Abstract
We report the case of a patient with pre-existing multiple sclerosis, who presented with horizontal diplopia, and a prior episode of progressive ataxia and dizziness lasting one week. While initially attributed to multiple sclerosis, subsequent imaging demonstrated a concurrent left cerebellar gangliocytoma, also known as Lhermitte-Duclos disease.
Keywords: Multiple Sclerosis, Lhermitte-Duclos disease, cerebellar gangliocytoma
CASE REPORT
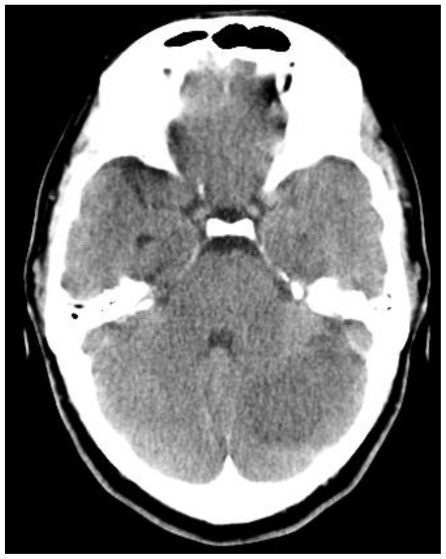
A 41 year old female with known multiple sclerosis (MS) presented to the Emergency Department with horizontal diplopia after one previous episode of progressive ataxia and dizziness, which was one week in duration. On physical exam, the visual acuity was decreased to 20/30 on the left eye. The patient showed decreased pinprick sensation in left arm and leg. No other neurological deficits were demonstrated at the time, and no cerebellar symptoms were noted. Computed tomography (CT) showed a hypodense area in the cerebellum, originally thought to represent a stroke or demyelination related to tumefactive MS (Fig.1).
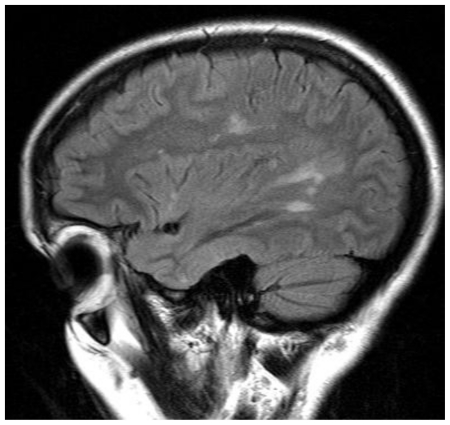
Figure 1.
41 yo female with history of multiple sclerosis presented with horizontal diplopia. Noncontrast enhanced axial CT slices (64 slice Somatom, Siemens Health Care, Erlangen, Germany, 3 mm axial reconstruction, 440 mA, 120 KVp) demonstrate a predominantly hypodense lesion in the left cerebellar hemisphere sparing the cerebellar peduncle with slight mass effect upon the fourth ventricle.
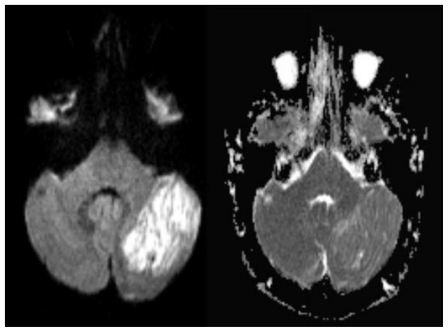
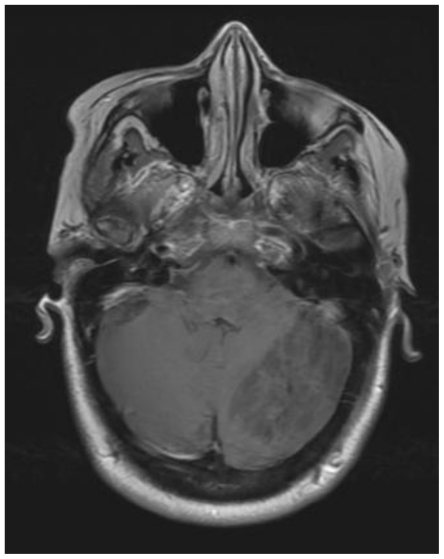
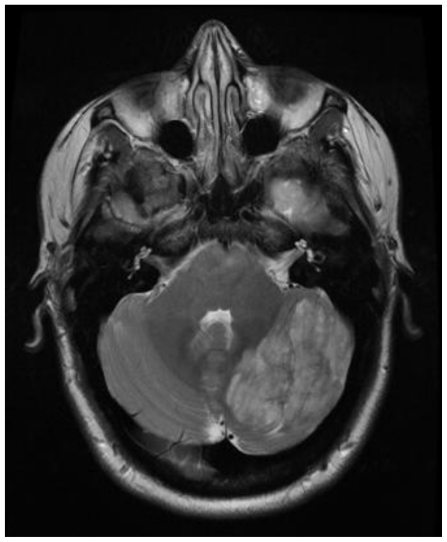
Subsequent magnetic resonance imaging (MRI) excluded stroke as a cause of the lesion, showing no diffusion restriction (Fig. 2), however characteristic findings suggestive of Lhermitte-Duclos disease (LDD) were seen. Images showed a striated mass in the left cerebellar hemisphere, with areas of alternating isointense and hypointense signal. This characteristic pattern seen in Lhermitte-Duclos was best visualized on axial T1-weighted images (Fig. 3). T2-weighted images demonstrated the same lesion with alternating isointense and hyperintense signal (Fig. 4).
