Abstract
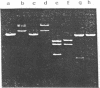
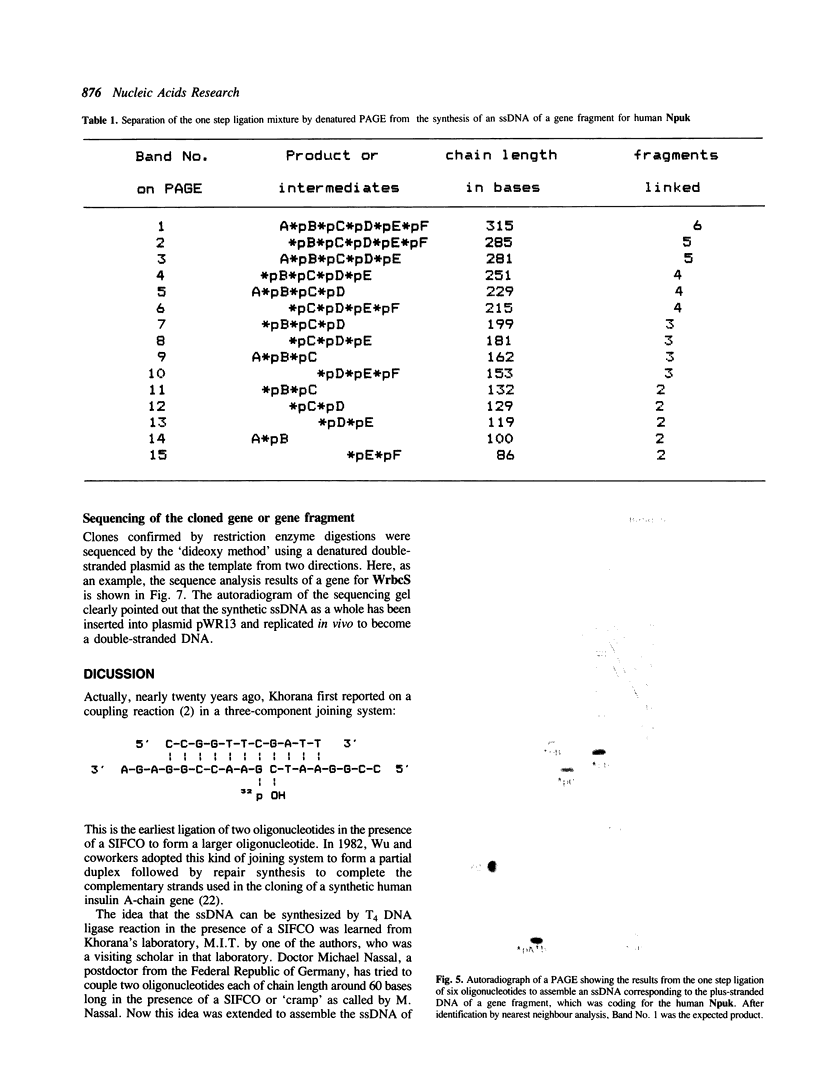
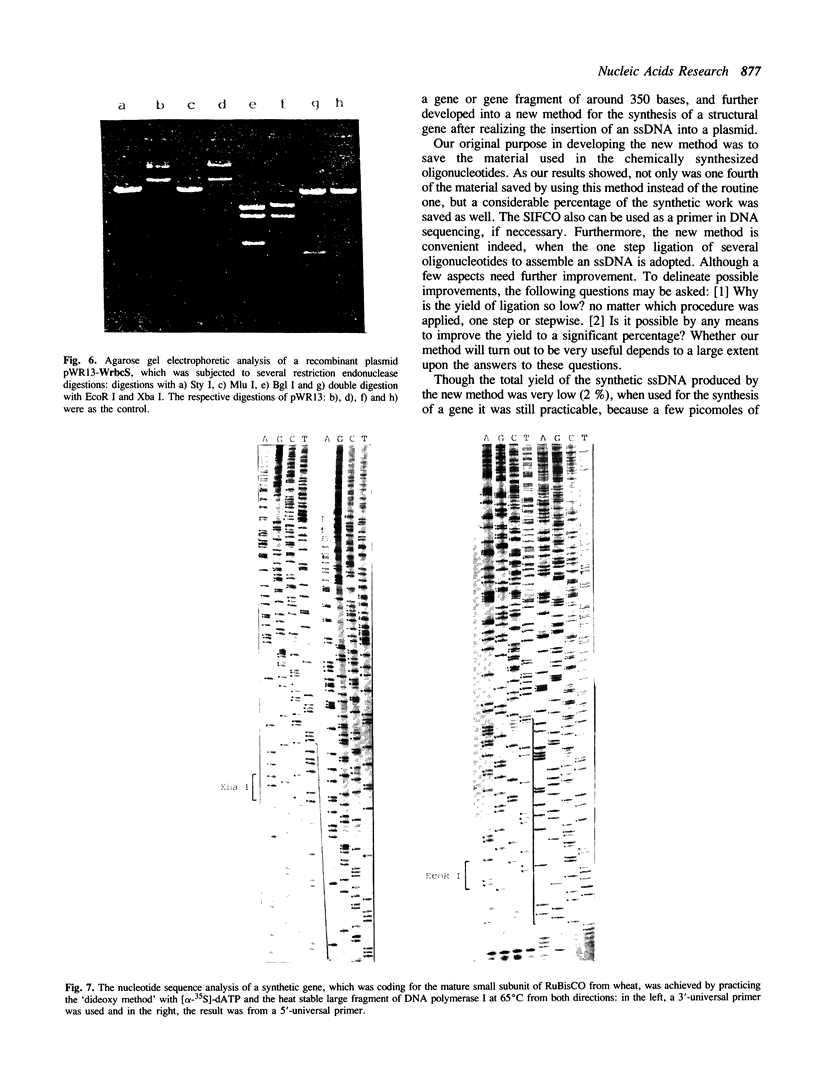
A novel method of synthesizing a structural gene or gene fragment, consisting of the first synthesis of a single-stranded DNA (ssDNA), has been developed. As a preliminary test of this method, four synthetic genes or gene fragments have been synthesized. The first one with 396 base pairs (b.p.) codes for the mature rbcS from wheat, the next two with 370 and 342 b.p. respectively, for two half molecules of a gene for trichosanthin and the last one with 315 b.p. for the N-terminal 1-102 residues of human prourokinase. In all these syntheses, a plus-stranded DNA of the target gene was generally assembled by a stepwise or one step T4 DNA ligase reaction of six oligonucleotides (A, *pB, *pC, *pD, *pE and *pF) of 30-71 nucleotides long in the presence of two terminal complementary oligonucleotides (Ab' and eF') and three short inter-fragment complementary oligonucleotides (bc, cd and de). After purification, the synthetic ssDNA was inserted into a cloning vector, pWR13. The resulting product was directly used to transform a host cell. The structure of the cloned synthetic gene was confirmed by DNA sequence analysis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal K. L., Büchi H., Caruthers M. H., Gupta N., Khorana H. G., Kleppe K., Kumar A., Ohtsuka E., Rajbhandary U. L., Van de Sande J. H. Total synthesis of the gene for an alanine transfer ribonucleic acid from yeast. Nature. 1970 Jul 4;227(5253):27–34. doi: 10.1038/227027a0. [DOI] [PubMed] [Google Scholar]
- Barone A. D., Tang J. Y., Caruthers M. H. In situ activation of bis-dialkylaminophosphines--a new method for synthesizing deoxyoligonucleotides on polymer supports. Nucleic Acids Res. 1984 May 25;12(10):4051–4061. doi: 10.1093/nar/12.10.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer B. F., Holmes W. M. Cloning of synthetic oligodeoxynucleotides may result in high frequency promoter mutations in E. coli. Nucleic Acids Res. 1989 Jan 25;17(2):812–812. doi: 10.1093/nar/17.2.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. L., Belagaje R., Ryan M. J., Khorana H. G. Chemical synthesis and cloning of a tyrosine tRNA gene. Methods Enzymol. 1979;68:109–151. doi: 10.1016/0076-6879(79)68010-2. [DOI] [PubMed] [Google Scholar]
