Abstract
Pseudoaneurysms of the cystic artery secondary to calculus cholecystitis are rare. In this report we describe a case of an elderly female who presented with abdominal pain, pyrexia, anaemia and jaundice. She had known chronic cholecystitis, but was not considered a suitable surgical candidate. Contrast enhanced computed tomography (CECT) demonstrated a probable aneurysm within the gallbladder fossa. The patient proceeded to digital subtraction angiography (DSA), which confirmed an aneurysm arising from the cystic artery and was subsequently managed with transcatheter arterial embolisation using coils. This case report reviews the diagnosis and management of this rare complication.
Keywords: Pseudoaneurysm, cystic artery, gallbladder, haemobilia, embolisation
CASE REPORT
A 78 year old female presented to the Accident & Emergency Department having had a fall. Further questioning revealed a one-week history of generalised abdominal pain, nausea, vomiting and melena. She had a previous history of chronic cholecystitis confirmed on ultrasound (US) and computed tomography (CT) three months previously and was felt not to be an appropriate surgical candidate in view of her cardiac history and colostomy formation for a colovescical fistula secondary to diverticular disease.
On examination the patient was haemodynamically stable but febrile with a temperature of 38°C, pale with mild jaundice and had epigastric pain and tenderness. Examination of the colostomy contents revealed melaena. Laboratory investigations showed haemoglobin 8.2 g/dL (N = 11.5–16 g/dL), MCV 71.3 fl (N = 76–96 fl), white cell count 29.7 × 109/L (neutrophils 27.8 × 109/L) (N = 4–11 × 109/L, neutrophils 40–60%), CRP 61 mg/L (N < 5 mg/L) and elevated liver function tests (bilirubin 59 μmol/L (N 3–22 = μmol/L), ALP 1400 IU/L (N = 42–98 IU/L), ALT 355 IU/L (N = 5–35 IU/L). The patient was initially resuscitated with intravenous fluids, blood transfusion and treated with intravenous broad spectrum antibiotics for sepsis secondary to cholecystitis.
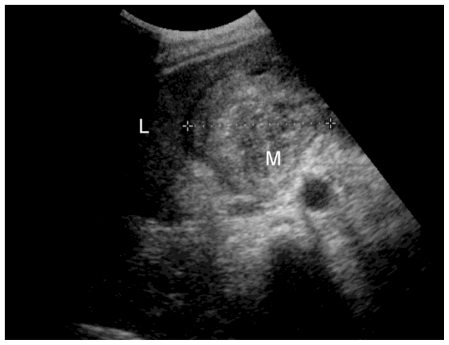
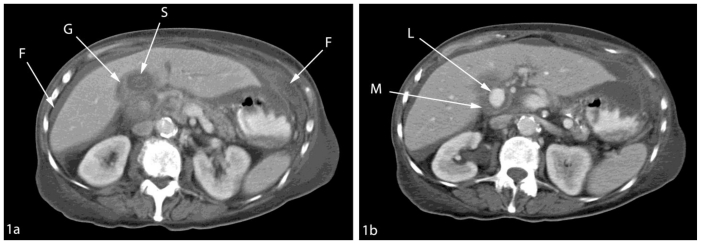
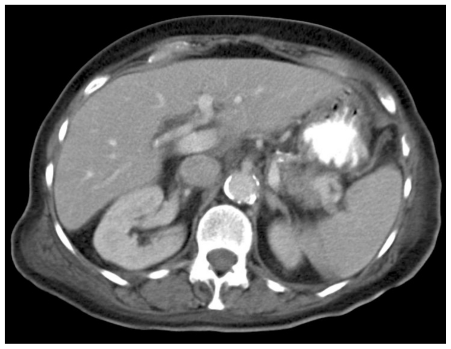
Initial investigation with upper gastrointestinal endoscopy was unremarkable. Ultrasound showed a 7 cm heterogeneous mass in the gallbladder fossa near a contracted gallbladder, which contained stones (Fig 1). There was no common bile duct or intrahepatic duct dilatation seen. Unfortunately, colour Doppler ultrasound was not performed at this stage. The patient proceeded to CECT, which revealed free fluid within the upper abdomen and a contracted thick-walled gallbladder containing stones (Fig 2a). In addition, a 7 cm soft tissue mass was seen in the gallbladder fossa with a 2.5 cm avidly enhancing nidus (Fig 2b), consistent with aneurysm formation and associated thrombus. These findings were not present on the previous CT examination (Fig 3). Urgent angiography was undertaken to confirm the diagnosis and to treat the aneurysm.
