Abstract
Extramedullary haematopoiesis is the production of blood elements outside the bone marrow cavity. In our case computed tomography and magnetic resonance imaging revealed the presence of a rare localization of extramedullary haematopoiesis with encasement of the biliary system in a 59 years-old male Caucasian patient, with chronic myelofibrosis and hepatic failure’s symptomatology. Computed tomography detected the presence of homogeneous hypodense tissue around intra-hepatic bile ducts with minimal contrast enhancement, strongly suggestive for extramedullary haematopoiesis. Magnetic resonance confirmed the presence of a solid tissue surrounding the biliary tree, showing late enhancement after gadolinium administration suggestive for non-active lesion of extramedullary haematopoiesis. Final diagnosis was established by percutaneous biopsy.
Keywords: Extramedullary haematopoiesis, diagnostic imaging, biliary system, computed tomography, magnetic resonance imaging, MRI
CASE REPORT
We report a 59 years-old male Caucasian patient with diagnosis of chronic myelofibrosis from 13 years, coming to our institute in February 2007, in order to plan a splenectomy, because of an inadequate response to pharmacological therapy. About 6 weeks before the admission, the patient went into hepatic failure with massive ascites.
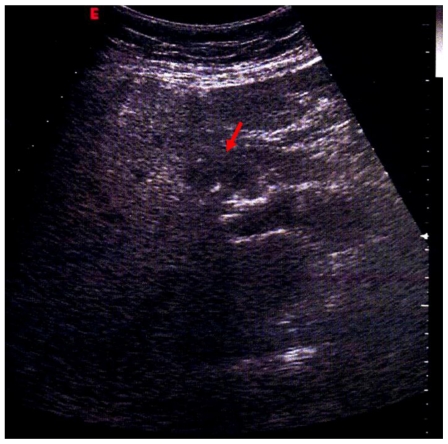
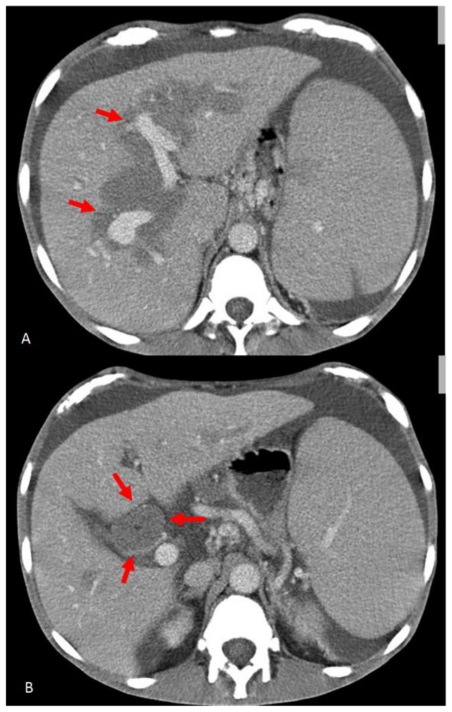
With a clinical suspicion of hepatic vein thrombosis an ultrasound (US) study was performed, which did not reveal vascular thrombosis; US scans showed near to the hepatic hilum hypoechoic tissue, with irregular margin (Fig. 1) A computed tomography (CT) of the abdomen was recommended. Subsequently, an unenhanced and contrast enhanced CT was performed (Sensation 16, Siemens, Erlangen, Germany), detecting the presence of hypo-attenuating tissue (41 HU), involving the intra-hepatic bile ducts and encasing the common duct and gallbladder. This tissue was homogeneous, showing minimal enhancement in delayed phase; no signs of vascular infiltration were visualized (Fig. 2); moreover CT examination identified extension into the perirenal space. Imaging findings, combined with the clinical history, were strongly suggestive of extramedullary haematopoiesis (EMH).
Figure 1.
59 years-old male Caucasian patient with extramedullary haematopoiesis. Abdominal ultrasonography (GE Healthcare, convex probe at 4.0 MHz) evidenced near the hilum of the liver, above the portal vein, solid tissue, with irregular margin, hypoechoic to the surrounding liver (red arrow).
Figure 2.