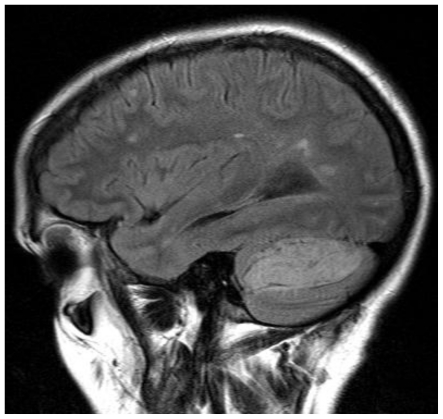
Figure 2.
41 yo female with history of multiple sclerosis presented with horizontal diplopia. Axial diffusion weighted imaging (left image, B-1000)(1.5 Tesla Siemens Espree, repetition time (TR) = 4500 ms; echo time (TE) = 112 ms) demonstrates a 3 × 4 cm large, well defined area of predominantly corduroy appearing, increased signal in the left cerebellar hemisphere with sparing of the cerebellar peduncle, causing slight mass effect upon the left aspect of the 4th ventricle. Corresponding apparent diffusion coefficient (ADC) map (right image) shows increased diffusion values proving lack of diffusion restriction. This excludes acute ischemia and is typically found in LDD. Medulloblastoma that may present with diffusion restriction becomes a less likely differential diagnosis.
Figure 3.
41 yo female with history of multiple sclerosis presented with horizontal diplopia. This axial T1-weighted image (General Electric Signa 3.0Tesla; TR = 2607ms, TE = 9.94 ms, inversion time (TI) 1100ms, number of excitations (NEX), 2; flip angle FA = 90) demonstrates alternating isointense and hypointense signal of the tumor within the left lobe of the cerebellum. This is the characteristic striated corduroy pattern of a tumor in Lhermitte-Duclos disease.
Figure 4.
41 yo female with history of multiple sclerosis presented with horizontal diplopia. This axial T2-weighted image (General Electric Signa 3.0Tesla; TR = 5200ms, TE = 101.82 ms, number of excitations (NEX), 2; flip angle FA = 90) demonstrates alternating isointense and hyperintense signals of the tumor within the left lobe of the cerebellum. This is the characteristic striated pattern of a tumor in Lhermitte-Duclos disease. The adjacent cerebellar fissures are affected from associated mass effect.
On recent examination, in 2010, our patient demonstrated visual acuity on the left of 20/50 and on the right of 20/30. She had slowed cognitive processing speed and word finding problems, as well as decreased verbal fluency. Symptoms of impulsivity and depression were apparent. She demonstrated no cerebellar abnormalities at this time. All other areas of neurological exam were normal. The patient has refused biopsy, as well as surgical resection of the tumor. She is being followed by her neurologist, currently with stable condition.
DISCUSSION
MS is a chronic demyelinating disease, thought to be caused by an autoimmune process in which the person’s own leukocytes destroy the myelin within the central nervous system, slowing or interrupting nerve conduction. The symptoms can include numbness, tingling, weakness, and paralysis, or it may be entirely asymptomatic. The course of this disease is variable, with some patients having years of minimal or no symptoms, while others rapidly lose function and quality of life. The life expectancy of a person with MS is normal. Differential diagnosis for MS is extensive (Table 1), and overlaps with many pathological processes responsible for ataxia (Table 2). On MRI imaging, MS typically presents with demyelinating T2 and FLAIR hyperintense plaques disseminated in the white matter radiating along the U fibers. They can demonstrate varying size and intensity, may show contrast enhancement as a sign of acute demyelination, and can disappear, usually progressing to hypointense T1 signal and moderately hyperintense T2 signal (Figs. 5 and 6). Clinical diagnostic criteria for a patient with suspected MS includes brain MRI with gadolinium, with additional MRI of the spinal cord if there is still indecision about the diagnosis. If the patient’s presenting symptoms are at the level of the spinal cord, a spinal MRI would be included in the initial workup regardless. Brain MRI with gadolinium is used to demonstrate new lesions on follow-up [1].
