Abstract
Schistosomiasis is a helminthic infection that is endemic in tropical and subtropical regions. Pulmonary involvement can be divided into two categories: acute or chronic compromise. Chronic and recurrent infection develops in persons living or travelling in endemic areas. In the lungs, granuloma formation and fibrosis around the schistosome eggs retained in the pulmonary vasculature may result in obliterative arteriolitis and pulmonary hypertension leading to cor pulmonale. Acute schistosomiasis is associated with primary exposure and is commonly seen in nonimmune travelers. The common CT findings in acute pulmonary schistosomiasis are small pulmonary nodules ranging from 2 to 15 mm and larger nodules with ground glass-opacity halo. Katayama fever is a severe clinical manifestation of acute involvement. We present a case of pulmonary involvement in schistosomiasis and provide a discussion about typical imaging findings in the acute and chronic form.
Keywords: schistosomiasis, lung disease, parasitic, computed tomography
CASE REPORT
A 28 year old male presented in the emergency department with dry cough for 2 ½ weeks, night sweats and subfebrile temperature. Three months ago he spent several weeks of vacation in central Africa, where he had already experienced short lasting fever. Malaria tests performed in Africa showed negative results.
On admission to our hospital there were no significant findings at physical examination. Laboratory tests revealed leukocytosis (12×109/l - normal range 3.5–10×109/l) with marked elevation of eosinophils (6.863×109/l - normal range 0–0.3×109/l). C-reactive protein (CRP), liver enzymes and electrolytes were within normal ranges.
The patient presented afebrile at admission in good health and with no significant clinical finding at physical exam.
Conventional chest radiography (Figure 3) was performed followed by chest CT (Figures 4–5).
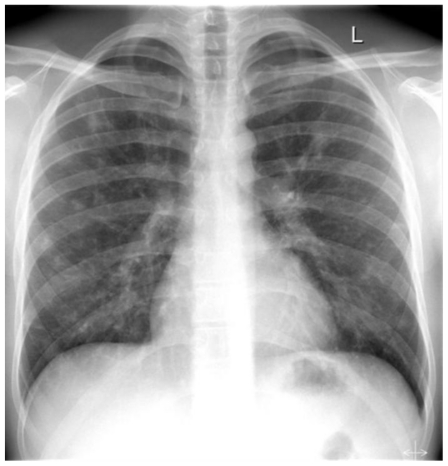
Figure 3.
28 y old male with pulmonary schistosomiasis. Chest radiography (pa) showing multiple small pulmonary nodules scattered over both lungs without obvious predilection.
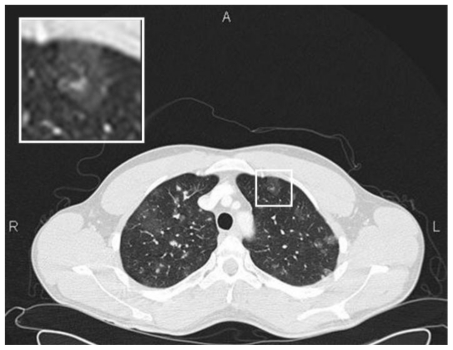
Figure 4.
28 y old male with pulmonary schistosomiasis. Chest CT of the upper lungs showing blurred ground glass nodules scattered over both lungs (Siemens Sensation 16. 120 kV, 100 mAs, 16×1.5 mm collimation, slice thickness 3 mm).
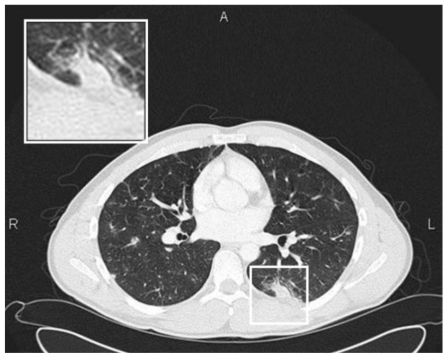
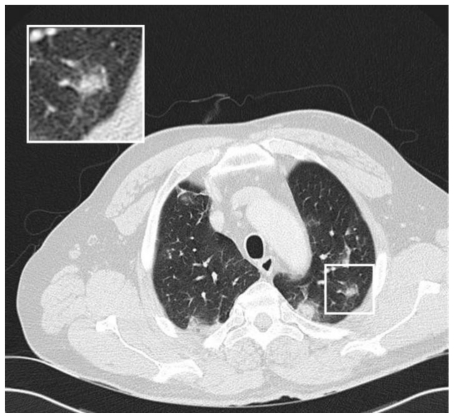
Figure 5.
