Abstract
The main cause of mortality among patients with breast cancer is the metastatic spread of the primary tumour. The urinary bladder is considered as an unusual site for breast cancer metastasis. A patient has presented with right breast tumour and qualified for surgical treatment. After removal of the mass, an intra-operative and final pathology evaluation indicated breast invasive lobular carcinoma. Adjuvant chemotherapy was given. Years later, an increase of serum CA15-3 tumour marker level was noted and physical examination revealed a lump at the mastectomy scar. A follow-up abdominal ultrasound scan demonstrated thickening of the urinary bladder wall segment, confirmed later by CT scan. A transurethral resection of bladder was performed, reaffirming a neoplastic mass, with histological assessment revealing invasive breast carcinoma. Palliative chemotherapy was given and another follow-up ultrasound scans were unremarkable. The patient is alive today.
Keywords: Breast cancer, breast invasive lobular carcinoma, recurrent lesion, transurethral resection, urinary bladder metastasis
CASE REPORT
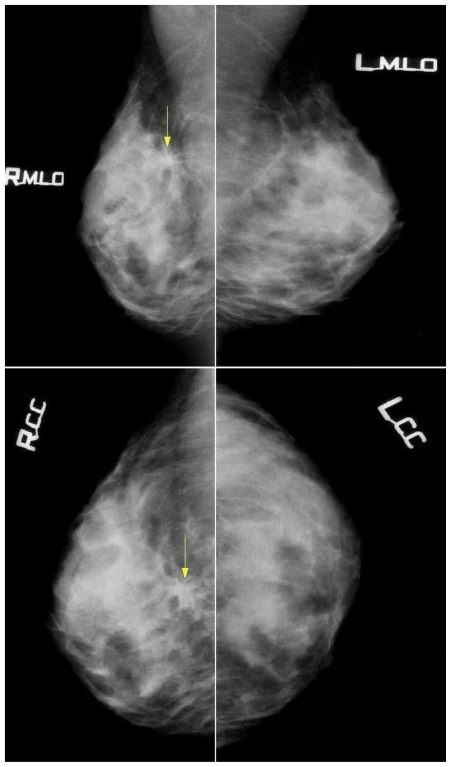
A 47 year old woman has presented with a palpable right breast tumour. Mammography demonstrated an architectural distortion with speculations measuring 30 mm located deep in the chest wall. Mammography of the left breast was unremarkable. No enlarged axillary lymph nodes were present (Fig. 1). Due to dense breast tissue texture and the palpable right breast lump, an ultrasound scan was performed, showing a hypoechogenic, retro-areolar heterogeneous mass, measuring up to 30mm. A guided fine needle aspiration biopsy revealed material suspected of malignancy. After the removal of the mass, an intra-operative pathology evaluation indicated breast invasive lobular carcinoma. Subsequently, right-sided radical mastectomy and lymphadenectomy were performed. The final morphological assessment of the surgical specimen confirmed the presence of bifocal invasive lobular carcinoma. The patient underwent adjuvant chemotherapy with CMF regimen (Cyclophosphamide, Methotrexat, Fluorouracil ).
Figure 1.
47-year-old female patient with invasive lobular breast carcinoma metastatic to the urinary bladder. Mammography images (26 kV, 51mAs for MLO; 26 kV, 48 mAs for CC) in Craniocaudal and Mediolateral Oblique views, demonstrating: In the right breast, at the borderline of the upper quadrants, deep in the chest wall about 7cm from the areola and 4cm from the skin surface - a visible architectural distortion with spiculations radiating from the common center, measuring up to 30mm, with no associated mass and minor area of focal retraction. No microcalcifications were noted. Mammography of the left breast is unremarkable. This finding was later confirmed as Right Breast Invasive Lobular Carcinoma.