Table 1.
Clinical Differential for Multiple Sclerosis [10]
| Cervical spondylosis |
| Fibromyalgia |
| Sleep disorders |
| Sjogren syndrome |
| Vitamin B12 deficiency |
| Stroke |
| Peripheral neuropathy |
| Lymphoma |
| Sarcoidosis |
| Tumor |
| Infection/abscess |
Table 2.
Differential for Ataxia [6]
| Alcoholic cerebellar degeneration |
| Drug induced ataxia |
| Ischemic or hemorrhagic stroke |
| Von Hippel-Lindau syndrome |
| Acute cerebellitis |
| Creutzfeldt-Jakob syndrome (ataxic variant) |
| Lhermitte-Duclos syndrome |
| Cerebellar abscess |
| Posterior fossa tumor |
| Multiple sclerosis |
| Wernicke-Korsakoff syndrome |
| Sjogren syndrome |
Figure 5.
41 yo female with history of multiple sclerosis presented with horizontal diplopia. This sagittal FLAIR image (1.5 Tesla Siemens Espree, TR = 9000 ms; TE = 123 ms; inversion time TI = 2500 ms; FA 180) demonstrates hyperintense lesions along the corona radiata and extending into the corpus callosum suggestive of Dawson’s fingers, which are characteristic of MS plaques. Subsequent images (see Figure 6) demonstrated Lhermitte-Duclos disease in concurrence with MS.
Figure 6.
41 yo female with history of multiple sclerosis presented with horizontal diplopia. This sagittal FLAIR (1.5 Tesla Siemens Espree, TR = 9000 ms; TE = 123 ms; TI = 2500 ms; FA 180) image demonstrates concurrent MS plaques along with a striated-appearing cerebellar tumor characteristic of Lhermitte-Duclos disease.
Lhermitte-Duclos disease (Table 3) is characterized by a rare slow growing dysplastic tumor, which is typically unilateral with a preponderance for the cerebellum [2]. Genetic inheritance of chromosome 10q23 may predispose to the development of LDD, as well of Cowden Syndrome [3]. Our patient did not have any signs of Cowden Syndrome, which include multiple hamartomas, fibrocystic changes of the breast, and mucocutaneous tumors [2]. The tumor of LDD has been described in many ways, such as hamartomablastoma, purkinjeoma, ganglio-neuroma, and diffuse hypertrophy of the cerebellar cortex. This rare cerebellar tumor is not well differentiated, with hamartomatous, dysplastic, and neoplastic features. The mass effect of the tumor leads to cerebellar symptoms, with compression of the 4th ventricle and may cause tonsillar herniation. Diagnostic findings include widening of the folia of the cerebellum with a striated appearance of MRI, which has also been described as “laminated” or “corduroy”. The striated appearance is due to alternating isointense and hypointense signals on T1 weighted images (Fig. 3). Rarely calcifications are seen. Biopsy can be performed to confirm diagnosis, and surgical resection of the tumor can be performed as a mode of symptom relief and treatment. On T2 weighted images, the striated appearance is due to isointense and hyperintense signals [4]. Contrast enhancement on MRI, an atypical appearance of LDD, has been reported as well [5].
Table 3.
Summary of Lhermitte-Duclos Syndrome
| Etiology | Undetermined, but thought to be either dysplastic, neoplastic, or hamartomatous |
| Life expectancy | As surgical removal is usually curative, life expectancy is normal |
| Age and Gender | There is no known age predilection or gender distribution |
| Risk Factors | Genetic inheritance of chromosome 10q23 |
| Treatment | Surgical resection of tumor |
| Findings on Imaging | Widening of the folia of the cerebellum with a striated appearance of MRI due to alternating isointense and hypointense signals on T1 images; on T2 images, the striated appearance is due to isointense and hyperintense signals; rarely calcifications are seen |
Despite the characteristic appearance of Lhermitte-Duclos disease there are a few reports in the literature describing medulloblastomas mimicking LDD [6][7]. Mittal et al. explain that this is due to the further lateral migration of primitive neuroectodermal cells in the adult population, out of which medulloblastoma arise. The authors found the presence of diffusion restriction in medulloblastoma useful in the differential diagnosis. This was not present in our case, supporting the diagnosis of Lhermitte-Duclos disease.
