Abstract
Malignant plasma cells in multiple myeloma are predominantly confined to the medullary space of the skeletal system, therefore the disease course will be dominated by signs and symptoms related to bone marrow infiltration and destructive bone lesions with their consequences as well as abnormal protein production. Visceral extramedullary plasmacytoma involving the gastrointestinal system and particularly the duodenum is a rare manifestation of the disease. We report a case of duodenal extramedullary plasmacytoma presenting with gastric outlet obstruction and painless jaundice, in a patient treated for multiple myeloma. Diagnosis was first suggested on imaging, and proved by endoscopic biopsy. The duodenal mass resolved following chemotherapy.
Keywords: Multiple myeloma, duodenal plasmacytoma, gastrointestinal involvement, extramedullary multiple myeloma, painless jaundice, gastric outlet obstruction
CASE REPORT
A 66-year-old gentleman presented to the emergency department of our institution complaining of severe nausea and vomiting in the setting of a two-week history of worsening fatigue, pruritus, and jaundice. The patient was known to have a history of multiple myeloma (MM) and had had bone marrow transplants twice, six and three years prior to presentation. He had recent evidence of recurrent disease based on the results of a bone marrow biopsy, obtained three weeks prior to presentation, and was started on Bortezomib (Velcade®). Patient was also known to have a personal history of cholelithiasis, hepatic steatosis, diabetes mellitus type II, and a history of benign esophageal stricture successfully treated using endoscopic dilation. On physical examination the patient was afebrile, and his vital signs were within normal limits; he had prominent scleral icterus and had mild abdominal distention with significant tenderness to deep palpation, and a palpable liver edge. Laboratory testing revealed abnormal liver function tests, with elevated total bilirubin of 12.7 mg/dl (Normal: 0.3–1.2 mg/dl), elevated alkaline phosphatase of 238 IU/L (Normal: 30–115 IU/L), elevated AST of 61 IU/L (Normal: 10–40 IU/L) and a normal ALT level, low calcium of 7.6 mg/dl (Normal: 8.7–10.7 mg/dl), and a low sodium of 126 mg/dl (Normal: 136–145 mg/dl). Results of routine hematologic tests were remarkable for hemoglobin of 7.4 g/dl (Normal: 14–18 g/dl) and hematocrit of 20.4 % (Normal: 42–52 %). Renal function tests were normal.
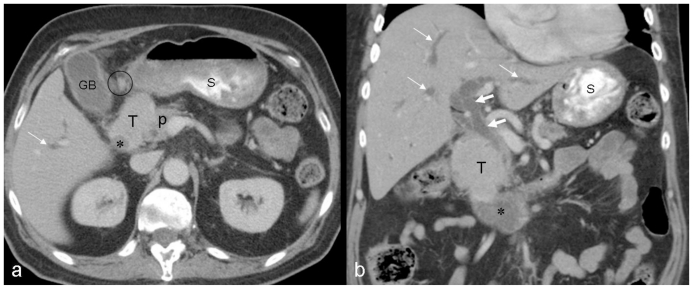
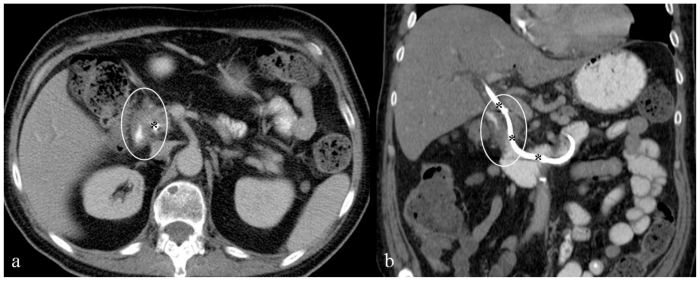
Given the patient's history, physical examination, and laboratory findings a CT scan of the abdomen and pelvis with intravenous and oral contrast was ordered for evaluation of possible biliary obstruction, hepatic pathology, and to rule out other gastrointestinal diseases and particularly bowel obstruction. CT scan demonstrated the presence of a 4.4cm × 6.0cm × 5.8cm irregular, soft tissue mass involving the second portion of the duodenum which was significantly narrowed, in close contact with the pancreatic head, with significant intrahepatic and extrahepatic biliary ductal dilation along with pancreatic ductal dilation; in addition there was associated mesenteric lymphadenopathy (Fig. 1).