59 years-old male Caucasian patient with extramedullary haematopoiesis. Contrast-enhanced computed tomography scans (Sensation 16 Siemens, Erlangen, 332 mA, 120 KV, slice thickness 1–5 mm) (Portal phase: acquisition after 60 s non-ionic iodinated i.v. contrast injection) of the upper abdomen (non-ionic iodinated contrast medium: Visipaque GE Healthcare 320 mg I/ml, 100 ml, flow 2 ml/s). Axial CT images demonstrate homogeneous hypo-attenuating soft tissue around intra-hepatic bile ducts (red arrows) (A), with minimal enhancement, encasing the common bile duct (red arrows) (B).
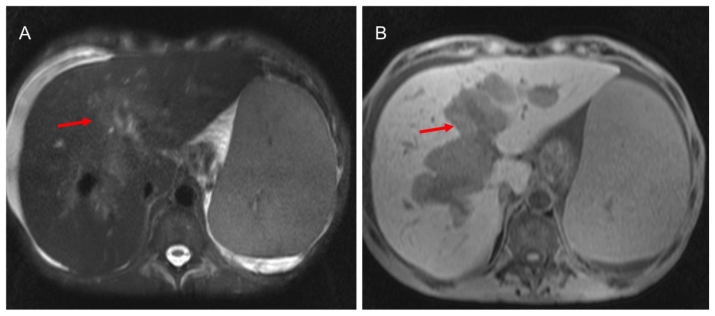
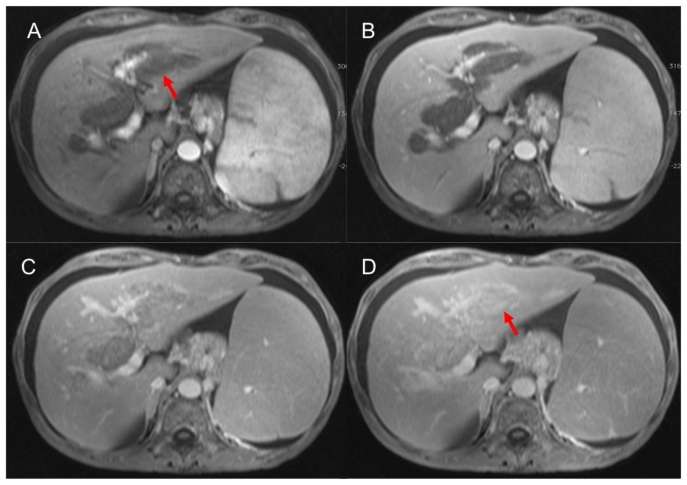
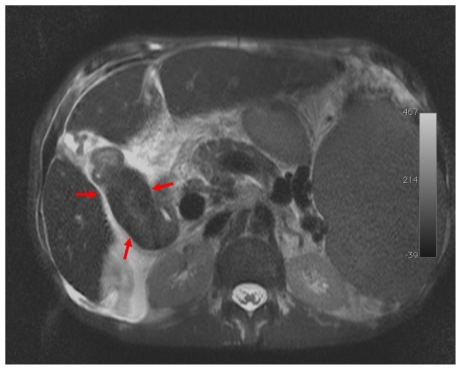
MRI of the abdomen was performed (Magnetom Symphony, Siemens, Erlangen, Germany), according to our standard protocol for hepatic-parenchyma study, that included T2-weighted (−w) TSE, T1-w 3D-GRE sequences and T1-w 3D-GRE sequences after gadolinium administration (Gd-BOPTA; Multihance, Bracco, Italy; 0,1 mmol/Kg; flow 2 ml/s) (arterial, portal and equilibrium phases and delayed phases at 5, 10 and 15 minutes after intravenous injection). MRI demonstrated solid tissue surrounding the biliary tree, slightly hyperintense on T2-weighted (−w), hypointense on T1-w images (Fig. 3). The tissue showed minimal contrast enhancement in early phases (arterial, portal and equilibrium) after i.v. injection and gradual enhancement on delayed scans at 5 and 10 minutes. The mass was inhomogeneously filled on 15 minutes after i.v. injection scans, showing hyperintense foci within the lesion (Fig. 4). The lumen of the gallbladder was nearly completely infiltrated by solid tissue (Fig. 5). No stenosis or dilatation of bile ducts was seen. Further findings on CT and MRI were massive splenomegaly (26.5 cm in craniocaudal length), hepatomegaly (19.6 cm in craniocaudal length), enlargement of the portal vein (with a diameter of 2.1 cm), oesophageal varicose veins, ascites and abdominal lymphadenopathy. A fine needle biopsy of the peribiliary mass under US guidance confirmed the diagnosis of EMH. The patient died of complication of the disease 9 months after splenectomy.
