Abstract
Peripheral primary neuroectodermal tumors (PNET) are uncommon tumors, with an overall incidence of 1% of all sarcomas. Renal PNET is extremely rare. It is an aggressive tumor, with a high incidence of local recurrence and early metastases. Radiological diagnosis can be challenging as it is often difficult to differentiate it from other primary renal neoplasms. Imaging features include a large heterogeneous mass with central low density areas due to necrosis. Intra tumor hemorrhage and calcification are rare. We present a rare case of a large PNET mimicking renal cell carcinoma in a 51 year old female that required arterial embolization and nephrectomy.
Keywords: Renal cancer, Primitive Neuroectodermal Tumor
CASE REPORT
A 51-year-old female presented with gross hematuria on and off for 2 months. Clinical and US examination (Fig. 1) revealed a very large left renal mass, measuring 10 cm transverse, 11.4 cm anterior to posterior, and 19 cm superior to inferior, with possible thrombosis of the proximal left renal vein. Renal vein extension of the hypervascular mass was demonstrated on a contrast enhanced CT (Fig. 2).
Figure 1.
51-year-old female with left renal neuroectodermal tumor. Ultrasound, 4 Mhz curvilinear probe. a) There is a large, reniform mass replacing the left kidney. The left renal mass measures approximately 19 cm in length, and shows heterogenous echotexture. B) Color Doppler overlay shows diffuse increased vascularity to the mass. C) Images at the interpolar region shows a beak of tissue extending medially (arrow). This corresponds to left renal vein extension.
Figure 2.
51-year-old female with left renal neuroectodermal tumor. MDCT, mAs 414, kVp 120, Slice Thickness 5mm, 100 cc of IV Iohexol for contrast enhancement. Unenhanced and contrast enhanced CT of the abdomen acquired during the cortical and excretory phases, at 2 levels. Images at the level of the left renal vein (a-c) and through the lower aspect of the tumor (d-f). There is a large, lobulated mass involving the upper and inter-polar region of the left kidney, which shows hypervascular enhancement. The mass does not extend beyond the perirenal fascia. There is extension of the mass into the left renal vein, but does not cross the midline. This thrombus enhances, consistent with tumor thrombus (arrow). There is no involvement of the IVC. Coronal reformatted image (g) shows the extent of the lobulated mass arising of the upper and interpolar regions of the left kidney to better advantage.
Considering the large size of the mass, arterial embolization prior to surgical resection of the mass was recommended, to reduce the blood flow, and hence decreases blood loss at surgery. Angiography prior to arterial embolization (Fig. 3) demonstrated a large, highly vascular left renal mass with neovascularity. Using super selective techniques, embolization of the left renal artery was performed by injection of 200–300 micron polyvinyl alcohol into supplying vessels (Fig. 4).
Figure 3.
51-year-old female with left renal neuroectodermal tumor. Visceral angiogram performed via a right common femoral artery approach, and using a 5FR sheath, a 5Fr Simmons 1 catheter was used to select and inject the left main renal artery. The left kidney is enlarged and is nearly completely replaced by a hypervascular mass that displays tumor vascularity. Contrast injection shows an upper and lower branch renal artery supplying the tumor.
Figure 4.
51-year-old female with left renal neuroectodermal tumor. Selective catheterization of each of the upper (a) and lower (b) feeder vessels was performed using a High Flow Renegade catheter. The catheter was advanced into the third order upper pole and lower pole branches. Following contrast injection, reflux embolization of the left renal artery was performed via the injection of 200–300 micron PVA through the microcatheter in each vessel. Post embolization angiogram through a Simmons 1 catheter in the left main renal artery show markedly reduced tumor vascularity (c).
Abdominal CT scan performed 2 weeks later revealed an 11.6 × 11.8 × 17.6 cm heterogeneous mass replacing the left kidney (Fig. 5–7), containing calcifications. It demonstrated heterogeneous enhancement during the arterial phase post contrast scan The mass extended into the left renal vein, with a left renal vein tumor thrombus demonstrated on the venous phase scan. There was mild fat stranding surrounding the mass, with no involvement of the adrenal gland or surrounding structures. The portal, splenic, and superior mesenteric veins were patent and there were no enlarged lymph nodes.
