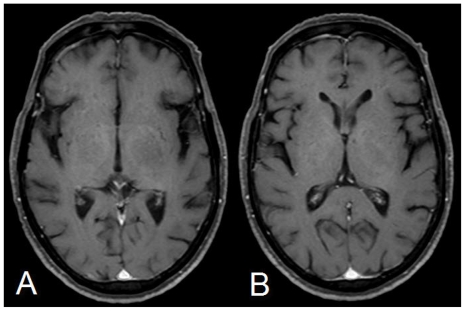
The post contrast images (using 20ml of Gadodiamide, OMNISCAN, GE Healthcare) revealed a small (14×12mm) heterogeneous ring enhancing focus anterosuperior to the optic chiasm on the left side (Fig. 3). Rest of the lesion showed no significant enhancement (Fig. 4). However, subtle meningeal enhancement was seen in the left temporal region.
Figure 3.
68 yrs old female with glioblastoma of the optic pathways. Post Contrast T1W (TR = 845, TE = 9, SL 5mm) Axial (A & B), Coronal (TR = 825, TE = 11, C & D) and sag. (TR = 825, TE = 9, E & F) after injecting 20cc 0.3mmol/kg of Gadodiamide (OMNISCAN, GE healthcare) showing small heterogeneous ring enhancing focus anterosuperior to the optic chiasm on the left side Subtle left temporal meningeal enhancement is seen (Fig. 7). There is no enhancement seen in the optic nerves, tracts and the pathways.
Figure 4.
68 yrs old female with glioblastoma of the optic pathways. Post Contrast T1W Axial images showing no significant enhancement in the rest of the lesion.
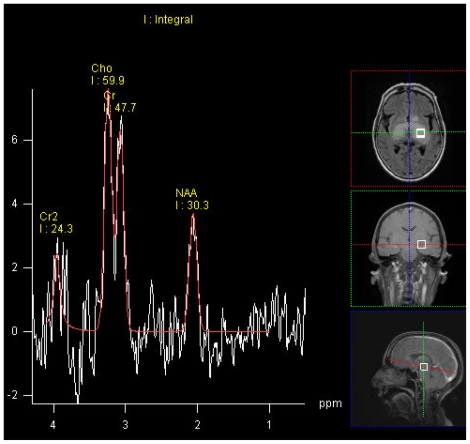
SV MR Spectroscopy of the lesion taken at multiple places (TE 30,135 and 270 ms) showed elevated Cho with raised Cho/NAA ratio (1.3 – 1.9). (Fig. 5)
Figure 5.
68 yrs old female with glioblastoma of the optic pathways. SV MR Spectroscopy of the lesion taken at left thalamus (TE 135 ms) showed elevated Cho with raised Cho/NAA ratio (1.3 – 1.9).
The patient was then taken up for an open biopsy. Using the left pterional approach, a large extra - axial pinkish tumor, which was soft and well demarcated, was visualized. Tumor surface was then coagulated and was entered into. It was vascular and could be easily sucked into. It extended posteromedially. Finally, a generous biopsy was carried out.
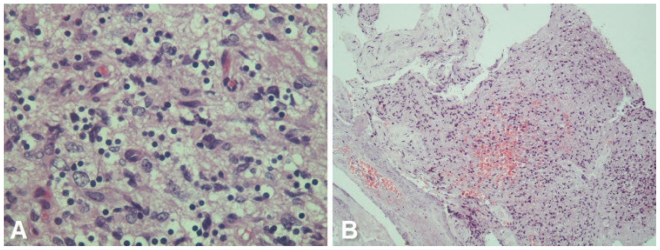
Histopathological examination of the biopsy (Fig. 6) showed cerebral tissue with varying degree of cellularity, characteristic cyto-morphology, abundant mitotic figures, increased vascularity and multi-focal areas of tumor necrosis consistent with a diagnosis of glioblastoma multiforme.
Figure 6.
68 yrs old female with glioblastoma of the optic pathways. A - Photomicrograph showing anaplastic tumor cells in a fibrillary background - tumor cells show abundant cytoplasmic clearing - somewhat reminiscent of ‘fried-egg’ appearance. However, cellular pleomorphism was pronounced (H &E, 400X). B - Photomicrograph showing focal necrosis and hemorrhage within the tumor tissue (H & E, 100X).