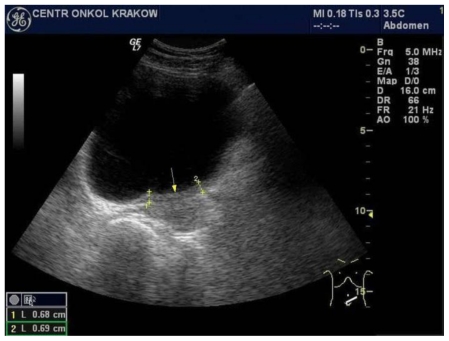
Six years later, an increase of blood serum CA 15-3 tumour marker level was noted (from 21.98 U/ml to 43.2 U/ml) during a routine follow-up. The physical examination revealed a 1cm lesion within the post-mastectomy scar which after excision was diagnosed microscopically as disease recurrence. Despite removal of the recurrent mass, the serum CA 15-3 concentration constantly increased up to 179.7 U/ml. A follow-up abdominal ultrasound scan, performed one month later, demonstrated thickening up to 9mm of a 6cm long left-posterior urinary bladder wall segment (Fig. 2; Fig. 3).
Figure 2.
47-year-old female patient with invasive lobular breast carcinoma metastatic to the urinary bladder. Trans-abdominal grayscale ultrasound image of urinary bladder (GE 4 MHz, Convex Transducer) in transverse plane, presenting: Irregular, isoechoic to bladder tissue, left segment urinary bladder wall thickening, involving left ureter outlet. This finding is consistent with breast cancer metastasis to the bladder.
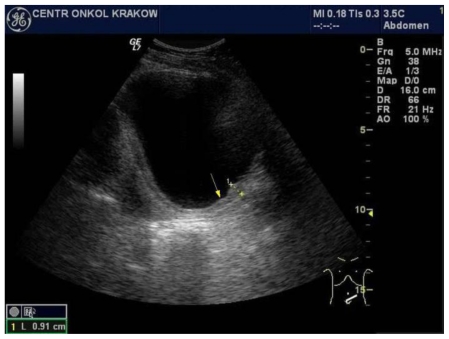
Figure 3.
47-year-old female patient with invasive lobular breast carcinoma metastatic to the urinary bladder. Trans-abdominal grayscale ultrasound image of urinary bladder (GE 4 MHz, Convex Transducer) in sagittal plane, presenting: Irregular, isoechoic to bladder tissue, posterior and inferior segment urinary bladder wall thickening up to 9mm. This finding is consistent with breast cancer metastasis to the bladder.
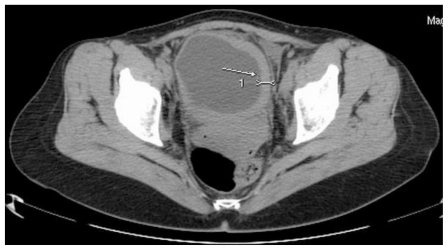
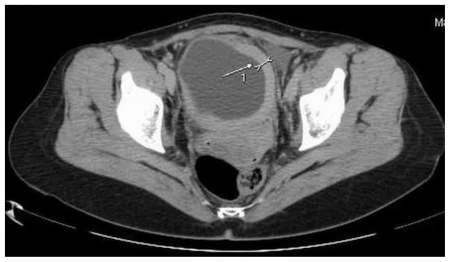
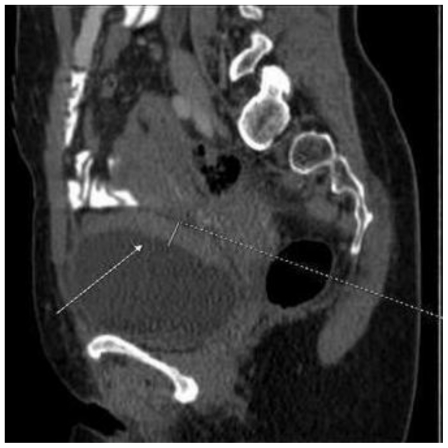
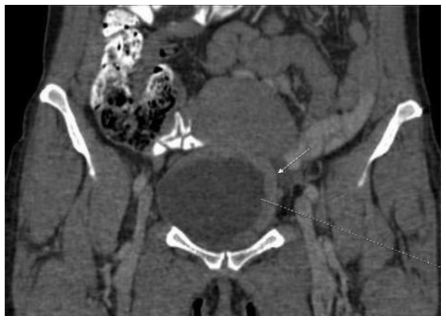
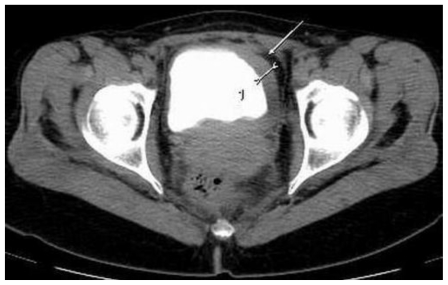
This abnormality was later confirmed by computed tomography scan presenting an irregular bladder wall thickening on 2/3 of its circumference, involving both ureteral outlets. No hydronephrosis was observed at this stage and the patient remained completely asymptomatic (Fig. 4; Fig. 5; Fig. 6; Fig. 7; Fig. 8). As a next step a transurethral resection of bladder (TURB) was performed, reaffirming a neoplastic mass mainly around the left ureteral site, with all lesions being resected to the muscular layer. Histological assessment of the specimen revealed a disperse cancer infiltration of the bladder mucosa.