Figure 1.
66-year-old male patient presented with severe nausea and vomiting in the setting of a two-week history of worsening fatigue, pruritus, and jaundice. Axial (a) and coronal (b) contrast enhanced (IV and PO) CT images through the mid abdomen obtained in the equilibrium phase show a large solid enhancing soft tissue tumor, measuring approximately 4.4 × 6.0 × 5.8 cm. It appears originating from the medial wall of the descending portion of the duodenum narrowing its lumen (asterisk), involving the medial aspect of the common bile duct and pancreatic head, resulting in significant dilatation of the intrahepatic (thin white arrows) and extrahepatic (thick white arrows) biliary tree; the pancreatic duct is also dilated (not shown). Enlarged regional mesenteric lymph nodes were noted (black cursor). Note significant fluid residue in the stomach. GB: gallbladder; T: tumor; P: pancreatic head; S: stomach. [Technique: KVp = 120; mA = 356; Slice Thickness = 4.00 mm; Dose of intravenous contrast: Iopamidol (Isovue-300), 100 ml].
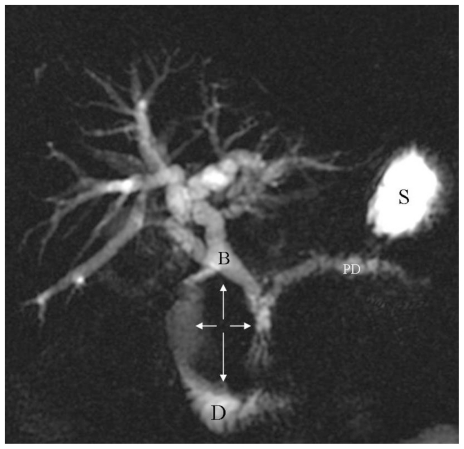
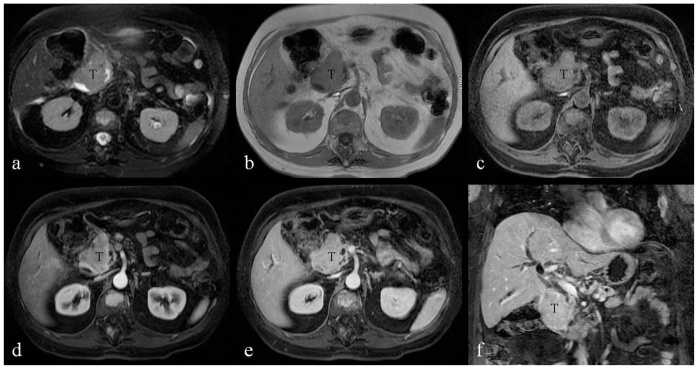
A Magnetic Resonance Cholangiopancreatography (MRCP) was then performed to further characterize the tumor and its relationship to the biliary tree and pancreas; MRCP demonstrated a 7cm × 5.5cm × 3.5 cm soft tissue mass arising from the medial wall of the second portion of the duodenum, significantly narrowing its lumen (Fig. 2, 3). The medial aspect of the mass was involving the region of the ampulla of Vater and impinging on the head of the pancreas resulting in severe dilation of the pancreatic duct as well as dilation of the intrahepatic and extrahepatic biliary tree.
Figure 2.
66-year-old male patient presented with severe nausea and vomiting in the setting of a two-week history of worsening fatigue, pruritus, and jaundice. Single three-dimensional Radial MRCP Breath Hold image (TR/TE = 2857.85 msec./1027.58 msec. flip angle = 90.0 degrees) demonstrates significant intra- and extrahepatic biliary ductal dilatation as well as main pancreatic duct dilatation. There is a large, non-cystic, space occupying mass (orthogonal white arrows) impinging on the duodenal lumen. Noted is significant fluid residue in the stomach. B: biliary tree; PD: pancreatic duct; D: duodenum; S: stomach.
Figure 3.