28 y old male with pulmonary schistosomiasis. Chest CT of the middle lungs showing ground glass nodules and subpleural consolidation of the left superior lower lobe, both consistent with acute eosinophilic infiltration in the context of acute pulmonary schistosomiasis (Siemens Sensation 16. 120 kV, 100 mAs, 16×1.5 mm collimation, slice thickness 3 mm).
Chest x-ray showed multiple patchy, ill-defined opacities in both lungs with non-specific distribution. The size varied from about 5 to 15 mm. Besides slight bronchial thickening with bronchial tram-tracking was described, consistent with peribronchitis. Otherwise no obvious abnormalities were detected, especially no hilar lymphadenopathy and no pleural effusions.
Chest CT confirmed multiple nodules throughout both lungs involving upper, middle and lower zones, most of them ill-defined, some though with sharp delineation. Many nodules demonstrated either a halo or were fully consisting of groundglass opacity. Some of the nodules were pleural-based, two lesions in the superior segment of the left lower lobe were wedge-shaped, pleural-based consolidations with a maximum diameter 25 mm.
DISCUSSION
Schistosomiasis is a rare helminthic infection in western countries but one of the most prevalent infectious diseases worldwide. It is also named bilharzia, named after a German physician who was the first to describe the life circle of the parasite in 1851.
In tropical countries it affects more than 200 million individuals, 20 million of whom have severe disease. Because of intensive and continuous control efforts infection rates are decreasing in all endemic areas [1].
The major forms of human schistosomiasis are caused by five species of water-borne flatworms, or blood flukes, called schistosomes:
Schistosoma (S.) mansoni occurs in Africa, the Eastern Mediterranean, the Caribbean and South America.
S. japonicum group of parasites (including S. mekongi in the Mekong river basin), is endemic in South-East Asia and in the Western Pacific region.
S. intercalatum has been reported from central African countries.
S. haematobium, endemic in Africa and the Eastern Mediterranean.
The parasites mainly affecting human beings are S. haematobium (fig. 1), S. mansoni (fig. 2), and S. japonicum. The most prevalent area for schistosomiasis is Africa, where S. haematobium and S. mansoni are endemic. Schistosomes enter the body following contact with infested surface water. This particularly affects people engaged in agriculture and fishing or people swimming in contaminated water.
Figure 1.
Egg of schistosoma haematobium showing the characteristic terminal spine. (Magnification factor 500)
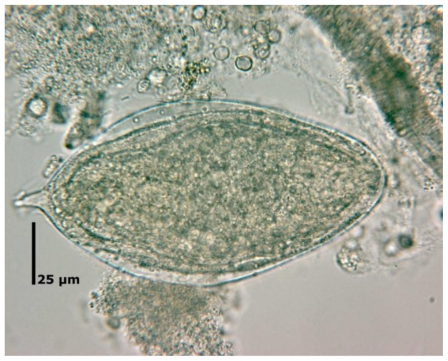
Figure 2.
Egg of schistosoma mansoni with a characteristic shape and a prominent lateral spine near the posterior end. (Magnification factor 500)
Schistosoma life cycle
Infected individuals shed schistosome eggs into fresh water via urine or feces. The larvae hatching from these eggs penetrate aquatic snails, the intermediate hosts, and subsequently undergo several cycles of multiplication. The larval stage infective for humans, the cercariae, is released into the water and will penetrate the skin of the final host. Thus infection is acquired through exposure to water contaminated with cercariae. When invading the skin, the cercariae shed their tail and turn into the juvenile form, schistosomulae. Schistosomulae first migrate to the lungs before reaching the liver by the end of the first week. In the portal system they mature into adult flukes within 6 weeks. Thereafter the flukes descend either to the vesicle beds (in case of S. haematobium) or the mesenteric veins (in case of S. mansoni and S. japonicum). The life span of the adult fluke is a matter of controversy, but it is known to be several years and can be up to 30 years [2].
Most of the eggs laid by the adult fluke are excreted by the host either via the urine or via the feces (depending on S. subtype). Eggs remaining in the host's tissue may lead to the formation of granulomas, esp. in the bladder, bowel, liver or lung, potentially giving rise to clinical symptoms.
