Abstract
The purpose of this paper is to demonstrate the potential role of CT in the early diagnosis of swine-origin influenza A (H1N1) virus (S-OIV) pneumonia. We present a case of acute influenza-like illness in which the CT findings of peribronchovascular and subpleural ground-glass opacities and consolidation resembled organizing pneumonia, and lead the radiologist to prospectively and correctly suggest the diagnosis of S-OIV infection.
Keywords: Swine-origin influenza A, H1N1, Viral infection, CT, Radiography, Organizing pneumonia, Ground-glass opacity
CASE REPORT
A 53-year-old male presented to our emergency department with a four-day-long complaint of low grade fever, sweats, cough, shortness of breath and hemoptysis. His past medical history was significant for diabetes mellitus, hypertension, dyslipidemia, ischemic heart disease and a remote triple coronary artery bypass graft procedure. The patient had no known underlying chronic lung disease. Apart from low-grade fever and mild tachypnea, his vital signs were stable. He had normal oxygen saturation on room air. His physical exam was unremarkable, apart from minimal bilateral basal lung crackles on auscultation. The patient had normal white blood cell, neutrophil and platelet counts. Lymphopenia (absolute lymphocyte count, < 1.0 × 103/L) was observed. The serum lactate dehydrogenase and creatine kinase levels were normal. The patient was admitted to the hospital and initially treated with broad-spectrum antibiotics. Subsequently, his blood and sputum cultures were negative for abnormal flora.
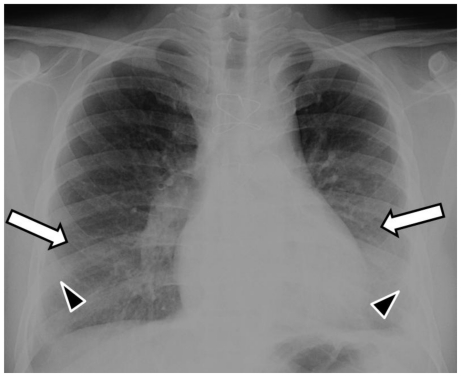
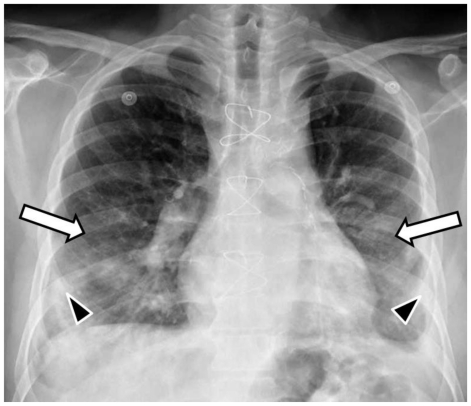
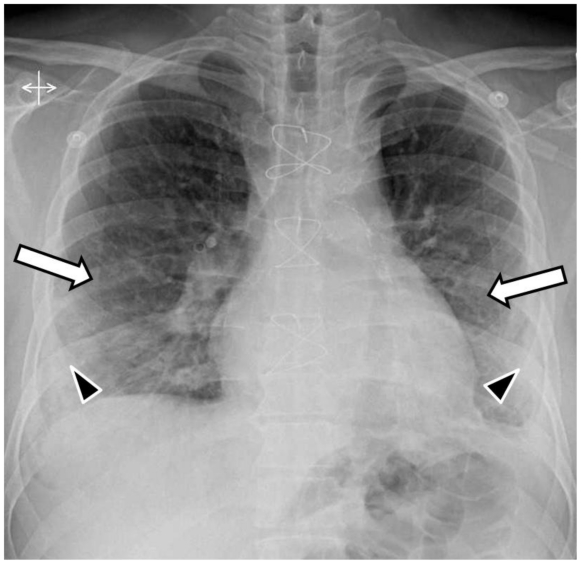
The admission posteroanterior- and lateral-projection chest radiographs demonstrated bilateral mid and lower lung zones ground-glass opacities (hazy opacities not obscuring the underlying lung vessels) and minimal superimposed patchy consolidation (dense opacities obscuring the underlying vessels) (Fig. 1). The radiographs 2 and 3 days later showed progression of the disease in the form of increasing and more confluent bilateral mid and lower lung zones consolidation (Fig. 2 and 3). Small bilateral pleural effusions were observed on all radiographs (Fig. 1, 2 and 3). These were clinically felt to be a result of resolving pulmonary edema.
