Abstract
Endometriosis of the liver is an uncommon disease characterized by the presence of endometrial tissue in the liver. There are no pathognomonic radiological features for hepatic endometriosis and preoperative diagnosis is difficult by imaging. Most cases are diagnosed after surgery. We report atypical imaging features of hepatic endometriosis in a 61 year-old female that mimic metastatic disease to the liver. She was referred to our institution with a presumed diagnosis of metastatic neuroendocrine tumors to the liver. After imaging guided core biopsy and histologic and immunohistochemical analysis, the diagnosis of hepatic endometrial stromal proliferation was made. We review the literature and provide imaging features that may help in reaching the correct diagnosis of hepatic endometriosis.
Keywords: endometrioma, liver
CASE REPORT
A 61-year-old woman with a history of hysterectomy 21 years ago, right salpingo-oophorectomy 14 years ago and bowel loop resection due to obstruction 9 years ago from endometriosis presented with the chief complaint of epigastric pain especially after eating. The pain lasted for several days then resolved spontaneously. She underwent a CT scan that showed multiple, irregularly shaped, heterogeneous, low density lesions scattered throughout the liver (Figure 1). These lesions demonstrated mild enhancement during the arterial phase without significant washout. Additional imaging findings include pelvic and mesenteric masses (Figure 2) and superior mesenteric vein (SMV) thrombosis (Figure 3).
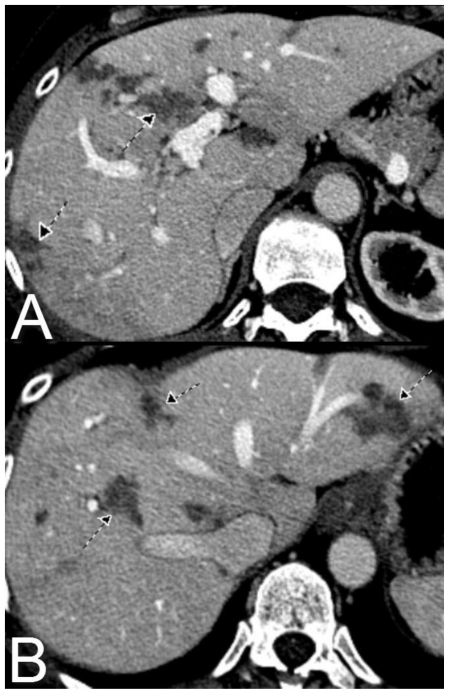
Figure 1.
61 year old Female with hepatic endometriosis. Axial contrast enhanced (IV and oral) CT of the abdomen during the portal-venous phase. There are multiple irregular poorly enhancing low attenuation areas in multiple segments of the liver (arrows). [Technique: CT (GE 16 slice scanner), KVp = 140; mA = 343; Slice Thickness = 2.5 mm; Dose of intravenous contrast: ioversol 350, 150 ml].
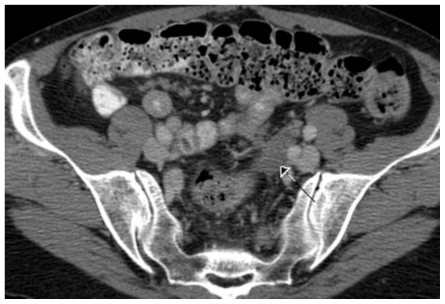
Figure 2.
61 year old Female with hepatic endometriosis. Axial contrast enhanced (IV and oral) CT of the pelvis during the portal-venous phase. There is a soft tissue mass lesion in the sigmoid mesocolon consistent with endometriosis (arrow). [Technique: CT (GE 16 slice scanner), KVp = 140; mA = 376; Slice Thickness = 2.5 mm; Dose of intravenous contrast: ioversol 350, 150 ml].
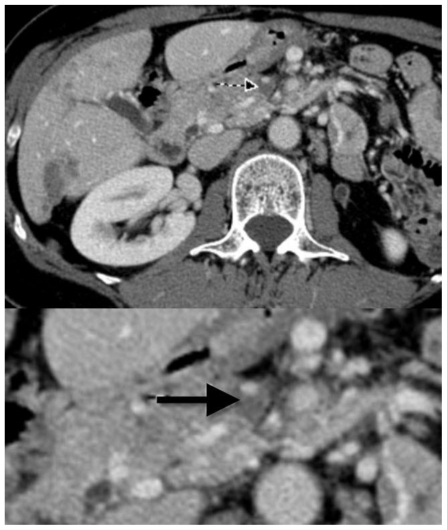
Figure 3.