Although the lungs are not a final target organ in the life cycle of the schistosome flukes, pulmonary pathology exists. Pulmonary compromise in schistosomiasis is divided into early (acute) and late (chronic) forms.
Acute form
Acute pulmonary schistosomiasis shows a wide variance in clinical presentation, but most patients are asymptomatic. The more severe clinical presentation at initial infection is also called Katayama fever. This previous name of the syndrome may be a misnomer since the absence of fever does not rule out acute pulmonary compromise [3].
The early pulmonary disease at first exposure occurs 3–8 weeks after parasitic penetration and results from allergic reaction due to immune complexes (type 3) and proinflammatory cytokines [4]. The severe manifestations including pulmonary symptoms and febrile illness are thought to be an immunologic reaction following the onset of egg-laying of the female worm and migration of the schistosomula. The eggs are thought to be highly immunogenic.
Patients usually present with nocturnal fever, dry cough, wheezing, shortness of breath, myalgia, abdominal tenderness and headaches [5;6]. Patients with acute pulmonary schistosomiasis present rather with restrictive than obstructive pulmonary compromise, again suggesting interstitial pulmonary involvement [4]. Early pulmonary compromise is mostly limited to nonimmune patients, usually travelers from developed countries that have never been exposed before. The nonspecific clinical presentation makes the disease likely to be misdiagnosed. History of contact with stagnant water 16–84 days before presentation may be a hint to diagnosis. Katayama disease is usually selflimiting and subsides over a few weeks, although a longer clinical course and occasional fatalities have been described. The majority of infected travelers remain asymptomatic, as their infections are light, with few adult worms and therefore small egg burden.
Eosinophilia is recognized in all patients, as well as increases in serum tumor necrosis factor-, interleukin (IL)-1, IL-6, and interferon- levels [4].
Imaging studies show ill-defined small nodular lesions, less commonly a reticulonodular pattern or bilateral diffuse groundglass opacities [5].
Chronic form
Chronic pulmonary disease is a granulomatous reaction to eggs trapped in the pulmonary vasculature, which leads to pulmonary fibrosis, pulmonary hypertension and eventually cor pulmonale [7]. Histologically it is a delayed-type hypersensitivity reaction.
In addition to many pathological features of idiopathic pulmonary arterial hypertension, pulmonary biopsies from patients with chronic pulmonary schistosomiasis show evidence of granulomas, granulomatous lesions and fibrosis. Severe intimal, medial, and adventitial hypertrophy and proliferation of a variety of inflammatory cells occur in the pulmonary vasculature [6]. Embolized ova may obstruct pulmonary arterioles, resulting in obliterative arteriolitis and leading to pulmonary hypertension [8].
Patients with chronic pulmonary schistosomiasis have unspecific clinical and radiographic findings, the latter being related rather to pulmonary hypertension than to infection related features. The majority of reported chronic pulmonary compromises are due to S. mansoni, with a few case reports due to S. hematobium or S. japonicum. The type of chronic disease and typical end-organ involvement depends on the schistosome species involved, with S. mansoni and S. japonicum classically affecting the hepatic portal system and S. hematobium affecting the genitourinary system [9]. Therefore possible associated disease must always be worked up.
The typical symptoms of associated end-organ involvement as diarrhoea, abdominal pain, or rectal bleeding (S. mansoni or S. japonicum) or terminal haematuria (S. haematobium) may be absent or develop very slowly [10]. However, without treatment, patients may develop chronic infection leading to portal hypertension with S. mansoni or hydro-ureter, hydronephrosis or transitional cell carcinoma of the bladder with S. haematobium.
Diagnosis is made by identifying eggs in stool or urine samples or at rectal biopsy. In early pulmonary disease, sensitivity is very low for examination of stool and urine for eggs. At this stage rectal biopsy may help improve the diagnosis. Serologic tests are of limited value as they cannot differentiate past infections from current disease.
The pulmonary eosinophilias are a wide group of disorders characterized by pulmonary infiltrates which are rich in eosinophils. Various parasitic infestations are known to cause eosinophilic pulmonary infiltrations, also known as Loeffler syndrome. Patients may be asymptomatic or have mild symptoms of cough, dyspnea and blood eosinophilia. Chest radiographs are always abnormal.
Since there is a wide variety of causative agents, Loeffler syndrome, also described as simple pulmonary eosinophilia (SPE), is therefore classified as a benign acute eosinophilic pneumonia of unknown cause.