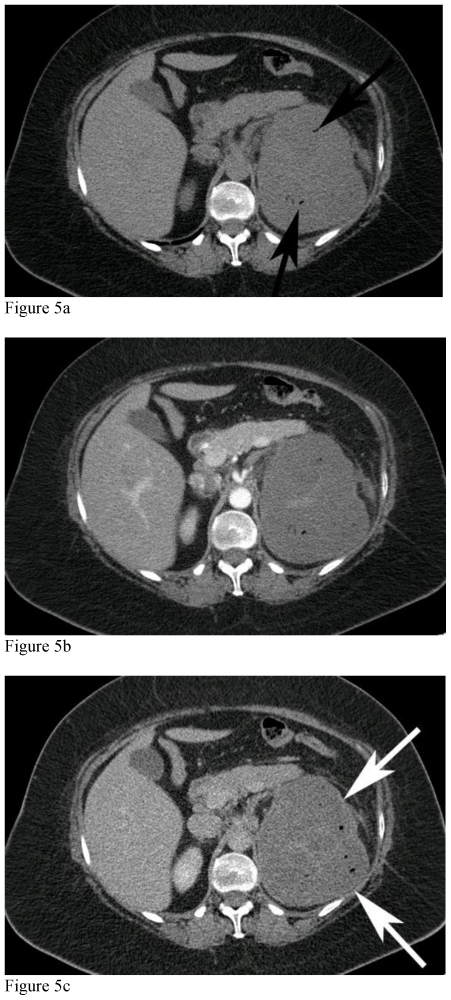
Figure 5.
51-year-old female with left renal neuroectodermal tumor following selective embolization. MDCT, mAs 376, kVp 120, Slice Thickness 3mm, 100 cc of IV Iohexol for contrast enhancement. Unenhanced and contrast enhanced CT of the abdomen acquired during the cortical phase and nephrographic phases through the upper mass. CT of the abdomen performed 2 weeks following selective embolization shows marked decreased in enhancement of the mass, with a thin, peripheral enhancing rim of the tumor from capsular vessels (white arrows), with tiny pockets of gas (black arrows) secondary to necrosis.
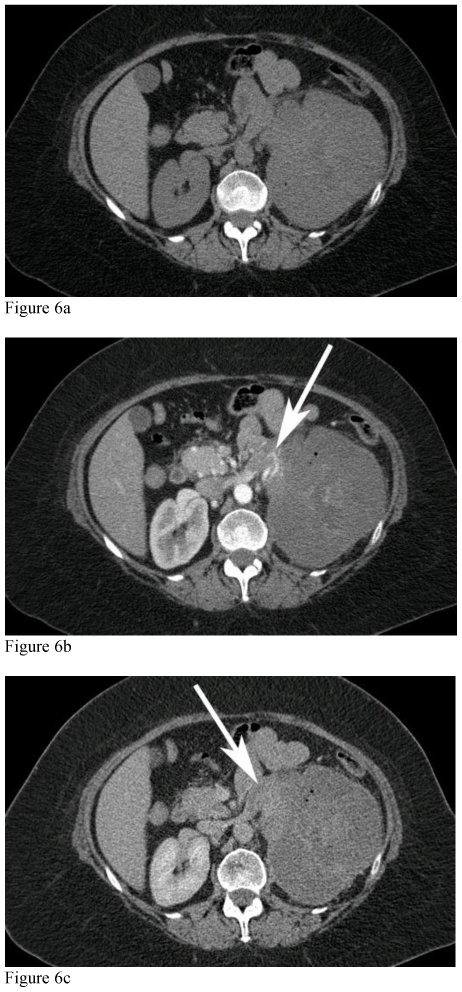
Figure 7.
51-year-old female with left renal neuroectodermal tumor following selective embolization. Unenhanced and contrast enhanced CT of the abdomen acquired during the cortical phase and nephrographic phases through the lower mass. CT of the abdomen performed 2 weeks following selective embolization shows marked decreased in enhancement of the mass, with faint peripheral capsular enhancement (white arrows) secondary to embolization and capsular feeder vascularity.
Left open radical nephrectomy, adrenalectomy, and paraaortic lymph node dissection was performed 1 week later. The left kidney with attached Gerota’s fascia, left adrenal gland and 6 para aortic lymph nodes were submitted for pathological examination.