Figure 1.
78 year old female with a cystic artery pseudoaneurysm. US scan image (curvilinear probe 5-2 MHz) showing the liver (L) and a 7 cm heterogeneous mass (M) in the gallbladder fossa. The gallbladder is contracted and contains stones.
Figure 2.
78 year old female with a cystic artery pseudoaneurysm. Axial contrast enhanced CT (130 KV, 110 mAs) taken during the portal venous phase showing (a) thick walled contracted gallbladder (G) containing stone (S) with free fluid within the peritoneum (F) and (b) hyperdense cystic artery pseudoaneurysm showing lumen (L) and mural thrombus (M).
Figure 3.
78 year old female with a cystic artery pseudoaneurysm. Axial contrast enhanced CT (130 KV, 110 mAs) taken during the portal venous phase demonstrates that there was no cystic artery pseudoaneurysm present on the patient’s CT prior to admission.
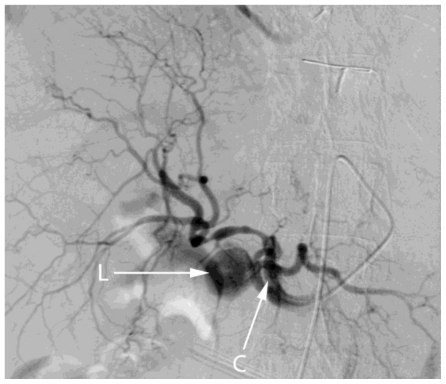
DSA was performed using a right femoral approach. Selective hepatic artery angiogram showed a pseudoaneurysm in relation to the cystic artery (Fig 4). Access to the cystic artery was attempted using a microcatheter to insert coils and embolise the aneurysm however, the remnant of the cystic artery was found to be only 2 mm and therefore too small for stable positioning of the coils.
Figure 4.
78 year old female with a cystic artery pseudoaneurysm. Digital subtraction angiogram performed via injection of the celiac axis illustrates the lumen (L) of cystic artery pseudoaneurysm. Note the tip of the catheter within the coeliac axis (C).
Access to the coeliac trunk using a sidewinder Simmons 2 (Cordis, UK) catheter was gained and a Terumo hydrophilic guidewire and 4F hydrophilic Cobra (Terumo, UK) were used to selectively catheterise the right hepatic artery prior to deployment of 6 mm 0.35 Tornado coils (Cook, UK) (Fig. 5) distal and proximal to the origin of the cystic artery. Post procedure imaging confirmed successful embolisation of the pseudoaneurysm.
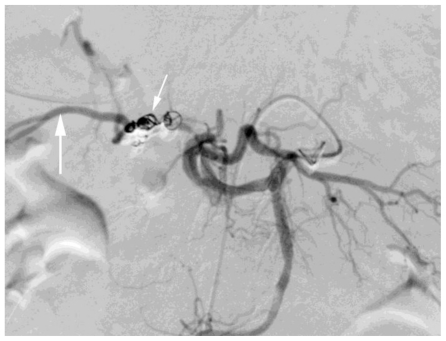
Figure 5.
78 year old female with a cystic artery pseudoaneurysm. Digital subtraction angiogram performed via celiac axis injection. Post embolisation image illustrating successful treatment of the cystic artery pseudoaneurysm with microcoils (small white arrow). Note the celiac axis (black arrow) and collateral filling of the right hepatic artery (large white arrow).