Figure 3.
59 years-old male Caucasian patient with extramedullary haematopoiesis. Magnetic resonance imaging of the upper abdomen (Symphony Siemens 1.5 T, Erlangen). A: Axial T2-weighted images TSE fat sat (TE: 84 ms, TR: 111060 ms; slice thickness: 5.0 mm) the pathological tissue shows inhomogeneous hyperintense signal (red arrow). B: Axial T1-weighted image, 3D-GRE, (TR= 5.12 ms, TE= 2.51 ms, slice thickness= 3.5 mm): the pathological tissue shows hypointense signal (red arrow).
Figure 4.
59 years-old male Caucasian patient with extramedullary haematopoiesis. Magnetic resonance imaging of the upper abdomen (Symphony Siemens 1.5 T, Erlangen). Axial T1-weighted image, 3D-GRE, (TR= 5.12 ms, TE= 2.51 ms, slice thickness= 3.5 mm): plain acquisition in which extramedullary haematopoiesis appears hypointense to liver parenchyma; after intravenous administration of gadolinium-based contrast agent (Gd-BOPTA; Multihance, Bracco, Italy; 0,1 mmol/Kg; flow 2 ml/s) on scans acquired in arterial (A) and portal (B) phases the lesion reveal minimal enhancement. The pathological tissue shows progressive enhancement 5 (C) and 15 (D) minutes after i.v. injection (red arrows).
Figure 5.
59 years-old male Caucasian patient with extramedullary haematopoiesis. Magnetic resonance imaging of the upper abdomen (Symphony Siemens 1.5 T, Erlangen). Axial T2-weighted images TSE fat sat (TE: 84 ms, TR: 111060 ms; slice thickness: 5.0 mm ): the usual hyperintense T2-w gallbladder signal was fully replaced by slightly high signal of pathological tissue (black arrows).
DISCUSSION
EMH is the production of blood elements outside the bone marrow cavity. EMH generally occurs in patients with deficient bone marrow haematopoiesis secondary to either peripheral red cell destruction or marrow replacement and it derives from the escape of progenitor cells from marrow and lodgement in other organs [1]. EMH is most commonly seen in the reticuloendothelial system (liver, spleen and lymph nodes), but rarely occurs in any other organ of the body. Most often, it is microscopic and asymptomatic, but it can sometimes manifest with enlarged organs and tumour-like masses.
In patients with chronic myelofibrosis homogeneous hepatomegaly is commonly found (25% at initial diagnosis and 39% at follow-up) [2], but patterns of focal myeloid metaplasia of the liver have been described in a few case reports [3]. To our knowledge, no cases of peribiliary metaplasia have been reported.
Only 14 cases of intrahepatic EMH correlated with CT and/or MRI findings, have been reported in the literature [3–15]; table 1 summarizes the imaging features in these patients.
Table 1.