Figure 1.
53-year-old male with swine-origin influenza A (H1N1) viral infection and remote coronary artery bypass graft procedure. Admission frontal (PA upright) chest radiograph shows bilateral mid and lower lung zones ground-glass opacities (white arrows) and minimal superimposed patchy consolidation (black arrowheads). Note small bilateral pleural effusions.
Figure 2.
53-year-old male with swine-origin influenza A (H1N1) viral infection and remote coronary artery bypass graft procedure. Frontal (PA upright) chest radiograph obtained 2 days after Figure 1 shows bilateral mid and lower lung zones ground glass opacities (white arrows) with increased and more confluent superimposed consolidation (black arrowheads). Note small bilateral pleural effusions.
Figure 3.
53-year-old male with swine-origin influenza A (H1N1) viral infection and remote coronary artery bypass graft procedure. Frontal (PA upright) chest radiograph obtained 3 days after Figure 1 shows bilateral mid and lower lung zones ground glass (white arrows) opacities with further increase in the superimposed consolidation (black arrowheads). Note small bilateral pleural effusions.
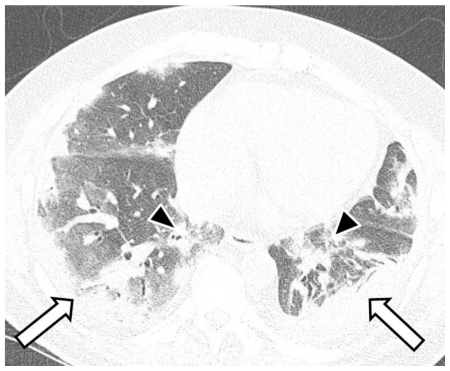
A thin-section non-enhanced helical computed tomography (CT) scan of the chest was performed on a multidetector scanner three days after the initial radiograph. The CT showed multifocal bilateral areas of ground-glass opacities and consolidation with basal predominance. The abnormalities had a predominantly peribronchovascular and subpleural distribution (Fig. 4 and 5), resembling organizing pneumonia [1]. Based on the CT findings and the patient’s acute influenza-like presentation, the radiologist strongly suggested the possibility of S-OIV and recommended rRT-PCR testing.
Figure 4.
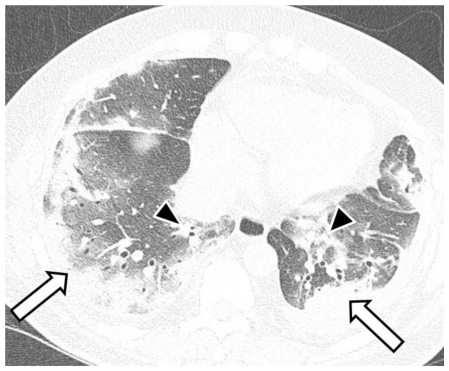
53-year-old male with swine-origin influenza A (H1N1) viral infection. Unenhanced trans-axial chest CT lung window image (64-MDCT Siemens Definition scanner, end inspiratory acquisition, 120 kV, 200 mAs, 1 mm reformation and window width/level of 1500/−700 HU) at the mid lung zone obtained 3 days after Figure 1 shows ground-glass opacities and consolidation in predominant subpleural (white arrows) and peribronchovascular (black arrowheads) distribution, resembling organizing pneumonia.
Figure 5.
53-year-old male with swine-origin influenza A (H1N1) viral infection. Unenhanced trans-axial chest CT lung window image (64-MDCT Siemens Definition scanner, end inspiratory acquisition, 120 kV, 200 mAs, 1 mm reformation and window width/level of 1500/−700 HU) at the lower lung zone obtained 3 days after Figure 1 shows ground-glass opacities and consolidation in predominant subpleural (white arrows) and peribronchovascular (black arrowheads) distribution, resembling organizing pneumonia.