On balance, it was felt that the development of the cystic artery pseudoaneurysm in our patient was due to calculus cholecystitis and that this was unrelated to the patient’s initial presentation of a fall. The patient remained stable post embolisation, but unfortunately died a few months later from a cardiac event.
DISCUSSION
A pseudoaneurysm or false aneurysm is an outpouching of a blood vessel in which there is disruption of one or more layers of the arterial wall, rather than expansion of all of the wall layers. There are a number of causes for pseudoaneurysm formation including iatrogenic injury, trauma, infection, inflammation (e.g. visceral, arteritis etc), atherosclerosis and infarction. Damage to the adventitia and thrombosis of the vasa vasorum results in localized weakness in the vessel wall making it prone to rupture.
There are only eleven cases in the reported literature of haemobilia secondary to cystic artery pseudoaneurysm associated with cholecystitis (1). Most cases of cystic artery pseudoaneurysms are related to mechanical or thermal injury post-cholecystectomy or angiographic intervention (2). Pseudoaneurysms of the cystic artery secondary to acute or chronic cholecystitis are rare despite the high incidence of cholecystitis, although the reasons for this are unclear. It is thought that these non-traumatic pseudoaneurysms develop secondary to erosion of the cystic artery by gallstones or inflammation and may be due to early thrombosis of the cystic artery in response to inflammation (3–6).
Rupture of extrahepatic pseudoaneurysms may cause bleeding into the abdominal cavity or biliary system (7). Most haemobilia is minor and self limiting. Major bleeding can present as haematemesis or melaena with abdominal pain and obstructive jaundice (Quincke’s triad) (8, 9). Our patient had all three of these clinical signs.
Upper gastrointestinal endoscopy does not usually reveal abnormalities in the stomach and duodenum, unless there is active bleeding with haemorrhage through the ampulla of Vater. Bleeding from the papilla on side viewing endoscopy is reported in the majority (60%) of cases in the literature (1) however, it may be missed if bleeding is intermittent or directly into the duodenum.
The presence of cystic artery pseudoaneurysm may be suspected on initial US scans. Colour Doppler US is the modality of choice in suspected pseudoaneurysm as it successfully demonstrates the vascular nature of the mass and may demonstrate the ‘yin-yang’ sign that is characteristic of pseudoaneurysms and the typical ‘to and fro’ sign on Doppler waveform analysis (10, 11). However, it is often difficult to accurately localize the exact site of the pseudoaneurysm.
CECT and CT Angiography (CTA) are highly accurate methods for investigating the hepatobiliary system and can clearly delineate a vascular lesion in the upper abdomen and is a particularly useful investigation in those cases with equivocal Doppler examinations. It is minimally invasive and can be performed in an emergency, without preparation, particularly in massive gastrointestinal bleeding (12). Pseudoaneurysms on CECT are usually seen as hyperdense lesions without contrast and enhance in the arterial phase. The typical appearances suggestive of a pseudoaneurysm were demonstrated on CECT conducted on our patient. MRI is another non-invasive imaging modality, which can be used to accurately depict the presence and delineate the vascular anatomy of a pseudoaneurysm.
Selective hepatic artery angiography is the diagnostic modality of choice for confirming CECT/CTA findings in suspected pseudoaneurysms, as it enables the aneurysm to be directly visualised and allows therapeutic intervention. Understanding of the normal vascular variants and of the extensive collateral circulations in the visceral circulation is mandatory to achieve successful isolation of an aneurysm.
The objective of embolisation is to isolate the pseudoaneurysm from the circulation (thereby preventing further bleeding) by interrupting both antegrade and retrograde flow to the lesion (13). Hepatic angiography with embolisation in comparison to surgical management has a lower morbidity and mortality than surgery, with higher success rates in controlling haemorrhage and identifying the origin of bleeding (14).
