Abstract
Portal vein aneurysms are a relatively uncommon entity and often an incidental, asymptomatic finding. Recognition of this finding can help to avoid potential confusion with abdominal masses of other etiologies. We would like to present four cases of portal vein aneurysms, and discuss the natural history, imaging findings, and treatment of this condition. One of the cases of portal vein aneurysm presented occurred after liver transplantation, which, to the best of our knowledge, has only been described once in the English-language literature.
Keywords: Portal vein aneurysm, MRI, CT, cirrhosis, ultrasound, liver transplant
CASE SERIES
CASE 1
An 80 year-old woman diagnosed with right lower lobe pulmonary adenocarcinoma was admitted for surgery. Her past medical history was significant for hypertension, diabetes, chronic obstructive pulmonary disease (COPD), aortic stenosis, and remote myocardial infarction. There was no history of alcohol or substance abuse. Physical examination was normal, specifically demonstrating no abdominal distention, organomegaly, peripheral edema, caput medusa or spider angiomas. Laboratory values were also normal.
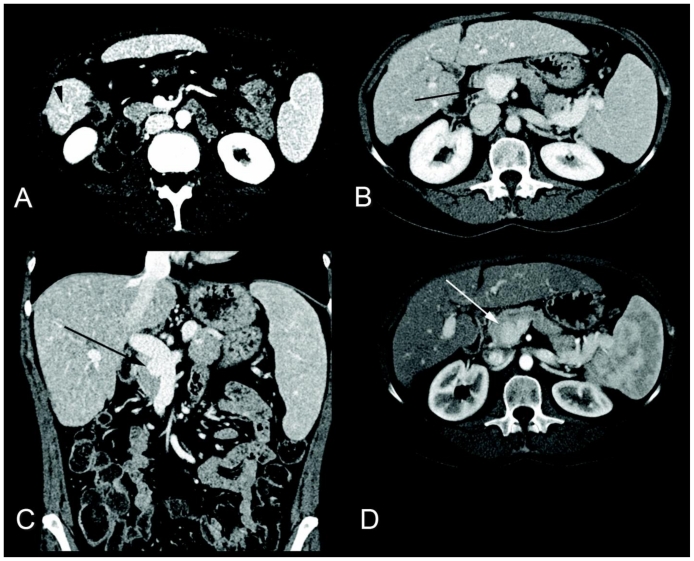
After a posterior right lower lobe segmentectomy, her hospital course was complicated by failure to extubate due to CO2 retention on the basis of her COPD. Her hemoglobin was also decreasing. A computed tomogram (CT) was ordered to exclude intra-abdominal or pelvic hemorrhage. Contrast-enhanced CT of the abdomen and pelvis demonstrated a cirrhotic-appearing liver, and evidence for portal hypertension, as demonstrated by a recanalized paraumbilical vein. There was also out pouching at the bifurcation of the main portal vein, consistent with a portal vein aneurysm (Figure 1). No source of hemorrhage was identified. The patient was eventually found to have a bleeding peptic ulcer, explaining the decreasing hemoglobin. This was treated with a proton pump inhibitor.
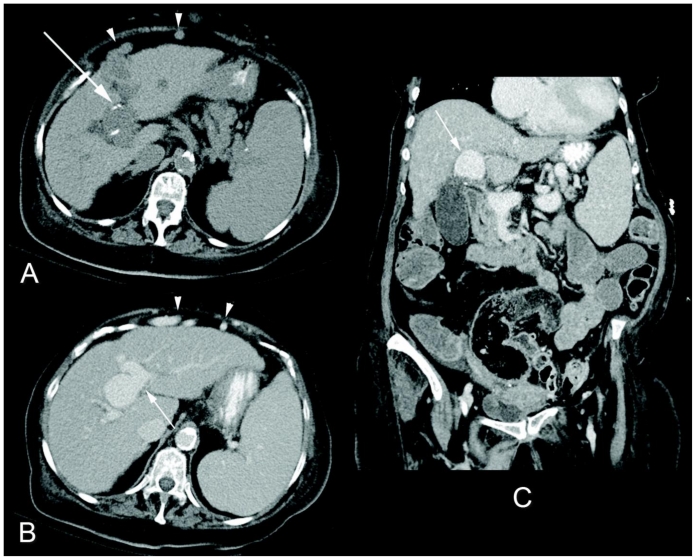
Figure 1.