Macroscopically, the kidney specimens showed a large lobulated tumor mass (Fig. 8), located mostly at the upper pole, but also invading the lower pole, occupying more than 85% of the kidney parenchyma by visual estimation. Microscopically, the tumor was composed of uniform cells with little cytoplasm, arranged as broad sheets, having a somewhat organized trabeculae (Fig. 9). Subtle pseudo rosette formation was identified. Tumor cells were present in the vasculature, with extension into the perihilar fat. Immunohistochemical stains were performed and stained positive for neuro-specific enolase and CD99 antigen.
Figure 8.
51-year-old female with left renal neuroectodermal tumor. Bivalved gross specimen demonstrating a heterogenous fleshy tumor mass with hemorrhage and necrosis.
Figure 9.
51-year-old female with left renal neuroectodermal tumor. a) Sheets of uniform small blue cells with geographic necrosis, (H&E 200x) b) The tumor cells have a brisk mitotic rate. An immunohistochemical stain for CD99 was positive in the tumor cells. Neuroendocrine markers and WT-1 were negative. The diagnosis was confirmed by RT-PCR demonstration of the EWSR1-FLI1 fusion transcript (H&E 1000x).
A final pathologic diagnosis of primitive neuroectodermal tumor was made, and a second opinion (outside consultation) concurred with this diagnosis.
DISCUSSION
Primitive neuroectodermal tumors (PNETs) are a rare group of small round cell tumors, which are classified into two types based on location in the body: peripheral PNET and central nervous system (CNS) PNET. Hart and Earle first introduced the term PNET in 1973 to describe undifferentiated tumors of the cerebellum that did not match the diagnostic criteria for neuroblastoma, ependymoblastoma, medulloblastoma or pineal parenchymal tumors (1).
Peripheral PNET are uncommon, with an overall incidence of 1% of all sarcomas. The most common locations of peripheral PNETs have been the thoracopulmonary region, the retroperitoneal paravertebral soft tissues, the soft tissues of the head and neck, the intra-abdominal and intra-pelvic soft tissues, and extremities. The incidence of peripheral PNET in the abdomen and pelvis, including the retroperitoneum, is about 14% of all peripheral PNETs(2).
The peak age incidence (of peripheral PNETs) is in adolescence and young adulthood, but can occur at any age, with no gender predilection (2).
Renal PNET is an aggressive tumor, with a tendency towards local recurrence and early metastases to regional lymph nodes, lungs, liver, and bone. The overall 5-year disease-free survival rate is between 45 and 55%, but in patients with metastatic disease at initial presentation, the median relapse-free survival is only 2 years (3).
Grossly, PNET of the kidney are encapsulated and contain focal areas of hemorrhage and/or necrosis. The tumor is usually sharply demarcated from the normal kidney (4). Microscopically, the tumor consists of small round cells with indistinct cell borders. The latter gives a ‘syncytial’ appearance. Some tumor cells may arrange themselves as perivascular pseudo-rosettes or Homer-Wright rosettes (3). These tumors typically express high amounts of the MIC2 antigen (CD99), and exhibit highly characteristic chromosomal translocation between chromosome 11 and 22. They stain positive with neuron-specific enolase, which made them a distinct pathologic entity and differentiated them from other small round cell tumors, such as Ewing’s sarcoma and neuroblastoma (2,5).
Radiological diagnosis of PNET of the kidney can be challenging as it is difficult to differentiate it from other primary renal neoplasms, such as Wilms’ tumor and renal cell carcinoma.
CT scans show a large and heterogeneous mass with central low density areas due to necrosis. Areas of high attenuation within the tumor, due to hemorrhage, have been observed. There have been reports of tumor calcification which may be multiple, peripheral, or linear (3) and tumor extension into the renal vein, inferior vena cava, right atrium and ventricle. Hepatic metastases, when present, manifest as low attenuation lesions (6).
MRI shows a low-signal-intensity solid mass with lobulated contours on T1-weighted images. On T2-weighted images, the tumor has heterogeneous signal intensity, separated by irregular septa, which invariably enhance after contrast administration. The presence of peripheral necrosis and hemorrhage, rather than central necrosis, which is found in most large renal tumors, as a feature of renal PNETs (7).