Summary table of extramedullary haematopoiesis (EMH)
| Etiology | Clonal stem-cell disorder (clonal myeloproliferation) accompanied by reactive myelofibrosis (bone marrow fibrosis) and by extramedullary haematopoiesis in the spleen or in multiple organs. |
| Incidence | Between 0.5 and 1.5 per 100,000 population (myeloid metaplasia); only 5% develop extramedullary haematopoiesis |
| Gender ratio | No predilection |
| Age predilection | Approximately 65 years |
| Risk factors | Thalassemia |
| Treatment | Irradiation of the EMH site, splenectomy, Autologous Stem-Cell Transplantation (palliative procedure) |
| Prognosis | Marked variation in survival, depending on the presence or absence of well-defined indicators of an adverse prognosis (advanced age, anemia, presence of hypercatabolic symptoms, leukocytosis, leukopenia, circulating blasts, increased numbers of granulocyte precursors, thrombocytopenia, abnormalities in the karyotype). |
On unenhanced CT, these liver masses have been described as hypodense lesions. Contrast-enhanced CT showed patchy and heterogeneous enhancement in three [4,9,10] out of eight cases reported, no enhancement in three [11,12,15], homogeneous enhancement in one [14] and intense enhancement in one [13]. There is a lack of knowledge on the MR features of intrahepatic EMH; only 5 cases have been described. In the first case the lesion was isointense on T1-w and slightly hyperintense to liver on T2-w, showing heterogeneous enhancement after gadolinium administration [3]. Kumar et al reported a patient with thalassemia and hepatic iron overload who had a solitary intrahepatic EMH lesion that was isointense to liver and hypointense to muscle on both T1-w and T2-w images [5]. In the third report the mass was hyperintense to liver and isointense to the muscle on T1-w scans and was heterogeneously hyperintense in T2-w scans [9]. Lee et al reported one mass that was hypointense on T1-w and hyperintense on T2-w, showing homogeneous and intense enhancement on the arterial phase without signal drop [13]. In the last report, intrahepatic EMH was isointense to the muscle but hyperintense to the liver on T1-w because of hepatic iron overload [14].
In the current case, the mass was hypodense on CT plain acquisition and revealed a minimal heterogeneous and delayed enhancement. On MR images lesion was hypointense on T1-w and hyperintense on T2-w, showing gradual and delayed enhancement after gadolinium administration, according to the presence of fibrotic tissue within the lesion. The peculiar characteristic was the distribution of lesion, because the pathological tissue was spread around the gallbladder and biliary tree, until up to the smaller ramification of the bile ducts. Even the normal hyperintense T2-w signal of the lumen of the gallbladder was fully replaced by slightly high signal intensity of pathological tissue.
The main differential diagnosis was intrahepatic cholangiocarcinoma, characterized by growth along a dilated or narrowed bile duct and by a gradual centripetal enhancement after injection of iodinate and gadolinium contrast [16].
Differential possibility also included sclerosing cholangitis (which would show multifocal strictures, segmental ectasias, ductal wall thickening, and irregular beading of the intra- and extrahepatic bile ducts) [17] [Table 2 of differential diagnosis].
Table 2.
Differential diagnosis table of extramedullary haematopoiesis (EMH)
| US | CT | MRI | Contrast enhancement | |
|---|---|---|---|---|
| EMH | Hypoechogenic tissue | Hypodense tissue | Hypointense on T1-w; hyperintense on T2-w | Negative (fibrosis); heterogeneous enhancement (active tissue) |
| Cholangiocarcinoma and Klatskin tumor | Biliary tree dilatation with heterogeneous hyperechoic mass | Hypodense tissue | Iso/hypointense on T1-w; hypointense on T2-w | Late enhancement |
| Primary sclerosis cholangitis | Thickened and hyperechoic ductal wall | Biliary tree dilatation with thickened ductal wall | Hypointense on T1-w; hyperintense on T2 | Mural enhancement of bile ducts |
| Biliary tree dilatation | Hypoechogenic tissue | Hypo-attenuating tissue | Hypointense on T1-w; hyperintense on T2-w | None |
In conclusion, sonographic and CT appearance of EMH lesions is usually heterogeneous respectively hypoechoic and hypodense. On the other hand, MR findings are variable and influenced by liver iron deposition, cellular or fibrous or fatty components of haematopoietic tissue. MR features of EMH localizations, in particular contrastographic appearances, could be related to haematopoietic tissue percentage within the lesion and degree of tissue’s activity. Active lesions show intermediate signal intensity on T1-w, mild high signal intensity on T2-w, and variable enhancement after intravenous contrast injection, because they are formed by immature and mature cells consisting mainly of erythroid and myeloid cells. Older lesions may show low signal intensity on T1-w and T2-w, due to iron deposition or high signal intensity on both sequences due to fatty infiltration; older lesions with fatty infiltration or iron deposition may not show any enhancement, whereas fibrotic lesions may show later and gradual enhancement [18]. As liver involvement can lead to hepatic insufficiency, recognition of different sites of EMH and degree of their activity is important for a prompt diagnosis and a suitable treatment. Unfortunately no imaging pattern is pathognomonic, and a biopsy is often mandatory to establish a definitive diagnosis.