Figure 4.
47-year-old female patient with invasive lobular breast carcinoma metastatic to the urinary bladder. Non-Enhanced Computed Tomography image (GE LightSpeed 16-Slice Scanner; 290mAs, 120 kV, 5.0mm slice thickness). Axial section demonstrating: Hyperdense segmental urinary bladder wall thickening involving left lateral (white arrow), inferior and right lateral bladder wall. There are no signs of involvement of adjacent structures (uterus and adnexa).
Figure 5.
47-year-old female patient with Invasive Lobular Breast Carcinoma. Contrast Enhanced Computed Tomography image in Arterial Phase. (GE LightSpeed 16-Slice Scanner; 290mAs, 120 kV, 5.0mm slice thickness, Ultravist intravenous contrast agent - dose 60ml administered at a rate of 3ml/sec). Axial section demonstrating: Hyperdense segmental urinary bladder wall thickening involving left lateral (white arrow), inferior and right lateral bladder wall. The contrast enhancement is 82jH (from 38jH before i.v contrast injection). There are no signs of involvement of adjacent structures (uterus and adnexa).
Figure 6.
47-year-old female patient with invasive lobular breast carcinoma metastatic to the urinary bladder. Contrast Enhanced Computed Tomography image of the pelvis in arterial phase (GE LightSpeed 16-Slice Scanner; 290 mAs, 120 kV, 1.2mm slice thickness, Ultravist intravenous contrast agent - dose 60ml administered at a rate of 3ml/sec). Sagittal reconstruction demonstrating: Hyperdense segmental urinary bladder wall thickening up to 13mm, involving posterior (white arrow) and superior bladder wall. There are no signs of adjacent structure involvement.
Figure 7.
47-year-old female patient with invasive lobular breast carcinoma metastatic to the urinary bladder. Contrast Enhanced Computed Tomography image of the pelvis in arterial phase (GE LightSpeed 16-Slice Scanner; 290 mAs, 120 kV, 1.2mm slice thickness, Ultravist intravenous contrast agent - dose 60ml administered at a rate of 3ml/sec). Coronal reconstruction demonstrating: Hyperdense segmental urinary bladder wall thickening up to 13mm, involving left lateral (white arrow) and superior bladder wall. There are no signs of adjacent structure involvement.
Figure 8.
47-year-old female patient with invasive lobular breast carcinoma metastatic to the urinary bladder. Contrast Enhanced Computed Tomography image of the pelvis in delayed excretory phase (GE LightSpeed 16-Slice Scanner; 290mAs, 120 kV, 5.0mm slice thickness, Ultravist intravenous contrast agent - dose 60ml administered at a rate of 3ml/sec). Axial section demonstrating: Hyperdense segmental urinary bladder wall thickening involving left lateral (white arrow) and inferior bladder wall. There are no signs of involvement of adjacent structures (uterus and adnexa).
The neoplasm consisted of cells which cytologically and immunohistochemically corresponded with the features of the primary lobular breast carcinoma (Fig. 9). The patient was qualified for palliative chemotherapy with AT regimen (Adriamycin, Taxotere).