The antibiotics were stopped and the patient was started empirically on a 5-day course of oral oseltamivir. A nasal swab was collected, processed and tested by real-time reverse-transcriptase-polymerase chain reaction (rRT-PCR) according to the Centers for Disease Control and Prevention guidelines [2, 3]. The results came back positive for S-OIV 48hrs later. The patient’s condition promptly improved, and he remained afebrile even after completing the antiretroviral medication course. The patient was discharged home after a 9-day-long non-complicated hospital course.
DISCUSSION
Since the documentation of its outbreak in the spring of 2009, swine-origin influenza A (H1N1) virus (S-OIV) infection has been a major global concern. As of January 31, 2010, the World Health Organization (WHO) has documented more than 15,174 S-OIV related deaths [4]. S-OIV is considered a highly contagious disease, which is transmitted from person-to-person via large respiratory droplets or contact with contaminated surfaces [5].
S-OIV infection typically manifests with fever, cough, sore throat, chills, rhinorrhea, shortness of breath, headache, fatigue, myalgias, arthralgias, and vomiting or diarrhea [5]. Most cases are mild; however, some patients may show a rapidly declining course, which mandates intubation and mechanical ventilation [6]. There is a higher risk of acquiring more severe disease in children younger than 5 years old, adults 65 years old or older, patients with chronic underlying conditions (e.g., asthma, diabetes, cardiac disease, renal disease, neurocognitive disease, and neuromuscular disorders), pregnant women, and immunosuppressed patients [5–7]. Laboratory findings in patients with S-OIV include lymphopenia, thrombocytopenia, elevated serum lactate dehydrogenase level, and increased serum creatine kinase level [6].
Nasopharyngeal swabs, nasal aspirates, endotracheal swabs and bronchoalveolar lavage are considered appropriate respiratory specimens to be tested for S-OIV [8]. Rapid influenza diagnostic tests (RIDT) and immunofluorescence assays (IFA) are widely available investigative methods with short processing time. However, these tests have variable sensitivities (10–93%) despite relatively high specificities (>95%) for detecting S-OIV, where a negative test does not necessarily exclude the disease [8]. Furthermore, RIDT and IFA do not distinguish S-OIV from other influenza types or subtypes [8]. Isolation of the virus in cultured specimens is considered a more specific way of testing; however, this test has limited availability and requires 2 to 10 days to yield a result [8]. The rRT-PCR is the most sensitive and specific test for detecting S-OIV but requires 48 to 96 hours of processing and is not widely available [8].
S-OIV can be effectively treated by oseltamivir phosphate (Tamiflu, Roche Laboratories) and zanamivir (Relenza, GlaxoSmithKline) [9]. The current recommendation is to start antiretroviral treatment empirically in persons suspected to have S-OIV infection and a severe initial presentation, rapidly deteriorating course, underlying risk factors or requiring hospitalization [9]. The earlier the antiretroviral medications are started, the better the results [9]. The use of imaging modalities in early detection of S-OIV cases becomes very important, since empiric treatment should be started as early as possible in selected S-OIV cases and diagnostic testing might be unavailable, falsely negative, nonspecific, or require lengthy processing time.
The initial chest radiograph is normal in a large proportion of S-OIV cases [10, 11]. Although the follow-up radiograph might remain normal in some patients [11], development of radiographic abnormalities is well-documented on the 1 to 4 day follow-up radiograph in severe or high-risk cases [10, 11]. On chest radiographs, S-OIV infection is more commonly bilateral than unilateral, and the lower lung zones are most commonly involved [10, 11]. Early in the course of the disease, ground-glass opacities predominate [11], with superimposed patchy consolidation often developing on follow-up radiographs and becoming the predominant finding in severe cases [10, 11].