TEACHING POINT
Extramedullary haematopoiesis spreading the intrahepatic biliary tree is an unusual finding and in the majority of cases magnetic resonance imaging is useful in the differential diagnosis. The final diagnosis of extramedullary haematopoiesis must be confirmed by biopsy of the lesions.
Table 3.
Imaging features of reported case of hepatic EMH
| Study | Solitary / Multiple | Ultrasound | Non-enhanced CT | Enhanced CT | MRI T1 WI | MRI T2 WI | Enhanced MRI (gadolinium) |
|---|---|---|---|---|---|---|---|
| Warshaueret al.[3] | Multiple | Hypoechoic | Hypodense | N/A | Isointense | Hyperintense | Heterogeneous enhancement |
| Dewaret al.[4] | Solitary | Heterogeneous, hypoechoic | Heterogeneous, hypodense | Patchy enhancem ent | N/A | N/A | N/A |
| Kumaret al.[5] | Solitary | N/A | Hyperdense | N/A | Hypointense to M | Hypointense to M | N/A |
| Kopekyet al.[6] | N/A | N/A | Hypodense | N/A | N/A | N/A | N/A |
| Wieneret al.[7] | Solitary | Heterogeneous, solid | Hypodense | N/A | N/A | N/A | N/A |
| Kobayashiet al.[8] | Multiple | Hypoechoic | Hypodense | N/A | N/A | N/A | N/A |
| Wonget al.[9] | Solitary | Hypoechoic | Heterogeneous, hypodense | Heterogen eous enhancem ent | Hyperintense to L | Heterogeneous | Heterogeneous enhancement |
| Kwacet al.[10] | Solitary | Heterogeneous, hypoechoic | Hypodense | Heterogen eous enhancem ent | N/A | N/A | N/A |
| Navarroet al.[11] | Solitary | Hyperechoic | Hypodense | No enhancem ent | N/A | N/A | N/A |
| Guptaet al.[12] | Multiple | N/A | Hypodense | No enhancem ent | N/A | N/A | N/A |
| Leeet al.[13] | Multiple | N/A | Hypodense | Intense enhancem ent | Hypointense | Hyperintense | Homogeneous enhancement |
| Jelaliet al.[14] | Multiple | Not revealed | Hypodense | Homogen eous enhancem ent | Hyperintense to M | Hyperintense to M | Moderate enhancement |
| Aytaçet al.[15] | Solitary | Heterogeneous | Hypodense | No enhancem ent | N/A | N/A | N/A |
| Our case | Solitary | Hypoechoic | Hypodense | Minimal and delayed enhancem ent | Hypointense | Hyperintense | Delayed enhancement |
Legend: N/A= not performed or evaluated; T1 w= T1 weighted image; T2 w= T2 weighted image; M = Muscle; L= liver.
ABBREVIATIONS
- CT
computed tomography
- US
ultrasound
- EMH
extramedullary haematopoiesis
- MR
magnetic resonance
- -w
-weighted
REFERENCES
- 1.Lichtman M, Beutler E, Kaushansky K, et al. Williams Hematology. 7th edn. New York: McGraw-Hill; 2005. [Google Scholar]
- 2.Siniluoto TM, Hyvärinen SA, Päivänsalo MJ, Alavaikko MJ, Suramo IJ. Abdominal ultrasonography in myelofibrosis. Acta Radiol. 1992;33:343–346. [PubMed] [Google Scholar]
- 3.Warshauer DM, Schiebler ML. Intrahepatic extramedullary hematopoiesis: MR, CT and sonographic appearance. J Comput Assist Tomogr. 1991;15:683–685. [PubMed] [Google Scholar]
- 4.Dewar G, Leung NW, Ng HK, Bradley M, Li AK. Massive, solitary, intrahepatic, extramedullary hematopoietic tumor in thalassemia. Surgery. 1990 Jun;107(6):704–7. [PubMed] [Google Scholar]
- 5.Kumar A, Aggarwal S, de Tilly LN. Thalassemia major with extramedullary hematopoiesis in the liver. Semin Roentgenol. 1995;30(2):99–101. [PubMed] [Google Scholar]