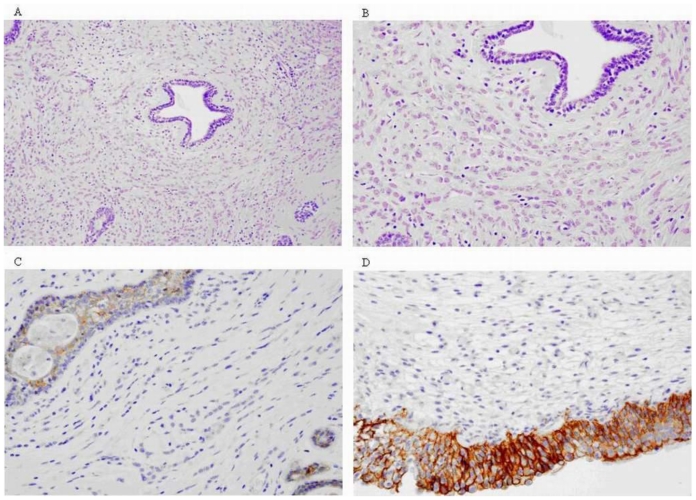
Figure 9.
47-year-old female patient with invasive lobular breast carcinoma metastatic to the urinary bladder. (A-C) Post-TURB Microscopic images presenting typical histological features of Breast Invasive Lobular Carcinoma: (A,B) - Single infiltrating tumour cells forming distinctive indian files and targetoid patterns (HE Stain; Magnification 100x and 200x respectively). (C) - Cancer cells’ lack of cohesion and E-cadherin expression (Immunohistochemical Stain; Magnification 200x). (D) Post-TURB Microscopic image presenting histological picture of metastatic lobular cancer to the urinary bladder: positive reaction to E-cadherin visible in the bladder epithelia only (Immunohistochemical Stain; Magnification 200x).
Another follow-up abdominal ultrasound scan did not present any pathological changes and metastatic workups were negative. The patient is alive today with no signs of recurrent disease.
DISCUSSION
Breast cancer is by far the most common cancer affecting women, with more than one million being diagnosed every year and more than 410,000 dying from the disease worldwide[1,2]. The main cause of mortality among these patients is attributed to metastatic spread of the primary tumour. The urinary bladder is considered as an unusual site of breast cancer metastasis, and such a spread has been reported in the literature very rarely.
The first examples of such atypical neoplastic involvement were discovered through autopsy reports[3,4]. A review of the English literature up to 2008 revealed only 34 cases of living patients with primary breast cancer metastatic to the bladder[5,6]. Breast cancer commonly spreads to the lymph-nodes, lungs, bones, liver and skin, less frequently involving the brain, adrenal glands, ovaries, spleen, pancreas, kidneys, thyroid and heart. On the other hand, the most common primary tumours that metastasize to the urinary bladder are stomach, lung and skin (melanoma)[7,8].
In the majority of reported cases, the urinary bladder lesions from breast cancer were part of systemic dissemination. This observation indicates that bladder metastases are typically late complications of primary disease[9]. Nevertheless the presented case is an unusual example of solitary distant organ metastasis. Some authors have linked the likelihood of developing bladder metastasis with prolonged steroid therapy due to the possible influence of immuno-suppressive effects on carcinoma spreading routes[10].
The metastatic pattern of breast carcinoma may be related to the histologic type of cancer[7]. It has been suggested that infiltrating lobular carcinoma (ILC), which according to multiple sources accounts for 8–14% of all cases of breast primary tumors, has a higher bladder metastatic rate in comparison with infiltrating ductal carcinoma representing 65–85% of breast cancers. It is postulated that breast neoplasm metastasizes to the bladder via retroperitoneal lymphatic involvement, observed more frequently in ILC cases[11,12,13].
In patients with a history of breast cancer, particularly with a lobular subtype, even very subtle lower urinary tract symptoms (LUTS) should be thoroughly evaluated for the possibility of bladder metastasis. Clinical presentation may vary, ranging from the most common painless haematuria, stress and urge incontinence, frequency and nocturia to rare signs like dysuria and back pain[14]. In the described case the patient was completely asymptomatic as far as LUTS are concerned, with first clues of bladder involvement being revealed on abdominal ultrasound scan. Other helpful diagnostic investigations should include cystoscopy and biopsy of any suspicious findings. Feldman et al. stated that assessment should also include further imaging studies, citing a case with a negative cystoscopy picture despite strong evidence of bladder involvement from the patient’s symptoms, ultrasound and CT scans[7]. Extending the range of imaging modalities by MRI scan would help in definitive confirmation of neoplastic bladder infiltration. Moreover, it would help in precise local cancer staging and in the exclusion of adjacent structure involvement (ie uterus and adnexa).