- Ferretti L., Karnik S. S., Khorana H. G., Nassal M., Oprian D. D. Total synthesis of a gene for bovine rhodopsin. Proc Natl Acad Sci U S A. 1986 Feb;83(3):599–603. doi: 10.1073/pnas.83.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M., Kent S., Caruthers M., Dreyer W., Firca J., Giffin C., Horvath S., Hunkapiller T., Tempst P., Hood L. A microchemical facility for the analysis and synthesis of genes and proteins. Nature. 1984 Jul 12;310(5973):105–111. doi: 10.1038/310105a0. [DOI] [PubMed] [Google Scholar]
- Karnik S. S., Sakmar T. P., Chen H. B., Khorana H. G. Cysteine residues 110 and 187 are essential for the formation of correct structure in bovine rhodopsin. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8459–8463. doi: 10.1073/pnas.85.22.8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorana H. G., Agarwal K. L., Büchi H., Caruthers M. H., Gupta N. K., Kleppe K., Kumar A., Otsuka E., RajBhandary U. L., Van de Sande J. H. Studies on polynucleotides. 103. Total synthesis of the structural gene for an alanine transfer ribonucleic acid from yeast. J Mol Biol. 1972 Dec 28;72(2):209–217. doi: 10.1016/0022-2836(72)90146-5. [DOI] [PubMed] [Google Scholar]
- McGraw R. A., 3rd Dideoxy DNA sequencing with end-labeled oligonucleotide primers. Anal Biochem. 1984 Dec;143(2):298–303. doi: 10.1016/0003-2697(84)90666-3. [DOI] [PubMed] [Google Scholar]
- Nassal M., Mogi T., Karnik S. S., Khorana H. G. Structure-function studies on bacteriorhodopsin. III. Total synthesis of a gene for bacterio-opsin and its expression in Escherichia coli. J Biol Chem. 1987 Jul 5;262(19):9264–9270. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpulla R. C., Narang S., Wu R. Use of a new retrieving adaptor in the cloning of a synthetic human insulin A-chain gene. Anal Biochem. 1982 Apr;121(2):356–365. doi: 10.1016/0003-2697(82)90493-6. [DOI] [PubMed] [Google Scholar]
- Sgaramella V., Khorana H. G. CXII. Total synthesis of the structural gene for an alanine transfer RNA from yeast. Enzymic joining of the chemically synthesized polydeoxynucleotides to form the DNA duplex representing nucleotide sequence 1 to 20. J Mol Biol. 1972 Dec 28;72(2):427–444. doi: 10.1016/0022-2836(72)90155-6. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]