- 6.Kopecky KK, Moriarty AT, Antony AC, Baker MK. Extramedullary hematopoiesis in acute lymphocytic leukemia masquerading as hepatic, renal, and splenic microabscesses. AJR Am J Roentgenol. 1986 Oct;147(4):846–7. doi: 10.2214/ajr.147.4.846. [DOI] [PubMed] [Google Scholar]
- 7.Wiener MD, Halvorsen RA, Jr, Vollmer RT, Foster WL, Roberts L., Jr Focal intrahepatic extramedullary hematopoiesis mimicking neoplasm. AJR Am J Roentgenol. 1987 Dec;149(6):1171–2. doi: 10.2214/ajr.149.6.1171. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi A, Sugihara M, Kurosaki M, Ishida Y, Takayanagi N, Matsui O, Takashima T. CT characteristics of intrahepatic, periportal, extramedullary hematopoiesis. J Comput Assist Tomogr. 1989 Mar-Apr;13(2):354–6. doi: 10.1097/00004728-198903000-00037. [DOI] [PubMed] [Google Scholar]
- 9.Wong Y, Chen F, Tai KS, et al. Imaging features of focal intrahepatic extramedullary haematopoiesis. Br J Radiol. 1999;72(861):906–910. doi: 10.1259/bjr.72.861.10645201. [DOI] [PubMed] [Google Scholar]
- 10.Kwak HS, Lee JM. CT findings of extramedullary hematopoiesis in the thorax, liver and kidneys, in a patient with idiopathic myelofibrosis. J Korean Med Sci. 2000;15:460–462. doi: 10.3346/jkms.2000.15.4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Navarro M, Crespo C, Perez L, Martinez C, Galant J, Gonzalez I. Massive intrahepatic extramedullary hematopoiesis in myelofibrosis. Abdom Imaging. 2000;25:184–186. doi: 10.1007/s002619910041. [DOI] [PubMed] [Google Scholar]
- 12.Gupta P, Naran A, Auh YH, Chung JS. Focal intrahepatic extramedullary hematopoiesis presenting as fatty lesions. AJR Am J Roentgenol. 2004;182:1031–1032. doi: 10.2214/ajr.182.4.1821031. [DOI] [PubMed] [Google Scholar]
- 13.Lee IJ, Kim SH, Kim DS, Lee JM, Han JK, Choi BI. Intrahepatic extramedullary hematopoiesis mimicking a hypervascular hepatic neoplasm on dynamic- and SPIO- enhanced MRI. Korean J Radiol. 2008;9(Suppl):S34–S38. doi: 10.3348/kjr.2008.9.s.s34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jelali MA, Luciani A, Kobeiter H, et al. MRI features of intrahepatic extramedullary haematopoiesis in sickle cell anaemia. Cancer Imaging. 2006;6:182–185. doi: 10.1102/1470-7330.2006.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aytaç S, Fitoz S, Akyar S, Atasoy C, Erekul S. Focal intrahepatic extramedullary hematopoiesis: color Doppler US and CT findings. Abdom Imaging. 1999 Jul-Aug;24(4):366–8. doi: 10.1007/s002619900515. [DOI] [PubMed] [Google Scholar]
- 16.Chung YE, Kim MJ, Park YN, Choi JY, Pyo JY, Kim YC, Cho HJ, Kim KA, Choi SY. Varying appearances of cholangiocarcinoma: radiologic-pathologic correlation. Radiographics. 2009 May-Jun;29(3):683–700. doi: 10.1148/rg.293085729. [DOI] [PubMed] [Google Scholar]
- 17.Menias CO, Surabhi VR, Prasad SR, Wang HL, Narra VR, Chintapalli KN. Mimics of cholangiocarcinoma: spectrum of disease. Radiographics. 2008 Jul-Aug;28(4):1115–29. doi: 10.1148/rg.284075148. [DOI] [PubMed] [Google Scholar]
- 18.Tsitouridis J, Stamos S, Hassapo-poulou E, et al. Extramedullary paraspinal hematopoiesis in thalassemia: CT and MRI evaluation. Eur J Radiol. 1999;30:33–38. doi: 10.1016/s0720-048x(98)00101-6. [DOI] [PubMed] [Google Scholar]