The (18F-FDG) Positron Emission Tomography (PET) imaging in localized bladder tumours has been considered of limited value due to urinary excretion of the tracer, making the evaluation of bladder wall lesions difficult[15]. However, efforts are made to overcome this problem with interventions such as catheterization, bladder irrigation and enforced diuresis. High 18F-FDG concentration in the bladder tumour indicate that PET and particularly PET/CT scanning might be feasible, contributing significantly to the imaging evaluation of patients with bladder neoplasm. Other studies have recently reported 11C-choline as a new tracer with lack of urinary excretion[16].
Patient assessment should also include verification of blood serum CA15-3 level which remains the most sensitive tumour marker in breast cancer follow up. Its main function is to monitor patient response to treatment and watch for cancer recurrence[17].
Awareness of such a metastatic route of breast cancer is important, since haematuria among patients receiving cyclophosphamide, a common cytotoxic drug used in adjuvant settings, may be easily dismissed as cystitis secondary to chemotherapy, leaving the metastatic bladder spread undiagnosed[18].
In conclusion, the evaluation of patients with a history of breast cancer developing urinary symptoms should include the potential presence of urinary bladder metastasis. Investigations undertaken in these patients should therefore include imaging studies (ultrasound and CT followed by MRI scan), cystoscopy and also mapping biopsy whenever bladder involvement is suspected.
TEACHING POINT
In patients with a history of breast cancer, particularly with a lobular subtype, a thorough radiological examination (ultrasound, CT, MRI followed by cystoscopy and mapping biopsy) of even the most unlikely secondary sites should be performed. Very subtle lower urinary tract symptoms may be the first signs of possible bladder metastasis.
Table 1.
Summary of cases with invasive lobular carcinoma metastatic to the urinary bladder (published up to 2008).
| Author, year | Patient umber | Primary tumour subtype | Signs of bladder involvement | Time to bladder involvement | Treatment for bladder metastasis | Follow-up from bladder involvement |
|---|---|---|---|---|---|---|
| Lin et al. (2007) [3] | 1 | IDC | Irregular mucosa and nodular lesions | 3 years | chemotherapy | 2 years |
| Kleinmann et al. (2005) [4] | 2 | IDC | Papillary tumour | 29 months | not stated | not stated |
| Gatti et al. (2005) [5] | 3 | IDC and ILC | Ulcerated mass | 5 years | chemotherapy | 1 year |
| Lawrentschuk et al. (2005)[6] | 4 | ILC | Abnormal lesions | not stated | not stated | not stated |
| 5 | IDC | Abnormal lesions | not stated | not stated | not stated | |
| Feldman et al. (2002) [7] | 6 | ILC | Irregularity | 10 years | radiation | 2 years |
| Silverstein et al. (1997) [8] | 7 | Smooth, raised, hard, immobile lesion | ||||
| 8 | Nodular masses | |||||
| Zagha et al. (2006) [9] | 9 | IDC | Ulcerated mass | 95 months | surgery+hormone | not stated |
| Soon et al. (2004) [14] | 10 | ILC | Poorly compliant bladder | not stated | hormone therapy | not stated |
| Schapira et al. (1980) [18] | 11 | not stated | Teleangiectasia and whitish plaque Large tumour left lateral wall |
5 years | radiation | not stated |
| Ganem et al. (1956) [19] | 12 | |||||
| Perez-Mesa et al. (1965) [20] | 13 | Ulcerating Posterior wall | ||||
| 14 | Infiltrated tumour | |||||
| Pontes et al. (1970) [21] | 15 | not stated | Tumour mass | 1 year | chemotherapy | 2 months |
| 16 | Poorly anaplastic | Cauliflower-like tumour | 4 years | radiation | 1 month | |
| Haid et al. (1980) [22] | 17 | not stated | Irregular sessile tumours | 66 months | not stated | 1 month |
| 18 | not stated | Mucosal nodularities, restricted capacity | 32 months | not stated | 13 months | |
| 19 | not stated | Not performed | 32 months | chemotherapy | 1 month | |
| 20 | not stated | Walnut-sized tumour | 38 months | radiation+chemo | 7 months | |
| Mairy et al. (1982) [23] | 21 | Not performed | ||||
| Rigatti et al. (1991) [24] | 22 | Exophytic mass | ||||
| 23 | Small, elevated, reddened area | |||||
| Berger et et al. (1992) [25] | 24 | IDC | Abnormal lesions | not stated | not stated | not stated |
| 25 | not stated | Abnormal lesions | 6 years | radiation+chemo | 1 month | |
| 26 | ILC | Not performed | 5 years | chemotherapy | 9 months | |
| 27 | ||||||
| Williams et al. (1992) [26] | 28 | not stated | Large tumour | 6 and 30 years | only symptomatic | |
| Schneidaue et al. (1995) [27] | 29 | Diffuse bullous oedema | ||||
| Lucas et al. (1996) [28] | 30 | not stated | Large, hypervascular | 32 months | radiation | 1 month |
| Elia et al. (1999) [29] | 31 | IDC | Few small polyps | 5 years | hormone therapy | 1 year |
| Poulakis et al. (2001) [30] | 32 | Multiple invasive tumours | ||||
| Choudhary et al. (2003) [31] | 33 | Atrophic, haemorrhagic trigone | ||||
| Ryan et al. (2006) [32] | 34 | Rigid, infiltrating mass | ||||
Table 2.