66-year-old male patient presented with severe nausea and vomiting in the setting of a two-week history of worsening fatigue, pruritus, and jaundice. a: Transverse noncontrast, T2-weighted, fat-suppressed image (TR/TE = 2600 msec./160 msec.) demonstrates the duodenal tumor showing homogenous high signal intensity. b: Transverse noncontrast, T1-weighted, 2D fat- suppressed spoiled gradient-echo sequence (TR/TE = 177 msec./4.3 msec.; flip angle = 80 degrees) demonstrates the duodenal tumor showing homogenous signal intensity isointense to the paraspinal muscles. c–f: Transverse, T1-weighted, fast-suppressed, 3D spoiled gradient echo (LAVA) sequences (TR/TE = 4.216 msec./2.02 msec. flip angle = 12 degrees), before and after intravenous contrast (20 ml gadobenate dimeglumine, Multihance). Duodenal tumor demonstrates mild enhancement during the hepatic arterial-dominant phase (d), which becomes homogenous and more intense after one minute (e), and two-minute delay (f). T: duodeal tumor.
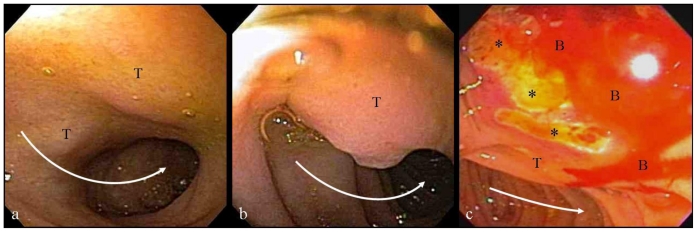
No focal liver or pancreatic lesions were visualized. The patient subsequently underwent Endoscopic Retrograde Cholangiopancreatography (ERCP) where areas of ulcerated duodenal mucosa and submucosal mass were biopsied (Fig. 4) and a biliary stent was placed (Fig. 5). The pathologic specimens showed evidence of tumor cells with prominent nucleoli staining positive for CD138 and kappa immunostains leading to the final pathologic diagnosis of plasmacytoma.
Figure 4.
66-year-old male patient presented with severe nausea and vomiting in the setting of a two-week history of worsening fatigue, pruritus, and jaundice. a, b: endoscopic images from the first and second segments of the duodenum showing an extrinsic compression on the medial wall of the duodenum (T) slightly narrowing the duodenal lumen (curved white arrows). c: Endoscopic image from the periampullary region showing mucosal ulceration (asterisks) and bleeding (B), overlying the extrinsic impression from the tumor (T).
Figure 5.
66-year-old male patient presented with severe nausea and vomiting in the setting of a two-week history of worsening fatigue, pruritus, and jaundice. Axial (a) and coronal (b) contrast enhanced (IV and PO) CT images through the mid abdomen obtained in the equilibrium phase, six weeks following chemotherapy, show resolution of the duodenal soft tissue tumor; there remains minimal circumferential duodenal wall thickening (white cursors). Noted is a biliary stent (asterisks) seen from the level of the porta hepatis across the ampulla of Vater, its distal tip formed in the fourth segment of the duodenum. [Technique: KVp = 120; mA = 275; Slice Thickness = 5.00 mm; Dose of intravenous contrast: Iopamidol (Isovue-300), 100 ml].
Subsequently, the decision was made by the patient and his care team to continue treating his MM with a modified VTD-PACE regimen (bortezomib, thalidomide, dexamethasone and 4-days continuous infusions of cis-platin, doxorubicin, cyclophosphamide, etoposide). The patient had significant improvement following chemotherapy; a 1-month follow-up CT revealed significant decrease in the intrahepatic ductal dilation, near complete resolution of the duodenal mass close to the pancreatic head, and absence of lymphadenopathy (Fig. 5).
Six months later the patient was admitted to the hospital for an autologous stem cell transplant from an unrelated donor. The patient experienced Tacrolimus-induced encephalopathy fourteen days after the procedure and his course was further complicated by aspiration pneumonia. The patient passed away eight weeks following his third stem cell transplant.
DISCUSSION
B-cell lymphocytes, part of the immune system, derive from hematopoietic stem cells in the bone marrow. In response to foreign antigens, B-cells will mature and transform into plasma cells. The role of plasma cells is to produce millions of copies of the same antibody called immunoglobulin that recognize the antigens and mark them for destruction by complement activation and phagocytosis. When the transformation of a stem cell to a B-cell is altered, it results in an abnormal or malignant plasma cell called plasmablast, a developmentally early form of plasma cell. These cells can proliferate in the bone marrow to form MM or, extramedullary in the soft tissues, to form plasmacytoma. Plasmacytoma can be either primary without evidence of bone marrow involvement or may occur simultaneously with MM, representing extramedullary spread of the disease (Table 1).