Considering the age of our patient, the primary diagnosis was renal cell carcinoma (RCC). RCC is the most common renal malignancy in adults and should be the first consideration in an adult presenting with hematuria and a flank mass. RCC is a vascular tumor that can attain a large size, and contain hemorrhage and necrosis. Calcification occurs in 10% of RCC and vascular invasion is a well-known feature. The differential diagnosis for hypervascular renal mass includes leiomyosarcomas, which are rare mesenchymal tumors, accounting for over 50% of renal sarcomas. Sarcomas present as large, infiltrative renal masses on imaging. This appearance is non-specific, and diagnosis depends on the cellular components. Other tumors that may present as large infiltrative renal masses include transitional cell carcinomas, lymphoma and renal metastases. Although the epicenters of transitional cell carcinomas are expected to be in the renal pelvis or upper ureter, this may be difficult to ascertain if the tumor is large. Lymphoma should be suspected when there is concomitant bulky perinephric disease, widespread lymphadenopathy or when contralateral renal involvement is seen. Metastases to kidneys present as small, multifocal, bilateral renal masses in a patient with a known primary malignancy such as lung cancer (8,9).
TEACHING POINT
Primitive neuroectodermal tumor is a rare primary tumor of the kidney. The imaging features are non specific and the definitive diagnosis is made by immunohistochemistry and electron microscopy, typically after surgical resection.
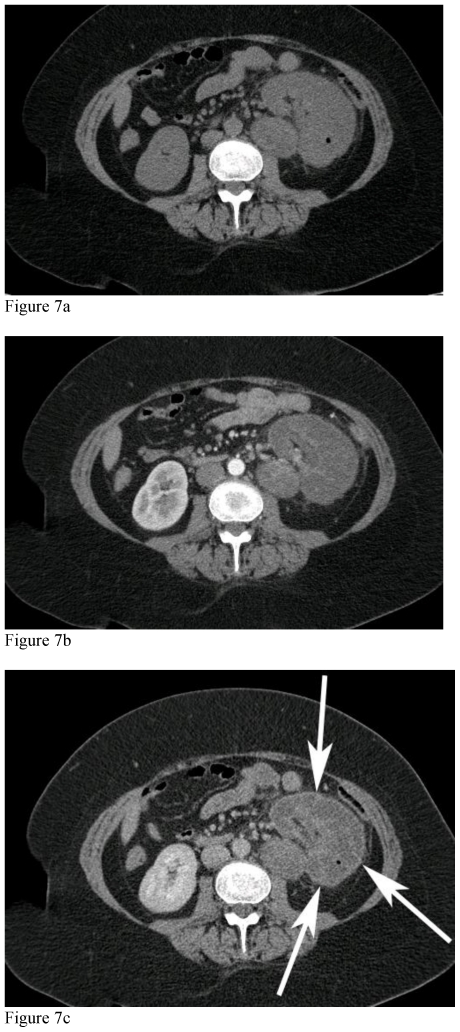
Figure 6.
51-year-old female with left renal neuroectodermal tumor following selective embolization. MDCT, mAs 376, kVp 120, Slice Thickness 3mm, 100 cc of IV Iohexol for contrast enhancement. Unenhanced and contrast enhanced CT of the abdomen acquired during the cortical phase and nephrographic phases at the level of the left renal vein. There is a thrombus within the left renal vein, which shows enhancement on the cortical phase of the study (white arrow), consistent with tumor thrombus.
Table 1.
Summary table for renal primitive neuroectodermal tumors
| Etiology | None |
| Incidence | 1% of all sarcomas |
| Gender ratio | None |
| Risk factors | None |
| Age predilection | Adolescence and young adulthood |
| Treatment | Nephrectomy and chemotherapy |
| Prognosis | The overall 5-year disease-free survival rate is 45–55% |
| Imaging findings | US: Hyperechoic with increased vascularity CT: Large and heterogeneous hypervascular mass with necrosis +/− calcification and Hemorrhage. Renal vein thrombus when mass large. MRI: Low T1W and heterogenous T2W with septation. Enhancement in arterial phase. |
Table 2.