61 year old Female with hepatic endometriosis. Axial contrast enhanced (IV and oral) CT of the abdomen during the portal-venous phase shows a small amount of contrast in the superior mesenteric vein, with surrounding low attenuation area represents superior mesenteric vein thrombosis (arrow). Lower image part represents magnification. [Technique: CT (GE 16 slice scanner), KVp = 140; mA = 398; Slice Thickness = 2.5 mm; Dose of intravenous contrast: ioversol 350, 150 ml].
The combination of pelvic, mesenteric and liver masses suggested the radiological diagnosis of metastatic neuroendocrine tumor.
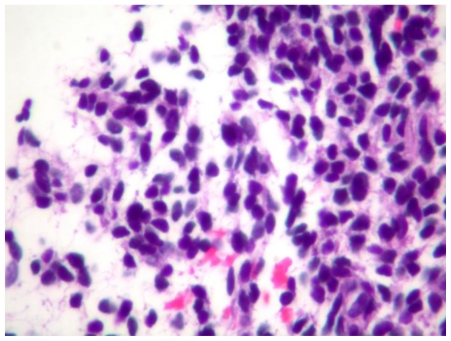
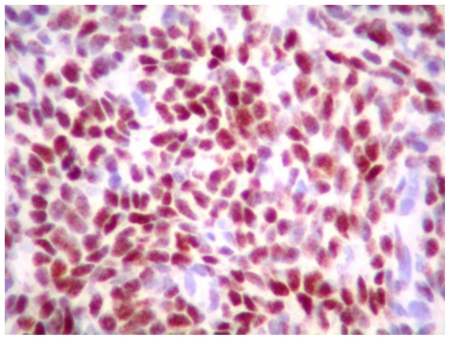
The combination of superior mesenteric vein thrombosis and irregular appearance of low attenuation areas suggested the radiological diagnosis of sub-segmental hepatic infarction. Percutaneous true-cut liver biopsy under CT guidance was performed. By gross examination the liver biopsy specimen had multiple, tan-brown, soft tissue measuring 1.3 × 0.5 × 0.5 cm in aggregate. The histological evaluation showed sheets of small, round cells with hyperchromatic nuclei and scant cytoplasm replacing hepatic parenchyma (Figures 4 and 5). The nuclei of these cells had staining for estrogen receptor and progesterone receptor (Figure 6) and there was cytoplasmic and membranous staining for CD10 by immunohistochemistry. No glandular component, mitosis or nuclear atypia was identified. The histologic and immunohistochemical features were compatible with endometrial stromal proliferation that could represent stromal endometriosis.
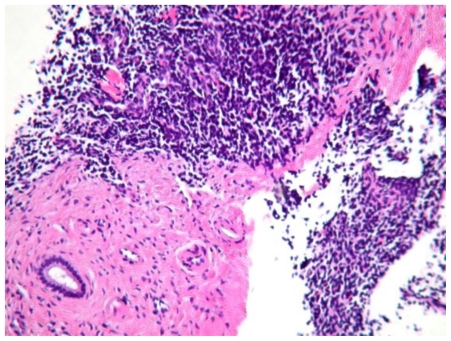
Figure 4.
61 year old Female with hepatic endometriosis. H&E stained section of the liver biopsy shows a bile duct with stromal cells and fibrosis. (Hematoxylin and eosin, intermediate power, 160x magnification)
Figure 5.
61 year old Female with hepatic endometriosis. Higher magnification of H&E stained section of the liver biopsy shows stromal cells with scant cytoplasm. (Hematoxylin and eosin, high power II, 320x magnification)
Figure 6.
61 year old Female with hepatic endometriosis. Stromal cells have nuclear staining for progesterone receptor by immunohistochemistry. (Hematoxylin and eosin, high power II, 320x magnification)
DISCUSSION
Endometriosis is a medical condition in women in which endometrial tissue is present outside the endometrium and myometrium [1]. Endometriosis usually involves ovaries, fallopian tubes, uterine serosa, small intestine, colon, and the serosal surface of the pelvic cavity, occasionally involves vagina, cervix, and bladder, and rarely involves remote sites including umbilicus, skin, diaphragm, lung, pleura, and brain [2]. Liver involvement by endometriosis is rare. The first case of hepatic endometriosis was reported by Finkel et al in 1986 [3]. To our knowledge there have only been a limited number of reported cases of hepatic endometriosis (Table 1) [1–12]. These patients range in age from 21 to 62 years of age and present with abdominal pain and may or may not have a prior history of endometriosis.
Table 1.