Unlike Loeffler syndrome the term acute eosinophilic pneumonia is reserved for a more severe clinical presentation characterized by marked symptoms of fever, dyspnea and hypoxemia [11]. The progress of the disease is usually rapid but there is good response to steroids. The eosinophil count is typically normal but there are usually high levels of eosinophils in bronchoalveolar lavage, and also a lung biopsy may reveal large numbers of eosinophils in the alveoli [11].
Imaging features of Loeffler syndrome consist of transient and migratory areas of consolidation that typically clear spontaneously within one month [12;13]. The consolidations are typically nonsegmental, may be single or multiple and usually have a predominantly peripheral distribution of the middle and upper lung zones (fig. 6). Single or multiple lung nodules may be depicted with surrounding ground glass opacities and ill-defined margins [11;14;15]. Pathologically there is an alveolar infiltrate which contains eosinophils and giant cells [13]. Small pulmonary nodules ranging from 2 to 15 mm were the most common finding in a case series of acute pulmonary schistosomiasis [8]. The nodules were more frequent within the lung bases and no central or peripheral predominance was present. In the series the smaller nodules were classically well defined, whereas the larger ones were often surrounded by a ground glass halo. This ground-glass halo is thought to be a nonspecific finding and could be related to immune complex deposition or eosinophilic infiltration of the lung parenchyma [9;16].
Figure 6.
56 y old male with angiostrongyloidosis. Chest CT of another patient with parasitic disease. Upper lungs showing ground glass nodules in peripheral distribution represeting eosinophilic accumulation as an unspecific feature in parasitic lung involvement in the context of Loeffler syndrome (Siemens Sensation 16. 120 kV, 120 mAs, 16×1.5 mm collimation, slice thickness 3 mm).
Differential diagnosis for migratory pulmonary opacities includes pulmonary hemorrhage, pulmonary vasculitis, cryptogenic organizing pneumonia, and recurrent aspiration [12]. Air space nodules with surrounding ground glass opacity may be seen in a wide variety of differential diagnosis including both infectious and non-infectious diseases.
Mediastinal or hilar lymphadenopathy seems not to be a characteristic associated feature [8].
For acute eosinophilic pneumonia imaging findings are very variable and nonspecific including air-space consolidation, diffuse bilateral patchy ground glass attenuation, bilateral reticular densities and septal lines and pleural effusions [11;15;17]. Again there is a wide radiologic differential diagnosis including hydrostatic pulmonary edema, respiratory distress syndrome, acute interstitial pneumonia and atypical bacterial or viral pneumonia [15].
TEACHING POINT
Imaging findings in schistosomiasis of the lung are unspecific. Dependent on the immune status of the affected person features related to bronchitis or small nodular lesions with ill defined borders may be observed in the acute setting. In chronic compromised patients unspecific findings related to pulmonary hypertension and eventually fibrosis will be main findings.
Table 1.
Summary table of Schistosomiasis
| Etiology | Schistosomiasis is a helminthic parasitic infection that is endemic mainly in tropical and subtropical regions. |
| Incidence | In tropical countries more than 200 million individuals are affected. |
| Gender ratio | No gender predilection |
| Age predilection | No age predilection. People particularly affected are those engaged in agriculture and fishing or people swimming in contaminated water. |
| Risk factors | Direct contact with contaminated water. |
| Treatment | To date Praziquantel is the only available and effective treatment against all forms of schistosomiasis with few and only transient side effects. |
| Prognosis | Prognosis depends on S. species and end-organ involved. In acute pulmonary schistosomiasis complete recovery is possible. Chronic lung involvement is associated with pulmonary fibrosis, pulmonary hypertension and eventually cor pulmonale. |
| Findings on imaging |
|
Table 2.