The main CT findings of S-OIV infection are ground-glass opacities, with or without superimposed areas of consolidation [10, 11]. When compared to radiography, CT typically better demonstrates the extent and distribution of S-OIV involvement [11]. On CT, the disease is noted to be more commonly bilateral in nature [10–12]. The lower lung zones are the areas which are most often involved, but central and diffuse patterns are also seen [10–12]. Of note, is a peribronchovascular and subpleural distribution that was observed by some authors [10, 11] and emphasized by others [11], an appearance which resembles that of organizing pneumonia [11]. This pattern and distribution of findings, together with the lack of centrilobular nodular opacities or tree-in-bud pattern typically seen in bronchopneumonias, and the patient’s acute influenza-like presentation, triggered the radiologist to strongly suggest S-OIV infection based on the CT of the case presented in this report.
Mediastinal or hilar lymph node enlargement is not a described feature of S-OIV [10–12]. Pleural effusions were described in some patients [10], but do not appear to be a predominant or consistent feature [11, 12]. The presence of pleural effusions might be explained by the associated co-morbidities -such as left-sided heart failure- rather than the S-OIV infection itself [9]. Nodular opacities were also noted on the radiograph and CT in a small number of patients [10], but were not a major feature [10–12]. Centrilobular nodules, tree-in-bud pattern or mosaic perfusion was not noted on CT in any of the reported cases [10–12]. S-OIV infection is similar in that regard to severe acute respiratory syndrome (SARS) [11, 13, 14]. It is important to highlight that a recent report demonstrated increase risk of pulmonary embolism in hospitalized patients with S-OIV infection [10].
In conclusion, it is important to recognize or at least suspect early S-OIV infection in severe cases or high-risk patients. In patients currently presenting with acute influenza-like illness, presence of patchy ground-glass opacities with or without superimposed consolidation on the radiograph with a characteristic peribronchovascular and subpleural distribution on CT should suggest the diagnosis of S-OIV pneumonia. An overall summary of S-OIV (Table 1) and a guide to relevant deferential diagnosis (Table. 2) are provided below.
Table 1.
Summary table for S-OIV pneumonia
| Etiology | Swine-Origin Influenza A (H1N1) Virus. |
| Incidence | The predominant circulating influenza type nowadays, with more than 15,174 reported deaths to date. |
| Gender ratio | No gender predilection. |
| Age predilection | No age predilection. |
| Risk factors | Children younger than 5 years old, adults 65 years old or older, patients with chronic underlying conditions (e.g., asthma, diabetes, cardiac disease, renal disease, neurocognitive disease, and neuromuscular disorders), pregnant women, and immunosuppressed patients. |
| Treatment | Most cases are self limited or require supportive treatment. However, Tamiflu and Relenza are effective in severe cases. |
| Prognosis | Most cases are mild and self-limited. However, some cases especially those under risk- are prone to develop severe and sometimes fatal illness. |
| Imaging findings |
|
Table 2.
Differential diagnosis of S-OIV pneumonia from an imaging point of view
| Disease | Clinical presentation | Laboratory findings | Radiographic findings | CT findings |
|---|---|---|---|---|
| S-OIV pneumonia |
|
|
|
|
| Atypical pneumonias (viral and non- viral) | Commonly presents with an influenza-like illness. |
|
|
Variable findings, with features of small airway involvement and septal thickening commonly seen. |
| Bacterial pneumonias | High grade fever and a productive cough are more suggestive of a bacterial cause. | Detecting the organism by sputum Gram stain or cultures of variable body secretions or tissues. |
|
|
| Organizing pneumonia from other causes (idiopathic, post-infectious, drug reaction, connective tissue disorder, post-organ transplant) |
|
|
|
|
TEACHING POINT
In patients currently presenting with acute influenza-like illness, presence of patchy ground-glass opacities with or without superimposed consolidation on the radiograph with a characteristic peribronchovascular and subpleural distribution on CT should raise the possibility of S-OIV pneumonia.