The imaging features of reported cases of hepatic endometriosis
| Author | Age (yrs) | Imaging features | History of endometriosis | ||
|---|---|---|---|---|---|
| US | CT | MRI | |||
| Finkel et al [3]. | 21 | NA | Large parenchymal hepatic cyst | NA | No |
| Inal et al [1]. | 25 | Well-defined mass with solid/cystic components, septations and fine nodular calcifications | Well-defined heterogeneous mass with septations and fine punctate calcifications at the periphery | Heterogeneous mass with heterogeneous enhancement after Gd injection | Yes |
| Khan et al [4]. | 31 | NA | large non-enhancing lobulated mass | NA | Yes |
| Jeanes et al [5]. | 31 | NA | Lobulated low density poorly enhancing hepatic mass lesion | NA | yes |
| Cravello et al [6]. | 34 | a 6-cm diameter mass | NA | NA | Yes |
| Verbeke et al [7]. | 34 | NA | Hepatic cystic lesion | Hepatic cystic lesion | No |
| Rovati et al [8]. | 37 | Cystic with low-level echoes and septation | Hepatic cystic lesion | NA | No |
| Chung et al [9]. | 40 | Multiseptated cyst | Septated hepatic cystic lesion | NA | Yes |
| Goldsmith et al [10]. | 48 | NA | Cystic mass with septations and irregular nodularites of the wall | high signals on TI and T2 (suggesting presence of blood) | Yes |
| Huang et al [11]. | 56 | cystic mass with irregular soft tissue | Well defined low density lobulated mass | cystic mass with irregular soft tissue | Yes |
| Khan et al [4]. | 61 | NA | Large low attenuating heterogeneous mass | NA | Yes |
| Verbeke et al [7]. | 62 | Cystic mass | NA | NA | No |
| The current case | 61 | NA | Multiple low densities lobulated lesions scattering throughout the liver | NA | Yes |
The pathogenesis of hepatic endometriosis is unknown and many theories tried to explain the extrapelvic endometriosis including retrograde menstruation, coelomic metaplasia, transplantation and blood/lymphatic dissemination [11]. The blood/lymphatic dissemination is the presumed pathway for intraparenchymal hepatic lesions and so commonly accepted [10].
Hepatic endometriosis may be seen in postmenopausal females having a strong history of pelvic endometriosis due to continuous exposure to estrogens in the post-menopausal period this may be due to either exogenous ingestion of hormones or by converting the circulating androstenedione to estrone, which is further converted to estradiol in extra-glandular tissues such as the adipose tissue and skin. Endometriosis is more common in overweight females [13].
The imaging features of hepatic endometriosis is variable and depend on the extent, age and the degree of its response to the normal hormonal fluctuations of the menstrual cycle [1,10]. We looked at the previous reported cases and concluded that there is no magnetic resonance imaging (MRI), computed tomography (CT), or ultrasound (US) characteristics exclusively specific to hepatic endometriosis, but the most common imaging features of hepatic endometriosis as seen in these reported cases are well defined lobulated cystic lesions with solid components and septations.
The clinical presentation of abdominal pain in a postmenopausal female with imaging findings of masses in the mesentery, pelvis and liver may suggest, as in this case, a diagnosis of multifocal metastatic disease. However, the lack of a primary tumor and the strong history of endometriosis, hepatic endometriosis should be considered as a part of the differential diagnosis.
In conclusion, the presence of either cystic lesions or irregular, lobulated low attenuation hepatic lesions in a female with a strong past history of pelvic endometriosis, the diagnosis of hepatic endometriosis should be suspected and histological confirmation is required.
TEACHING POINT
The detection of solid liver lesions in a patient without primary tumor should alert to a broader differential diagnosis. The clinical history will be essential, such as in this case of endometriosis, to raise the possibility of atypical diagnosis. The imaging features may overlap other common diagnosis. Thus, the suspicion of atypical diagnosis will require tissue sampling for confirmation.
Table 2.
Summary table of hepatic endometriosis
| Etiology | Unknown |
| Incidence | Extremely rare |
| Gender ratio | Females only |
| Age predilection | Range from 3rd to 6th decades of life |
| Risk factors | Strong history of pelvic endometriosis. |
| Treatment | Surgical excision – hormonal treatment (Danazol and Gonadotrophin analogues) |
| Prognosis | Good |
| Findings on Imaging | Multiple, poorly enhancing, irregularly shaped, heterogeneous, low density lesions scattered throughout the liver |
Table 3.