Differential Diagnosis of Bladder Wall Thickening.
| Clinical | Lab | US | CT | MRI | |
|---|---|---|---|---|---|
| Cystitis |
Gender: M<F. Most common symptoms: dysuria, frequency, urgency, hematuria. Etiology: Infectious (Bacterial, Tuberculosis, Viral, Fungal). Noninfectious (mechanical, drug-related, radiation-induced). |
Leukocyturia, bacteriuria, pyuria, hematuria | Hypoechoic edematous bladder wall. | Usually entire bladder wall thickening +/− hypodense wall. Emphysematous cystitis: gas in the bladder wall and/or lumen. | |
| Non-distention and Trabeculation | Indistinguishable by imaging alone | ||||
| Bladder Carcinoma |
Demographics: 50–60 years of age. Gender: M:F = 4:1 5 year survival rate: 82% in all stages combined. Most common symptom: painless hematuria. |
Positive urine dipstick. +/− Micro to normocytic anemia. | Hypo to normoechoic bladder wall thickening or endoluminal soft tissue mass. | Sessile or pedunculated soft tissue mass projecting into the lumen, with similar density to bladder wall. Fine punctate calcifications with tumour. +/− Enlarged metastatic lymph nodes. +/− Extravesical tumour extension. |
T1WI: Tumour has intermediate signal intensity, equal to muscle layer of bladder wall. Infiltration of perivesical fat has high signal intensity. T1 C+: Mild early enhancement in primary, perivesical, nodal or bone invasion. +/− Enlarged metastatic lymph nodes. T2WI: Tumour has intermediate signal intensity, higher than bladder wall and lower than urine. Infiltration of perivesical fat has either low or high signal intensity. |
| Blood Clot | Frequently post-trauma. | Mobile mass, does not cast an acoustical shadow. | Disappear with time, no enhancement | Low signal intensity, no infiltration. | |
| Extrinsic tumour | Rectal, ovarian, vaginal, uterus tumours or fibrosis overlying bladder may simulate bladder wall thickening, mimicking neoplastic involvement. | ||||
ABBREVIATIONS
- AT
Adriamycin / Taxotere (chemotherapy regimen)
- CA15-3
Cancer Antigen 15-3
- CMF
Cyclophosphamide, Methotrexat, Fluorouracil
- CT
Computed Tomography
- 18 F-FDG PET
18 Fluorodeoxyglucose Positron Emission Tomography
- ILS
Infiltrating Lobular Carcinoma.