Pseudoaneurysms because of their thin walls must be embolised carefully using a low pressure delivery system in order to avoid rupture. Injection of liquid polymer can be introduced under low pressure and provides instant occlusion however, there is a risk of flowing through of polymer with failure of distal occlusion as well as difficulty in handling the material. Mini balloons flow directed into the aneurysm mouth provide immediate occlusion, although the use of this technique is limited by the size of the vessel.
Gelfoam embolisation may be used for occlusion of small arterial aneurysms, however there is a risk of aneurysm wall rupture due to increased intra-aneurysm pressure during injection. Embolisation with coils is the preferred method of choice as they are available in several sizes allowing adjustment to the size of the vessel to be embolised and can be introduced without a pressure rise in the vascular lesion (15).
The mainstay of treatment of cystic artery pseudoaneurysm is cholecystectomy with ligation of the cystic artery. However, the presence of inflammation poses the risk of bleeding and injury to adjacent visceral structures. Definitive treatment of cystic artery pseudoaneurysms with percutaneous thrombin injection has been attempted, but failed after initial success (16).
It has been suggested that DSA should be used in the pre-operative planning prior to cholecystectomy and angiographic embolisation followed by elective cholecystectomy has been reported (17). Embolisation allows the surgeon to electively perform definitive surgery once the patient’s condition is stable.
In our patient, DSA confirmed the presence of a cystic pseudoaneurysm, however the remnant of the cystic artery was too small for stable positioning of the coils to be achieved and therefore selective catheterisation of the right hepatic artery was undertaken prior to coil deployment. Super-selective catheterisation of an artery and deployment of embolisation coils cause the vessel to thrombose, thereby controlling bleeding (18).
In conclusion, pseudoaneurysms of the cystic artery are rare and usually occur as the result of mechanical or thermal injury post surgery or angiographic intervention or more rarely secondary to cholecystitis. The rarity of cystic artery pseudoaneurysms, despite the high incidence of cholecystitis, may be secondary to early thrombosis of the cystic artery as a response to adjacent inflammation. Awareness of this complication allows accurate diagnostic evaluation with US, CECT, DSA as well as therapeutic intervention using less invasive angiographic techniques, particularly in those patients who are unfit for surgery. Currently, cholecystectomy with ligation of the pseudoaneurysm remains the treatment of choice.
TEACHING POINT
Cystic artery pseudoaneurysms are a rare complication of traumatic injuries, postbiliary surgery or more rarely cholecystitis. Colour Doppler US, CECT and CTA can all be used to diagnose pseudoaneurysms. In addition, DSA can be used to confirm the presence of a pseudoaneurysm and allows therapeutic intervention using less invasive angiographic techniques, particularly in those patients who are not surgical candidates.