Table 1.
Summary table of plasmacytoma
| Definition of plasmacytoma | Proliferation of an abnormal or malignant plasma cell in the extramedullary soft tissues |
| Incidence | Primary extramedullary plasmacytoma: 20% (Multiple myeloma Involving the gastrointestinal tract: up to 7%) |
| Clinical manifestations | Obstructing mass or Upper GI bleed |
| Diagnosis | Pathology and Immunohistology |
| Treatment | Isolated plasmacytoma: Radiotherapy Extramedullary spread of MM: Chemotherapy followed by stem cell transplantation |
| Prognosis | Poor despite aggressive management |
Primary extramedullary plasmacytoma is a rare form of the disease accounting for 20% of cases [1,2] with 7% only manifesting in the gastrointestinal tract [3,4]. Goldstein and Poker noted in a review of thirty six patients that the stomach was the most commonly involved gastrointestinal site followed by the jejunum, ileum, colon, rectum and finally rarely the duodenum [5]. Gastrointestinal plasmacytoma in the course of MM is extremely rare accounting for approximately 0.9% as Talamo et al. showed in a large retrospective study conducted on 2,584 recruited MM patients [6]. Duodenal involvement manifests usually as an obstructing mass or as upper gastrointestinal bleeding [7–11]. Our patient presented with gastric outlet obstruction secondary to a large duodenal mass significantly narrowing the duodenal lumen; he also had painless jaundice secondary to common bile duct obstruction by the tumor at the level of the ampulla of Vater. The imaging of duodenal MM is not reported in the literature since most of the published cases appeared in gastroenterology journals. The case we are presenting appeared as a large homogenous solid soft tissue mass arising from the duodenal wall, showing a CT density similar to liver, with homogeneous enhancement following intravenous contrast administration. On MRI, it appeared hyperintense on T2-weighted images and isointense on T1-weighted images compared to skeletal muscle, and demonstrated homogeneous enhancement on the equilibrium phase; the radiological diagnosis of duodenal plasmacytoma was suggested based on the patient's clinical history. The radiological differential diagnosis would obviously include more prevalent diseases such as duodenal adenocarcinoma and lymphoma (Table 2). Endoscopically, it may appear as a discrete ulcer, an ulcerated mass, thickened mucosal folds, or polyp [13]. In our patient, due to its location along the medial wall of the duodenum, the endoscopic appearance of the tumor simulated a pancreatic head mass invading the duodenal wall. As the endoscopic appearance of duodenal plasmacytoma is non specific, resembling more common conditions such as duodenal adenocarcinoma or like in our case pancreatic head tumor, pathological and immunohistological diagnosis are crucial in making the final diagnosis.
Table 2.
Differential diagnosis of duodenal plasmacytoma
| CT | MRI-T1 | MRI-T2 | MRI-DWI | Pattern of contrast enhancement | PET | |
|---|---|---|---|---|---|---|
| Plasmacytoma |
|
Intermediate | Variable | Restricted | Mild to moderate | Increased tracer uptake |
| Adenocarcinoma |
|
Intermediate | Variable | Restricted | Mild | Increased tracer uptake |
| Lymphoma |
|
Intermediate (homogeneous) | High (heterogeneous) | Restricted | Mild to moderate | Increased tracer uptake |
The treatment of duodenal plasmacytoma, as a primary disease or as extramedullary spread, will be to first address the local complications caused by the mass itself such as relief of biliary obstruction or control of bleeding if significant, followed by surgical resection if necessary [8]. Reports on management of solitary plasmacytoma favor radiation therapy over chemotherapy; however in cases of extramedullary spread, as in our patient, high-dose chemotherapy followed by stem-cell transplantation is the standard treatment [8, 14]. Of note is that even with aggressive treatment, once there is gastrointestinal involvement the prognosis will be very poor [6].
TEACHING POINT
Although very rare, duodenal involvement by multiple myeloma should be considered as differential diagnosis in the proper clinical settings; pathology will establish the final diagnosis. The prognosis of MM with evidence of gastrointestinal involvement is poor despite aggressive management.