DISCUSSION
Gliomas account for 40–50% of all primary and metastatic intracranial tumors. (1) Glioblastoma is the most common type of glioma.
WHO 2000 classification grades astrocytic tumors from grade I to grade IV. WHO Grade I tumor includes pilocytic astrocytoma, pleomorphic xanthoastrocytoma and subependymal giant cell astrocytoma. Other astrocytomas include diffuse astrocytoma (grade II), anaplastic astrocytoma (grade III) and glioblastoma (grade IV).
Gliomas generally demonstrate high signal intensity on T2w images and low signal intensity on T1w images, unless hemorrhage or calcification is present. Low grade astrocytoma (WHO grade I and II) tend to be well defined, nonhemorrhagic and demonstrate little mass effect, vasogenic edema or heterogeneity. Anaplastic astrocytomas (WHO grade III) are less well defined and demonstrate moderate amount of mass effect, heterogeneity and edema with varying degrees of enhancement. Glioblastoma (WHO grade IV) are poorly defined and often have considerable mass effect, vasogenic edema, hemorrhage and heterogeneity. Irregular ring enhancement with nodularity and necrotic foci are typical of Glioblastoma (2,3).
Apart from conventional and contrast MR, glioma grading have been further attempted on the basis of diffusion weighted images, MR perfusion and MR Spectroscopy.
The signal intensity of gliomas on DW images is variable (hyper-, iso-, or hypointense) (4). Tumor cellularity is probably a major determinant of ADC values of brain tumors. ADC values have not been found to be useful in individual cases to differentiate glioma types reliably. DW images and/or ADC maps have also not been reliable to distinguish neoplastic cell infiltration from peritumoral edema in patients with malignant glioma (4).
MR Spectroscopy has shown increased Cho values in glioma with associated decrease in NAA/Cho and Cr/Cho ratios with lipid-lactate peaks. One study has shown that specific metabolites, when standardized to water, are of diagnostic value in the division of tumors into three categories. Specifically, the lactate/water ratio can be used to differentiate GBMs, anaplastic astrocytomas, and low-grade tumors. The choline/water, choline/creatine, and lactate/creatine ratios can be used to distinguish high-grade from low-grade tumors (5).
MR perfusion studies have shown a correlation between relative cerebral blood volume and tumor grade (6).
Gliomas of the optic pathway are classified as (a) the relatively benign optic nerve glioma (typically occurring in the pediatric age group) and (b) the malignant optic glioma of adulthood (7). Benign optic nerve gliomas represent 4% of orbital tumors, 4% of intracranial gliomas, and 2% of intracranial tumors. They also constitute two thirds of all primary optic nerve tumors (8).
The benign optic nerve glioma is more common and is considered a low grade Astrocytoma (9). From 10 to 38% of pediatric patients with optic nerve glioma have NF-1; conversely, 15–40% of children with NF-1 have optic nerve glioma. Bilateral optic nerve gliomas are almost pathognomonic for NF-1 (10).
The malignant optic glioma of adulthood, on the other hand, is an extremely rare optic pathway tumor. Till 1990, only 30 cases were reported in this century (11). In a recent review of literature in 2004 by Bettina Wabbles et al, 45 cases of adult malignant optic gliomas have been described. Of these patients 51% were male, 49% females; the mean age at diagnosis was 54 years (median 59yrs, range 22 to 79yrs) (12) The sites of occurrence of malignant optic glioma in addition to the optic chiasma and the optic nerve(s) were found to be the hypothalamus in 50% of patients, the temporal lobe in 22.5% and the basal ganglia in 15%. Other more infrequent sites were the midbrain, parietal cortex, cerebellum and the cervical, thoracic and lumbar subarachnoidal spaces. (12) There was no reported association with neurofibromatosis with adult optic glioblastoma. The malignant optic glioma of adulthood is classified pathologically as either an anaplastic astrocytoma or a glioblastoma multiforme (7). In adults, malignant optic gliomas are rare and are rapidly fatal (13) visual pathway tumors. Anatomical classification for OPGs was proposed by Dodge et al in 1958, defining tumors as involving either the optic nerves alone (Stage 1), the chiasm with or without nerve involvement (Stage 2), and the hypothalamus or other adjacent structures (Stage 3). The modified Dodge classification further categories stage 1 into stage 1a (i.e. involvement of Single optic nerve), 1b (Bilateral optic nerve involvement), 1c (Cisternal segment of the optic nerve). Stage 2 is categorized into 2a (Central chiasmatic) and 2b (Asymmetric chiasmatic). Stage 3a is optic tracts involvement, 3b is asymmetric tract involvement. Stage 4 is diffuse posterior tract involvement and stage 4b is asymmetric posterior tract involvement. The Hypothalamic involvement, leptomeningeal dissemination and associated neurofibromatosis are also taken in account ( i.e. H+/−, LM+/− and NF1 +/−) (14).