Differential diagnosis table for renal primitive neuroectodermal tumors
| MASSES | US | CT | MRI T1W | MRI T2W |
|---|---|---|---|---|
| Renal primitive neuroectodermal tumor | Hyperechoic with increased vascularity | Large and heterogeneous mass with central necrosis. Hemorrhage and calcifications rare | Low-signal-intensity solid mass with lobulated contours with enhancement maximal in arterial phase | Heterogeneous signal intensity, separated by irregular septa |
| Renal cell carcinoma | Hypo/iso/hyper echoic | Hypodense soft tissue attenuating; presence of calcification; contrast enhancement less than renal parenchyma; | Hypointense; enhancement maximal in arterial phase | Hyperintense |
| Leiomyosarcoma/Other sarcoma | Hyperechoic | Soft tissue mass with nhancement | Hypointense with Enhancement. | Hyperintense |
| Renal metastasis | Hypo/hyperechoic | Multiple small low density and limited enhancement. Vascular mets (e.g. melanoma) rare | Hypointense with limited enhancement. Vascular mets (melanoma) rare | Hypointense |
| Lymphoma | Hypoechoic | Iso- to slightly hypointense with minimal enhancement | Hypointense |
ABBREVIATIONS
- PNET
Primitive neuroectodermal
- CT
Computed tomography
- MRI
Magnetic resonance imaging
- RCC
Renal cell carcinoma
REFERENCES
- 1.Hari S, Jain TP, Thulkar S, Bakhshi S. Imaging features of peripheral primitive neuroectodermal tumours. The British Journal of Radiology. 81:975–83. doi: 10.1259/bjr/30073320. [DOI] [PubMed] [Google Scholar]
- 2.Kim MS, Kim B, Park CS, Song SY, Lee EJ, Park NH, et al. Radiologic findings of peripheral primitive neuroectodermal tumor arising in the retroperitoneum. AJR American Journal of Roentgenology. 2006;186:1125–32. doi: 10.2214/AJR.04.1688. [DOI] [PubMed] [Google Scholar]
- 3.Chea YW, Agrawal R, Poh AC. Primitive neuroectodermal tumour of the kidney: radiologic-pathological correlations. Hong Kong Medical Journal = Xianggang Yi Xue Za Zhi/Hong Kong Academy of Medicine. 14(3):240–3. [PubMed] [Google Scholar]
- 4.Thyavihally YB, Tongaonkar HB, Gupta S, Kurkure PA, Amare P, Muckaden MA, et al. Primitive neuroectodermal tumor of the kidney: a single institute series of 16 patients. Urology. 71:292–6. doi: 10.1016/j.urology.2007.09.051. [DOI] [PubMed] [Google Scholar]
- 5.Ambros IM, Ambros PF, Strehl S, Kovar H, Gadner H, Salzer-Kuntschik M. MIC2 is a specific marker for Ewing’s sarcoma and peripheral primitive neuroectodermal tumors. Evidence for a common histogenesis of Ewing’s sarcoma and peripheral primitive neuroectodermal tumors from MIC2 expression and specific chromosome aberration. Cancer. 67:1886–93. doi: 10.1002/1097-0142(19910401)67:7<1886::aid-cncr2820670712>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 6.Ng AW, Lee PS, Howard RG. Case Report: Primitive neuroectodermal kidney tumour. Australasian Radiology. 48:211–213. doi: 10.1111/j.1440-1673.2004.01299.x. [DOI] [PubMed] [Google Scholar]
- 7.Wu CF, Wang LJ, Chen CS. Magnetic resonance imaging of primitive neuroectodermal tumor of the kidney. Urology. 55(2):284. doi: 10.1016/s0090-4295(99)00467-7. [DOI] [PubMed] [Google Scholar]
- 8.Thomas JC, Sebek BA, Krishnamurthi V. Primitive neuroectodermal tumor of the kidney with inferior vena cava and atrial tumor thrombus. The Journal of Urology. 168:1486–7. doi: 10.1016/S0022-5347(05)64481-3. [DOI] [PubMed] [Google Scholar]
- 9.Pickhardt PF, Lonergan GF, Davis CF, Kashitani N, Wagner BF. From the Archives of the AFIP Infiltrative Renal Lesions: Radiologic-Pathologic Correlation. Radiographics. 20:215–243. doi: 10.1148/radiographics.20.1.g00ja08215. [DOI] [PubMed] [Google Scholar]