ABBREVIATIONS
- IU
International units
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- CT
Computed tomography
- MRI
Magnetic resonance imaging
- cm
Centimeters
- MM
Multiple Myeloma
- ERCP
Endoscopic Retrograde Cholangiopancreatography
- MRCP
Magnetic Resonance Cholangiopancreatography
- GI
Gastrointestinal
REFERENCES
- 1.Pimmental R, vanstolk R. Gastric plasmacytoma: A rare cause of massive gastrointestinal bleeding. Am J Gastroenterol. 1993;88:1963–1964. [PubMed] [Google Scholar]
- 2.Chim CS, Wong WM, Nicholls J, Chung LP, Liang R. Extramedullary sites of invovelment in hematologic malignancies: case3. Hemorrhagic gastric plasmacytoma as the primary presentation in multiple myeloma. J Clin Oncol. 2002;20:344–347. doi: 10.1200/JCO.2002.20.1.344. [DOI] [PubMed] [Google Scholar]
- 3.Alexiou C, Kau RJ, Dietzfelbinger H, et al. Extramedullary plasmacytoma: tumor occurrence and therapeutic concepts. Cancer. 1999;85:2305–2314. [PubMed] [Google Scholar]
- 4.Dolin S, Dewer J. Extramedullary plasmacytoma. Am J Pathol. 1955;32:83–103. [PMC free article] [PubMed] [Google Scholar]
- 5.Goldstein WB, Poker N. Multiple myeloma involving the gastrointestinal tract. Gastroenterology. 1966;51:87–93. [PubMed] [Google Scholar]
- 6.Talamo G, Cavallo F, Zangari M, Barlogie B, Lee CK, Pineda-Roman M, et al. Clinical and biological features of multiple myeloma involving the gastrointestinal system. Haematologica. 2006;91:964–967. [PubMed] [Google Scholar]
- 7.Pentimone F, Camici M, Cini G, Levorato D. Duodenal plasmacytoma. A rare Primary Extramedullary Localization Simulating a Carcinoma. Acta Haemat. 1979;61:155–160. doi: 10.1159/000207649. [DOI] [PubMed] [Google Scholar]
- 8.Telakis E, Tsironi E, Tavoularis G, Papatheodorou K, Tzaida O, Nikolaou A. Gastrointestinal involvement in a patient with multiple myeloma: A case report. Annals Gastro. 2009;22(4):287–290. [Google Scholar]
- 9.Gradshir W, Recant W, Shapiro C. Obstructing Plasmacytoma of the Duodenum: First Manifestation of Relapsed Multiple Myeloma. Am J Gastroenterol. 1988;83(1):77–91. [PubMed] [Google Scholar]
- 10.Schoretsanitis G, Livingstone JI, El-Japourl JN, Watkins N, Wastell C. Duodenal plasmacytoma: a rare extramedullary localization simulating carcinoma of the head of the pancreas. Postgrad Med J. 1994;70:378–379. doi: 10.1136/pgmj.70.823.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siddique I, Papadakis KA, Weber DM, Glober G. Recurrent bleeding from a duodenal plasmacytoma treated successfully with embolization of the gastroduodenal artery. Am J Gastroenterol. 1999;94:1691–1692. doi: 10.1111/j.1572-0241.1999.01166.x. [DOI] [PubMed] [Google Scholar]
- 12.Hefferman A. Plasmacytoma of pancreas and duodenum causing acute intestinal obstruction. Lancet. 1947;1:910. doi: 10.1016/s0140-6736(47)91368-8. [DOI] [PubMed] [Google Scholar]
- 13.Esfandyari T, Abraham SC, Arora AS. Gastrointestinal plasmacytoma that caused anemia in a patient with multiple myeloma. Nat Clin Pract Gastroenterol Hepatol. 2007;4:111–115. doi: 10.1038/ncpgasthep0719. [DOI] [PubMed] [Google Scholar]
- 14.West RL, Sonneveld P, de Jonge V, Hordijk ML, Kuipers EJ. Gastrointestinal plasmacytomas: a rare finding with important consequences. Am J Gastroenterol. 2008;103:2413–2414. doi: 10.1111/j.1572-0241.2008.02010_13.x. [DOI] [PubMed] [Google Scholar]