Magnetic Resonance Spectroscopy (MRS) has been used to assess Lhermitte-Duclos disease [8]. Klisch et al. detected increased lactate and decreased myo-inositol and N-acetyl-aspartate (NAA) in LDD similar to other low grade gliomas. However, unlike in gliomas, LDD presents with decreased choline levels. Most tumors demonstrate increased choline levels representing increased membrane metabolism. Lack of lipid peaks combined with increased lactate is more suggestive of increased glucose metabolism within the tumor rather than cell necrosis, which the authors were also able to detect by increased glucose uptake on fluorodeoxyglucose positron emission tomography (FDG-PET) (Table 4). Lhermitte-Duclos disease is classified as a WHO grade 1 tumor with slow growth, with surgical resection usually being curative [9]. Symptoms and complications often arise from compression of the 4th ventricle with associated hydrocephalus.
Table 4.
Radiological Findings of Differential Diagnosis in Lhermitte-Duclos Disease [7]
| DWI | T1 and T2 | T1 with contrast | MRS-NAA level | MRS-lactate level | MRS-lipid level | MRS-choline level | |
|---|---|---|---|---|---|---|---|
| LDD | T2 shine through | hypointense on T1; hyper-intense on T2 | typically no enhancement | Decreased (−) | Increased (+) | no lipids | Decreased (−) |
| Medullo-blastoma | restricted diffusion | isointense on T2 | enhancement | Decreased (− − −) | Increased (++) | lipids | Increased (+) |
TEACHING POINT
Symptoms of diplopia, ataxia, and dizziness could all be attributed to multiplesclerosis, and Lhermitte-Duclos disease could have easily be overlooked. The key finding in Lhermitte-Duclos disease is the corduroy-like appearance of the cerebellar tumor on Magnetic Resonance Imaging.
ABBREVIATIONS
- CT
computed tomography
- LDD
Lhermitte-Duclos disease, MRI, magnetic resonance imaging
- MS
multiple sclerosis
- MRS
Magnetic Resonance Spectroscopy
- NAA
N-acetyl-aspartate
- FDG-PET
fluorodeoxyglucose positron emission tomography
- DWI
diffusion weighted imaging
REFERENCES
- 1.Consortium of MS Centers MRI Protocol for the Diagnosis and Follow-up of MS. www.mscare.org.
- 2.Grossman RI, Yousem DM. Neuroradiology: The Requisites. 2nd ed. Philadelphia: Mosby; 2003. pp. 137–138.pp. 143 [Google Scholar]
- 3.Robinson S, Cohen A. Cowden Disease and Lhermitte-Duclos Disease, Neurosurgery. 2000;46(2):371–83. doi: 10.1097/00006123-200002000-00021. [DOI] [PubMed] [Google Scholar]
- 4.Osborn AG. Brain. 1st ed. Section I-6. Salt Lake City: Amirsys; 2004. Diagnostic Imaging; pp. 70–3. [Google Scholar]
- 5.Awwad E, Levy E, Martin D, Merenda G. Atypical MR Appearance of Lhermitte-Duclos Disease with Contrast Enhancement. AJNR Am J Neuroradiol. 1995;16(8):1719–20. [PMC free article] [PubMed] [Google Scholar]
- 6.Douglas-Akinwande AC, Payner TD, Hattab EM. Medulloblastoma mimicking Lhermitte-Duclos disease on MRI and CT. Clin Neurol Neurosurg. 2009 Jul;111(6):536–9. doi: 10.1016/j.clineuro.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Mittal P, Gupta K, Saggar K, Kaur S. Adult medulloblastoma mimicking Lhermitte-Duclos disease: Can diffusion weighted imaging help? Neurol India. 2009;57:203–5. doi: 10.4103/0028-3886.51297. [DOI] [PubMed] [Google Scholar]
- 8.Klisch J, Juengling F, Spreer J, Koch D, Thiel T, Büchert M, et al. Lhermitte-Duclos disease: Assessment with MR imaging, positron emission tomography, single-photon emission CT, and MR spectroscopy. AJNR Am J Neuroradiol. 2001;22:824–30. [PMC free article] [PubMed] [Google Scholar]
- 9.Kulkantrakorn K, Awwad EE, Levy B, Selhorst JB, Cole HO, Leake D, Gussler JR, Epstein AD, Malik MM. MRI in Lhermitte-Duclos disease. Neurology. 1997 Mar;48(3):725–31. doi: 10.1212/wnl.48.3.725. [DOI] [PubMed] [Google Scholar]
- 10.“Disease Lookup” ' “Differential” Epocrates Online: https://online.epocrates.com/