Differential diagnosis table of Schistosomiasis on imaging
| Disease | Findings |
|---|---|
| Pulmonary schistosomiasis |
|
| Chronic pulmonary hypertension | Pulmonary artery dilation, right ventricular enlargement |
| Acute eosinophilic pneumonia | Septal lines, ground glass opacities, consolidation. |
| Hypereosinophilic Syndrome | Patchy ground glass opacities and small nodules |
| Simple pulmonary eosinophilia (Löffler Syndrome) | Migratory areas of consolidation, patchy bilateral ground glass opacities |
ABBREVIATIONS
- CT
Computed tomography
- CRP
C-reactive protein
- Fig
Figure
- IL
Interleukin
- S
Schistosome
- SPE
Simple pulmonary eosinophilia
REFERENCES
- 1.World Health Organization. Schistosomiasis Fact Sheet No. 115. Geneva: WHO; 2007. [Google Scholar]
- 2.Schwartz E. Pulmonary schistosomiasis. Clin Chest Med. 2002;23:433–443. doi: 10.1016/s0272-5231(01)00013-2. [DOI] [PubMed] [Google Scholar]
- 3.Leshem E, Maor Y, Meltzer E, Assous M, Schwartz E. Acute schistosomiasis outbreak: clinical features and economic impact. Clin Infect Dis. 2008;47:1499–1506. doi: 10.1086/593191. [DOI] [PubMed] [Google Scholar]
- 4.de Jesus AR, Silva A, Santana LB, et al. Clinical and immunologic evaluation of 31 patients with acute schistosomiasis mansoni. J Infect Dis. 2002;185:98–105. doi: 10.1086/324668. [DOI] [PubMed] [Google Scholar]
- 5.Martinez S, Restrepo CS, Carrillo JA, et al. Thoracic manifestations of tropical parasitic infections: a pictorial review. Radiographics. 2005;25:135–155. doi: 10.1148/rg.251045043. [DOI] [PubMed] [Google Scholar]
- 6.Butrous G, Ghofrani HA, Grimminger F. Pulmonary vascular disease in the developing world. Circulation. 2008;118:1758–1766. doi: 10.1161/CIRCULATIONAHA.107.727289. [DOI] [PubMed] [Google Scholar]
- 7.Phillips JF, Cockrill H, Jorge E, Steiner R. Radiographic evaluation of patients with schistosomiasis. Radiology. 1975;114:31–37. doi: 10.1148/114.1.31. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen LQ, Estrella J, Jett EA, Grunvald EL, Nicholson L, Levin DL. Acute schistosomiasis in nonimmune travelers: chest CT findings in 10 patients. AJR Am J Roentgenol. 2006;186:1300–1303. doi: 10.2214/AJR.05.0213. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz E, Rozenman J, Perelman M. Pulmonary manifestations of early schistosome infection among nonimmune travelers. Am J Med. 2000;109:718–722. doi: 10.1016/s0002-9343(00)00619-7. [DOI] [PubMed] [Google Scholar]
- 10.Doherty JF, Moody AH, Wright SG. Katayama fever: an acute manifestation of schistosomiasis. BMJ. 1996;313:1071–1072. doi: 10.1136/bmj.313.7064.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allen JN, Davis WB. Eosinophilic lung diseases. Am J Respir Crit Care Med. 1994;150:1423–1438. doi: 10.1164/ajrccm.150.5.7952571. [DOI] [PubMed] [Google Scholar]
- 12.Jeong YJ, Kim KI, Seo IJ, et al. Eosinophilic lung diseases: a clinical, radiologic, and pathologic overview. Radiographics. 2007;27:617–637. doi: 10.1148/rg.273065051. [DOI] [PubMed] [Google Scholar]
- 13.Bain GA, Flower CD. Pulmonary eosinophilia. Eur J Radiol. 1996;23:3–8. doi: 10.1016/0720-048x(96)01029-7. [DOI] [PubMed] [Google Scholar]
- 14.Johkoh T, Muller NL, Akira M, et al. Eosinophilic lung diseases: diagnostic accuracy of thin-section CT in 111 patients. Radiology. 2000;216:773–780. doi: 10.1148/radiology.216.3.r00se01773. [DOI] [PubMed] [Google Scholar]
- 15.Kim Y, Lee KS, Choi DC, Primack SL, Im JG. The spectrum of eosinophilic lung disease: radiologic findings. J Comput Assist Tomogr. 1997;21:920–930. doi: 10.1097/00004728-199711000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Hiatt RA, Sotomayor ZR, Sanchez G, Zambrana M, Knight WB. Factors in the pathogenesis of acute schistosomiasis mansoni. J Infect Dis. 1979;139:659–666. doi: 10.1093/infdis/139.6.659. [DOI] [PubMed] [Google Scholar]
- 17.King MA, Pope-Harman AL, Allen JN, Christoforidis GA, Christoforidis AJ. Acute eosinophilic pneumonia: radiologic and clinical features. Radiology. 1997;203:715–719. doi: 10.1148/radiology.203.3.9169693. [DOI] [PubMed] [Google Scholar]