Majority of the cases reported so far showed subtle to significant enhancement of the optic nerves/chiasm or the optic pathways (15). In this particular case, there was no enhancement of the optics nerves/pathways.
Most of the optic nerve gliomas of adulthood initially show some degree of enlargement and enhancements of one of the optic nerves. With progression, the optic chiasm becomes involved and then there is spread of the tumor to the contralateral optic nerve and subsequently to the optic pathways. Hypothalamic involvement with symptoms, such as polyuria and polydipsia, that may appear late in the clinical course suggest secondary invasion of the hypothalamus.
The symptoms and clinical presentation in our case follows the pattern of the syndrome of malignant optic glioma of adulthood, as defined by Hoyt et al (16). In this syndrome, the physical signs that would normally raise suspicion for an intraorbital mass are absent, or, when present, occur late in the clinical course and are mild in extent(15). In the syndrome of malignant optic glioma of adulthood, progressive loss of visual acuity, central retinal vein and/or artery occlusion, and monocular blindness occur in rapid succession, as occurs in our case.
From a clinical perspective, monocular visual loss at presentation implies origin in the optic nerve, whereas bilateral visual changes at presentation imply origin in the optic chiasm (16).
Glioblastomas of the optic pathways involving the optic chiasm may show contiguous spread to the hypothalamus, basal ganglia, and internal capsule. Leptomeningeal and subpial spread of malignant optic glioma of adulthood to the medial temporal lobes and brain stem has also been reported (17). Our case shows subtle Leptomeningeal enhancement in the left temporal region. One of the case reports (18) showed an enhancing focal lesion the left temporal lobe with an expanded enhancing left optic nerve, the left lateral aspect of the chiasm and the left optic pathway. Similar enhancing centrally necrotic lesion was seen in our case.
The treatment options available for optic nerve gliomas of adulthood include surgery, radiation, and chemotherapy. But the results of treatment are not very encouraging and it is invariably considered a lethal disease. Mean survival from the time of presentation is usually less than 1 year (13). Our patient survived for two months and was then lost to follow up.
TEACHING POINT
The diagnosis of optic pathway glioma should be kept in mind even with non enhancing lesions involving bilateral optic nerves, the chiasm and optic pathways. This will help in early diagnosis of an otherwise rapidly fatal malignancy.
ACKNOWLEDGEMENTS
We wish to thank Dr SPS Chawla and Dr Maneesh Bagai for their immense support during the preparation of the manuscript.