ABBREVIATIONS
- CECT
Contrast Enhanced Computed Tomography
- DSA
Digital Subtraction Angiography
- US
Ultrasound
- CT
Computed Tomography
- CTA
Computed Tomography Angiography
REFERENCES
- 1.Saluja SS, Ray S, Gulati MS, et al. Acute cholecystitis with massive upper gastrointestinal bleed: a case report and review of the literature. BMC Gastroenterol. 2007;7:12. doi: 10.1186/1471-230X-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saldinger PF, Wang JY, Boyd C, et al. Cystic artery stump pseudoaneurysm following laparoscopic cholecystectomy. Surgery. 2002;131:585–6. doi: 10.1067/msy.2002.115353. [DOI] [PubMed] [Google Scholar]
- 3.England RE, Marsh PJ, Ashleigh R, et al. Pseudoaneurysm of the cystic artery: A rare cause of hemobilia. Clin Radiol. 1998;53:72–5. doi: 10.1016/s0009-9260(98)80041-x. [DOI] [PubMed] [Google Scholar]
- 4.Wu TC, Liu TJ, Ho YJ. Pseudoaneurysm of the cystic artery with upper gastrointestinal hemorrhage: Case report. Acta Chir Scand. 1988;154:151–2. [PubMed] [Google Scholar]
- 5.Nakajima M, Hoshino H, Hayashi E, et al. Pseudoaneurysm of the cystic artery associated with upper gastrointestinal bleeding. J Gastroenterol. 1996;31:750–4. doi: 10.1007/BF02347630. [DOI] [PubMed] [Google Scholar]
- 6.Smague EA, Schulte F, Guse S. Recurrent hemobilia caused by a ruptured pseudoaneurysm of the cystic artery in the gallbladder. Chirurg. 1990;61:199–200. [PubMed] [Google Scholar]
- 7.Shanley CJ, Shah NL, Messina LM. Common splanchnic artery aneurysms: splenic, hepatic and celiac. Ann Vasc Surg. 1996;10:315. doi: 10.1007/BF02001900. [DOI] [PubMed] [Google Scholar]
- 8.Quincke H. Ein fall von aneurysma der leberarterie. Klin Wochenschr. 1871;8:349–351. [Google Scholar]
- 9.Harlaftis SJ, Akin JT. Haemobilia from ruptured hepatic artery aneurysms. Report of a case and review of the literature. Am J Surg. 1997;133:229–232. doi: 10.1016/0002-9610(77)90087-3. [DOI] [PubMed] [Google Scholar]
- 10.Barba CA, Bret PM, Hinchey EJ. Pseudoaneurysm of the cystic artery: A rare cause of hemobilia. Can J Surg. 1994;37:64–6. [PubMed] [Google Scholar]
- 11.Miura K, Hoshino T, Komatsu M, et al. A case of hemorrhage into the gallbladder probably due to rupture of pseudoaneurysm formed by cystic artery. Nippon Shokakibyo Gakkai Zasshi. 1998;95:450–4. [PubMed] [Google Scholar]
- 12.Ernst O, Bulois P, Saint-Drenant S, et al. Helical CT in acute lower gastrointestinal bleeding. Eur Radiol. 2003;13:114–7. doi: 10.1007/s00330-002-1442-y. [DOI] [PubMed] [Google Scholar]
- 13.Shramek KS, Cooper SG, Wickremsinghe PC. Massive haemobilia resulting from gallstone erosion of the cystic artery: percutaneous transcatheter embolisation as a temporising measure - case report. J Vasc Interv Radiol. 1991;16:205–214. doi: 10.1016/s1051-0443(93)71880-1. [DOI] [PubMed] [Google Scholar]
- 14.Lygidakis NJ, Okasaki M, Damtsios G. Iatrogenic haemobilia: how to approach it. Hepatogastroenterology. 1991;38:454–457. [PubMed] [Google Scholar]
- 15.Uflacker R. Transcatheter embolisation of arterial aneurysms. Br J Radiology. 1986;59:317–324. doi: 10.1259/0007-1285-59-700-317. [DOI] [PubMed] [Google Scholar]
- 16.Ghoz A, Kheir E, Kotru A, et al. Haemoperitoneum secondary to rupture of cystic artery pseudoaneurysm. Hepatobiliary Pancreat Dis Int. 2007;6(3):321–3. [PubMed] [Google Scholar]
- 17.Maeda A, Kunou T, Saeki S, et al. Pseudoaneurysm of the cystic artery with hemobilia treated by arterial embolization and elective cholecystectomy. J Hepatobiliary Pancreat Surg. 2002;9:755–8. doi: 10.1007/s005340200105. [DOI] [PubMed] [Google Scholar]
- 18.Delgadillo X, Berney T, Perrot M, et al. Successful treatment of a pseudoaneurysm of the cystic artery with microcoil embolisation. J Vasc Interv Radiol. 1999;10(6):789–92. doi: 10.1016/s1051-0443(99)70116-8. [DOI] [PubMed] [Google Scholar]