- LUTS
Lower Urinary Tract Symptoms
- TURB
Transurethral Resection of Bladder
REFERENCES
- 1.Coughlin S, Ekwueme D. Breast Cancer as a Global Health Concern. Cancer Epidemiology. 2009;33(5):315–318. doi: 10.1016/j.canep.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Anderson BO, Shyyan R, Eniu A, et al. Breast Cancer in Limited-Resource Countries: an Overview of the Breast Health Global Initiative 2005 Guidelines. Breast J. 2006;12(1):3–15. doi: 10.1111/j.1075-122X.2006.00199.x. [DOI] [PubMed] [Google Scholar]
- 3.Lin CW, Chen HJ. Urinary Bladder Metastasis from Breast Cancer with Heterogenic Expression of Estrogen and Progesterone Receptors. Journal of Clinical Oncology. 2007;25(27):4308–4310. doi: 10.1200/JCO.2007.12.9379. [DOI] [PubMed] [Google Scholar]
- 4.Kleinmann N, Mor Y, Laufer M, Duvdevani M, Fridman E, Ramon J. A Solitary Metastasis of Breast Cancer to the Urinary Bladder. The Breast Journal. 2005;11(6):497. doi: 10.1111/j.1075-122X.2005.00153.x. [DOI] [PubMed] [Google Scholar]
- 5.Gatti G, Zurrida S, Gilardi D, Bassani G, Rosali dos Santos G, Luini A. Urinary Bladder Metastases from Breast Carcinoma: Review of the Literature Starting from a Clinical Case. Tumori. 2005;(91):283–286. doi: 10.1177/030089160509100317. [DOI] [PubMed] [Google Scholar]
- 6.Lawrentschuk N, Chan Y, Bolton D. Metastatic Breast Cancer to the Bladder. The Breast Journal. 2005 Mar-Apr;11(2):143. doi: 10.1111/j.1075-122X.2005.21427.x. [DOI] [PubMed] [Google Scholar]
- 7.Feldman PA, Madeb R, Naroditsky I, Halachmi S, Nativ O. Metastatic Breast Cancer to the Bladder: a Diagnostic Challenge and Review of the Literature. Urology. 2002;59:138. doi: 10.1016/s0090-4295(01)01489-3. [DOI] [PubMed] [Google Scholar]
- 8.Silverstein LI, Plaine L, Davis JE, Kabakow B. Breast Carcinoma Metastatic to Bladder. Urology. 1997;29:544–547. doi: 10.1016/0090-4295(87)90048-3. [DOI] [PubMed] [Google Scholar]
- 9.Zagha R, Hamawy K. Solitary Breast Cancer Metastasis to the Bladder: An Unusual Occurrence. Urologic Oncology. 2007;25:236–239. doi: 10.1016/j.urolonc.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Bates AW, Baithun SI. Secondary Neoplasms of the Bladder are Histological Mimics of Nontransitional Cell Primary Tumours: Clinicopathological and Histological Features of 282 Cases. Histopathology. 2000;36(1):32–40. doi: 10.1046/j.1365-2559.2000.00797.x. [DOI] [PubMed] [Google Scholar]
- 11.Winston CB, Hadar O, Teitcher JB, et al. Metastatic Lobular Carcinoma of the Breast: Patterns of Spread in the Chest, Abdomen and Pelvis on CT. Am J Roentgenol. 2000;175:795–800. doi: 10.2214/ajr.175.3.1750795. [DOI] [PubMed] [Google Scholar]
- 12.Arpino G, Bardou JV, Clark MG, Elledge MR. Infiltrating Lobular Carcinoma of the Breast: Tumor Characteristics and Clinical Outcome. Breast Cancer Res. 2004;6(3):149–156. doi: 10.1186/bcr767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harake MD, Maxwell AJ, Sulkumar SA. Primary and Metastatic Lobular Carcinoma of the Breast. Clin Radiol. 2001;56(8):621–630. doi: 10.1053/crad.2001.0766. [DOI] [PubMed] [Google Scholar]
- 14.Soon PS, Lynch W, Schwartz P. Breast Cancer Presenting Initially with Urinary Incontinence: a Case of Bladder Metastasis from Breast Cancer. The Breast. 2004 Feb;13(1):69–71. doi: 10.1016/j.breast.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Anios DA, Etchebehere EC, Ramos D, Santos AO, Albertotti C, Camargo EE. 18F-FDG PET/CT delayed images after diuretic for restaging invasive bladder cancer. J Nucl Med. 2007 May;48(5):764–770. doi: 10.2967/jnumed.106.036350. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida S, Nakagomi K, Goto S, Futatsubashi M, Torizuka T. 11C-choline positron emission tomography in bladder cancer: Report of four cases. Int J Urol. 2006 Jun;13(6):829–31. doi: 10.1111/j.1442-2042.2006.01416.x. [DOI] [PubMed] [Google Scholar]