Differential diagnosis table of hepatic endometriosis
| Disease | US findings | CT findings | MRI findings |
|---|---|---|---|
| Hepatic endometriosis | Most of the reported cases show well defined lobulated cystic lesions with solid components and septations, but can be variable. | Most of the reported cases show Lobulated low density or cystic poorly enhancing hepatic mass lesion, but can be variable | Usually demonstrate signal intensity similar to that of normal endometrium on T1- and T2-weighted images. However, because endometrial implants can exhibit various degrees of hemorrhage due to hormonal stimulation, implants may demonstrate a spectrum of appearances depending on the age of the hemorrhage, but can be variable |
| Hepatic metastases | Homogeneous and of increased or decreased echogenicity. may have a surrounding hypoechoic rim giving a target appearance | The majority of metastases is of low attenuation on unenhanced images and show late enhancement. Hypervascular tumors are often visible as low attenuation lesions on unenhanced images and enhance transiently in the arterial phase, some becoming invisible in the portal phase | Majority of metastases are of low signal on T1w and high signal on T2w images, with the same pattern of contrast enhancement as seen on CT |
| Hepatic epithelioid hemangioendothelioma | The tumors appear solid and of low echogenicity | The lesions are visible as multiple peripheral heterogeneous areas of low attenuation with mild enhancement in post contrast study. | Lesions are hypointense on T1w images, and moderately hyperintense on T2w images with mild enhancement in post contrast study. |
ABBREVIATIONS
- SMV
superior mesenteric vein
- MRI
Magnetic Resonance Imaging
- US
Ultrasound
- CT
Computed Tomography
- H&E
hematoxylin and eosin
- NA
not available
- IV
intravenous
REFERENCES
- 1.Inal M, Bicakci K, Soyupak S, et al. Hepatic endometrioma: a case report and review of the literature. Eur Radiol. 2000;10(3):431–434. doi: 10.1007/s003300050070. [DOI] [PubMed] [Google Scholar]
- 2.Jelovsek JE, Winans C, Brainard J, Falcone T. Endometriosis of the liver containing mullerian adenosarcoma: case report. Am J Obstet Gynecol. 2004 Nov;191(5):1725–1727. doi: 10.1016/j.ajog.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 3.Finkel L, Marchevsky A, Cohen B. Endometrial cyst of the liver. Am J Gastroenterol. 1986 Jul;81(7):576–578. [PubMed] [Google Scholar]
- 4.Khan A, Craig M, Jarmulowicz M, Davidson B. Liver tumours due to endometriosis and endometrial stromal sarcoma. HPB (Oxford) 2002;4(1):43–45. doi: 10.1080/136518202753598735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeanes AC, Murray D, Davidson B, Hamilton M, Watkinson AF. Case report: hepatic and retro-peritoneal endometriosis presenting as obstructive jaundice with ascites: a case report and review of the literature. Clin Radiol. 2002 Mar;57(3):226–229. doi: 10.1053/crad.2001.0667. [DOI] [PubMed] [Google Scholar]
- 6.Cravello L, D'Ercole C, Le Treut YP, Blanc B. Hepatic endometriosis: a case report. Fertil Steril. 1996 Oct;66(4):657–659. doi: 10.1016/s0015-0282(16)58585-9. [DOI] [PubMed] [Google Scholar]
- 7.Verbeke C, Harle M, Sturm J. Cystic endometriosis of the upper abdominal organs. Report on three cases and review of the literature. Pathol Res Pract. 1996 Mar;192(3):300–304. doi: 10.1016/S0344-0338(96)80235-4. discussion 305. [DOI] [PubMed] [Google Scholar]
- 8.Rovati V, Faleschini E, Vercellini P, Nervetti G, Tagliabue G, Benzi G. Endometrioma of the liver. Am J Obstet Gynecol. 1990 Nov;163(5 Pt 1):1490–1492. doi: 10.1016/0002-9378(90)90611-a. [DOI] [PubMed] [Google Scholar]
- 9.Chung CC, Liew CT, Hewitt PM, Leung KL, Lau WY. Endometriosis of the liver. Surgery. 1998 Jan;123(1):106–108. [PubMed] [Google Scholar]
- 10.Goldsmith PJ, Ahmad N, Dasgupta D, Campbell J, Guthrie JA, Lodge JP. Case hepatic endometriosis: a continuing diagnostic dilemma. HPB Surg. 2009;2009:407206. doi: 10.1155/2009/407206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang WT, Chen WJ, Chen CL, Cheng YF, Wang JH, Eng HL. Endometrial cyst of the liver: a case report and review of the literature. J Clin Pathol. 2002 Sep;55(9):715–717. doi: 10.1136/jcp.55.9.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.N'Senda P, Wendum D, Balladur P, Dahan H, Tubiana JM, Arrive L. Adenosarcoma arising in hepatic endometriosis. Eur Radiol. 2000;10(8):1287–1289. doi: 10.1007/s003300000322. [DOI] [PubMed] [Google Scholar]
- 13.Magtibay PM, Heppell J, Leslie KO. Endometriosis-associated invasive adenocarcinoma involving the rectum in a postmenopausal female: report of a case. Dis Colon Rectum. 2001 Oct;44(10):1530–1533. doi: 10.1007/BF02234612. [DOI] [PubMed] [Google Scholar]