ABBREVIATIONS
- ADC
Apparent Diffusion Coefficient images
- Cho
Choline
- Cr
Creatinine
- DW
Diffusion Weighted images
- FLAIR
Fluid Attenuation Inversion Recovery
- GBM
Glioblastoma Multiforme
- HPE
Histopathological Examination
- MRI
Magnetic Resonance Imaging
- NAA
N=Acetyl Aspartate
- NF
Neurofibromatosis
- OPG
Optic Pathway Glioma
- Sag
Sagittal
- SV
Single Voxel
- TE
Time to echo
REFERENCES
- 1.Rubinstein LJ. Tumors of the Central Nervous System. Washington, DC: Armed Forces Institute of Pathology; 1972. [Google Scholar]
- 2.Dean BL, Drayer BP, Bird CR, et al. Gliomas: Classification with MR Imaging. Radiology. 174:411–415. doi: 10.1148/radiology.174.2.2153310. [DOI] [PubMed] [Google Scholar]
- 3.Richards J. Hicks: Supratentorial adult brain tumors. In: Edelman Robert, et al., editors. Clinical MRI. third ed. Philadelphia: PA Saunders Elsevier; 2006. pp. 1098–1100. [Google Scholar]
- 4.Sugahara T, Korogi Y, Kochi M, et al. Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J Magn Reson Imaging. 1999;9:53–60. doi: 10.1002/(sici)1522-2586(199901)9:1<53::aid-jmri7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Meyerand M, Pipas J, et al. Classification of Biopsy-Confirmed Brain Tumors Using Single-Voxel MR Spectroscopy. AJNR Am J Neuroradiol. 1999;20:117–123. [PubMed] [Google Scholar]
- 6.Shin JH, Lee HK, Kwun BD, et al. Using relative cerebral blood flow to evaluate the histopathologic grade of cerebral gliomas: preliminary results. Am J Roentgenol. 179:783–789. doi: 10.2214/ajr.179.3.1790783. [DOI] [PubMed] [Google Scholar]
- 7.Listernick R, Charrow J, Greenwald MJ, et al. Optic gliomas in children with neurofibromatosis type 1. J Pediatr. 1989 May;114(5):788–92. doi: 10.1016/s0022-3476(89)80137-4. [DOI] [PubMed] [Google Scholar]
- 8.Harper CG, Stewart-Wynne EG. Malignant optic gliomas in adults. Arch Neurol. 1978;35:731–735. doi: 10.1001/archneur.1978.00500350035007. [DOI] [PubMed] [Google Scholar]
- 9.Taylor T, Jaspan T, Milano G, Gregson R, Parker T, Ritzmann T, Benson C, Walker D. Radiological classification of optic pathway gliomas: experience of a modified functional classification system. The British Journal of Radiology. 2008;81:761–766. doi: 10.1259/bjr/65246351. [DOI] [PubMed] [Google Scholar]
- 10.Hollander MD, FitzPatrick M, O’Connor SG, et al. Optic gliomas. Radiol Clin North Am. 1999 Jan;37(1):59–71. doi: 10.1016/s0033-8389(05)70078-6. [DOI] [PubMed] [Google Scholar]
- 11.Millar WS, Tartaglino LM, Sergott RC, Friedman DP, Flanders AE. MR of malignant optic glioma of adulthood. AJNR Am. J. Neuroradiol. 1995 Sep;16:1673–1676. [PMC free article] [PubMed] [Google Scholar]
- 12.Hoyt WF, Meshel LG, Lessell S, Schatz NJ, Suckling RD. Malignant optic glioma of adulthood. Brain. 1973;96:121–132. doi: 10.1093/brain/96.1.121. [DOI] [PubMed] [Google Scholar]
- 13.Shapiro SK, Shapiro I, Wirtschafter JD, Mastri AR. Malignant optic glioma in an adult. Minn Med. 1982;65(3):155–159. [PubMed] [Google Scholar]
- 14.Woiciechowsky C, Vogel S, Meyer R, Lehmann R. Magnetic resonance imaging of a glioblastoma of the optic chiasm. J Neurosurg. 83:923–925. doi: 10.3171/jns.1995.83.5.0923. [DOI] [PubMed] [Google Scholar]
- 15.Taphoorn MJB, de Vries-Knoppert WAEJ, Ponssen H, Wolbers JG. Malignant optic glioma in adults. J Neurosurg. 1989;70:277–279. doi: 10.3171/jns.1989.70.2.0277. [DOI] [PubMed] [Google Scholar]
- 16.Bilaniuk LT, Atlas SW, Zimmerman RA. The orbit. In: Lee SH, Rao KCVG, Zimmerman RA, editors. Clinical MRI and CT. New York, NY: McGraw-Hill; 1992. pp. 119–191. [Google Scholar]
- 17.Albers GW, Hoyt WF, Forno LS, Shratter LA. Treatment response in malignant optic glioma of adulthood. Neurology. 1988:1071–1074. doi: 10.1212/wnl.38.7.1071. [DOI] [PubMed] [Google Scholar]
- 18.Wabbels B, Demmler A, Seitz J, Woenckhaus M, Bloss HG, Lorenz B. Unilateral adult malignant optic nerve glioma. Graefes Arch Clin Exp Ophthalmol. 242:741–748. doi: 10.1007/s00417-004-0905-z. [DOI] [PubMed] [Google Scholar]