- 17.Given M, Scott M, Mc Grath JP, Given HF. The Predictive of Tumour Markers CA 15-3, TPS and CEA in Breast Cancer Recurrence. The Breast. 2000;9(5):277–280. doi: 10.1054/brst.1999.0154. [DOI] [PubMed] [Google Scholar]
- 18.Schapira D, Asbury R, Wandtke J, Macintosh P. Hematuria Secondary to Perivesical Tumors. New York State Journal of Medicine. 1980;(1):67–69. [PubMed] [Google Scholar]
- 19.Ganem EJ, Batal JT. Secondary Malignant Tumors of the Urinary Bladder Metastatic from Primary Foci in Distant Organs. Journal of Urology. 1956;75:965–972. doi: 10.1016/S0022-5347(17)66911-8. [DOI] [PubMed] [Google Scholar]
- 20.Perez-Mesa C, Pickren JW, Woodruff MN, Mohallatee A. Metastatic Carcinoma of the Urinary Bladder from Primary Tumors in the Mammary Gland of Female Patients. Surg. Gynecol Obstet. 1965;21:813–818. [PubMed] [Google Scholar]
- 21.Pontes JE, Oldford JR. Metastatic Breast Carcinoma to the Bladder. Journal of Urology. 1970;104:839–842. doi: 10.1016/s0022-5347(17)61848-2. [DOI] [PubMed] [Google Scholar]
- 22.Haid M, Ignatoff J, Khandekar JD, Graham J, Holland J. Urinary Bladder Metastases from Breast Carcinoma. Cancer. 1980;46:229–232. doi: 10.1002/1097-0142(19800701)46:1<229::aid-cncr2820460138>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 23.Mairy Y, Opsomer R, Donnez J, Van Cangh PJ. Métastases Véscicales du Cancer du Sein, à propos de Deux Observations. Acta Urol Belg. 1982;50:87–90. [PubMed] [Google Scholar]
- 24.Rigatti P, Broglia L, Montorsi F, et al. Breast Cancer metastasis of the Urinary Bladder. International Journal of Tissue Reactions. 1991;13:159–163. [PubMed] [Google Scholar]
- 25.Berger Y, Nissenblatt M, Salwitz J, Lega B. Bladder Involvement in Metastatic Breast Carcinoma. Journal of Urology. 1992;147:137–139. doi: 10.1016/s0022-5347(17)37161-6. [DOI] [PubMed] [Google Scholar]
- 26.Williams JR, Stott MA, Moisey CU. Bilateral Hydronephrosis Secondary to Breast Carcinoma Metastasising to the Bladder. British Journal of Urology. 1992;69:97–98. doi: 10.1111/j.1464-410x.1992.tb15469.x. [DOI] [PubMed] [Google Scholar]
- 27.Schneidau T, Stroumbakis N, Choudhury M, Eshgi M, Mallouh C. Metastatic Breast Cancer to the Bladder. Int Urol Nephrol. 1995;27:297–300. doi: 10.1007/BF02564765. [DOI] [PubMed] [Google Scholar]
- 28.Lucas B, Simon H, Malhaire JP, Labat JP. Métastase Véscicale d'un Cancer du Sein. Presse Med. 1996;25:732. [PubMed] [Google Scholar]
- 29.Elia G, Steward S, Makhuli N, et al. Metastatic Breast Cancer Diagnosed During a Work-up for Urinary Incontinence: A Case Report. Int Urogynecol J Pelvic Dysfunct. 1999;10:39–42. doi: 10.1007/pl00004013. [DOI] [PubMed] [Google Scholar]
- 30.Poulakis V, Witzsch U, De Vries R, Becht E. Metastatic Breast Carcinoma to the Bladder: 5-year Follow-up. Journal of Urology. 2001;165:905. [PubMed] [Google Scholar]
- 31.Choudhary M, Ahmed AA, Williamson JG. Sole Bladder Metastasis from. Breast Cancer J Obstet Gynaecol. 2003;(23):212. [PubMed] [Google Scholar]
- 32.Ryan PD, Harisinghani M, Lerwill M, Kaufman D. Case Records of the Massachusetts General Hospital: Case 6-2006: A 71-year Old Woman with Urinary Incontinence and a Mass in the Bladder. The New England Journal of Medicine. 2006;354(8):850–856. doi: 10.1056/NEJMcpc059042. [DOI] [PubMed] [Google Scholar]