ABBREVIATIONS
- S-OIV
swine-origin influenza A (H1N1) virus
- CT
computed tomography
- rRT-PCR
real-time reverse-transcriptase-polymerase chain reaction
- WHO
world health organization
- RIDT
rapid influenza diagnostic tests
- IFA
immunofluorescence assays
REFERENCES
- 1.Ujita M, Renzoni EA, Veeraraghavan S, Wells AU, Hansell DM. Organizing pneumonia: perilobular pattern at thin-section CT. Radiology. 2004 Sep;232(3):757–61. doi: 10.1148/radiol.2323031059. [DOI] [PubMed] [Google Scholar]
- 2.United States Centers for Disease Control and Prevention. Interim guidance on specimen collection, processing, and testing for patients with suspected novel influenza A (H1N1) virus infection. [Accessed October 19, 2009]. www.cdc.gov/h1n1flu/specimencollection.htm. Published May 13, 2009.
- 3.World Health Organization. Global alert and response. CDC protocol of realtime RTPCR for influenza A (H1N1) [Accessed October 19, 2009]. www.who.int/csr/resources/publications/swineflu/CDCRealtimeRTPCR_SwineH1Assay-2009_20090430.pdf. Updated October 6, 2009.
- 4.World Health Organization. Global alert and response. Pandemic (H1N1) 2009 - update 86. [Accessed February 9, 2010]. http://www.who.int/csr/don/2010_02_5/en/index.html.
- 5.U.S. Centers for Disease Control and Prevention. Interim guidance for clinicians on identifying and caring for patients with swine-origin influenza A (H1N1) virus infection. [Accessed October 19, 2009]. www.cdc.gov/h1n1flu/identifyingpatients.htm. Published May 4, 2009.
- 6.Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, et al. INER Working Group on Influenza. Pneumonia and respiratory failure from swineorigin influenza A (H1N1) in Mexico. N Engl J Med. 2009;361:680–689. doi: 10.1056/NEJMoa0904252. [DOI] [PubMed] [Google Scholar]
- 7.U.S. Centers for Disease Control and Prevention. H1N1 Flu (Swine Flu) and You. 2009. [Accessed October 19, 2009]. www.cdc.gov/h1n1flu/qa.htm. Updated September 24, 2009.
- 8.U. S. Centers for Disease Control and Prevention. Interim recommendations for clinical use of influenza diagnostic tests during the 2009–10 influenza season. [Accessed October 19, 2009]. www.cdc.gov/h1n1flu/guidance/diagnostic_tests.htm. Published September 29, 2009.
- 9.U. S. Centers for Disease Control and Prevention. Updated interim recommendations for the use of antiviral medications in the treatment and prevention of influenza for the 2009–2010 Season. [Assessed October 19, 2009]. www.cdc.gov/h1n1flu/recommendations.htm. Published October 16, 2009.
- 10.Agarwal PP, Cinti S, Kazerooni EA. Chest radiographic and CT findings in novel swine-origin influenza A (H1N1) virus (S-OIV) infection. AJR Am J Roentgenol. 2009 Dec;193(6):1488–93. doi: 10.2214/AJR.09.3599. [DOI] [PubMed] [Google Scholar]
- 11.Ajlan AM, Quiney B, Nicolaou S, Müller NL. Swine-origin influenza A (H1N1) virus infection: radiographic and CT findings. AJR Am J Roentgenol. 2009 Dec;193(6):1494–9. doi: 10.2214/AJR.09.3625. [DOI] [PubMed] [Google Scholar]
- 12.Mollura DJ, Asnis DS, Conetta R, et al. Imaging findings in a fatal case of pandemic swine-origin influenza A (H1N1) AJR Am J Roentgenol. 2009 Dec;193(6):1500–3. doi: 10.2214/AJR.09.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Müller NL, Ooi GC, Khong PL, Nicolaou S. Severe acute respiratory syndrome: radiographic and CT findings. AJR Am J Roentgenol. 2003 Jul;181(1):3–8. doi: 10.2214/ajr.181.1.1810003. [DOI] [PubMed] [Google Scholar]
- 14.Müller NL, Ooi GC, Khong PL, Zhou LJ, Tsang KW, Nicolaou S. High-resolution CT findings of severe acute respiratory syndrome at presentation and after admission. AJR Am J Roentgenol. 2004 Jan;182(1):39–44. doi: 10.2214/ajr.182.1.1820039. [DOI] [PubMed] [Google Scholar]