80 year-old women with an incidentally noted intrahepatic portal vein aneurysm. Nonenhanced axial CT (a), and contrast-enhanced axial (b) and coronal (c) CT images in the portal venous phase (Siemens Sensation 64 detector scanner, 200 mAs, 120 kV, 5 mm slice thickness, 100 mL Isoview 370®, Bracco Diagnostics Inc.) demonstrate a 3.3 × 2.9 × 3.1 cm portal vein aneurysm at the bifurcation of the right and left portal venous branches (b and c, white arrow). Note the mural calcification on the nonenhanced images (a, white arrow). The liver has a nodular contour consistent with cirrhosis, and there is sequela of portal hypertension including splenomegaly and a recanalized umbilical vein (a and b, white arrowheads).
CASE 2
A 23 year-old woman had undergone an orthotopic liver transplantation at the age of 2 for cryptogenic hepatic failure. During routine surveillance, she presented with massive splenomegaly on physical exam, mildly elevated transaminases, and thrombocytopenia.
Evaluation with Doppler ultrasound (Figure 2) showed an enlarged liver transplant containing an anechoic mass with internal turbulent color flow. This mass was in communication with the intrahepatic portal vein. There was non-occlusive thrombus in dilated extrahepatic main portal vein. Splenomegaly was confirmed and there was no biliary dilatation. Magnetic resonance angiography/venography (MRA/MRV) demonstrated the large vascular mass arising from the right portal vein, as well as fusiform dilatation of the left portal vein (Figure 3). Of note, the mass did not enhance during arterial phase imaging, and there was no clear fistulous communication between the mass and the hepatic artery or hepatic veins, which were normal caliber. The spleen measured 21 cm and the splenic and superior mesenteric veins were also diffusely dilated.
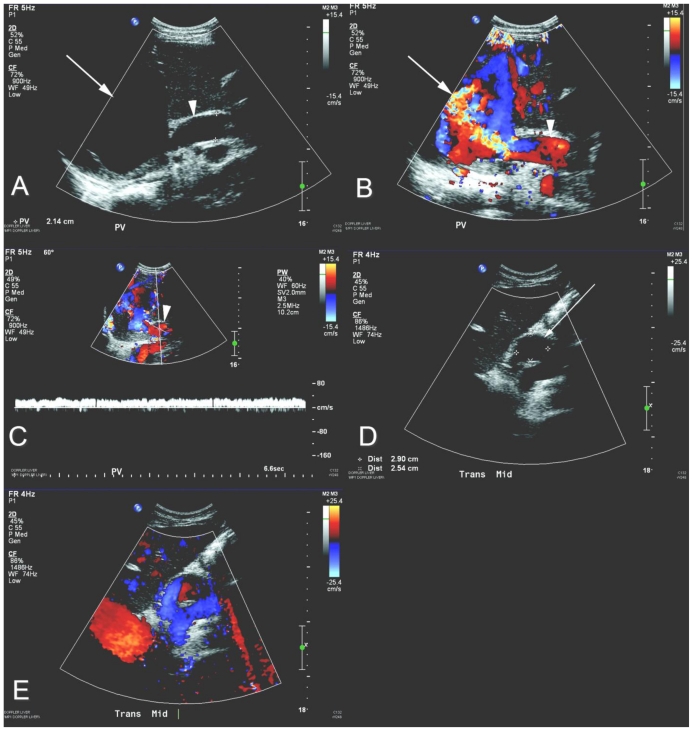
Figure 2.
23 year-old woman status post orthotopic liver transplantation presents with a partially thrombosed portal vein aneurysm. Grayscale (a and d), color Doppler (b and e), and pulsed Doppler (c) ultrasound images of the liver (curved array 5.0–2.0 MHz transducer). There is a 9.1 × 7.8 × 5.1 cm anechoic, intrahepatic structure (a, white arrow) which demonstrates internal color flow (b, white arrow). This structure is contiguous with the main portal vein (a, b, and c, white arrowhead), as evident by the monophasic waveform (c), consistent with a portal vein aneurysm. Mural-adherent intraluminal echogenic substance (d, white arrow) with surrounding color flow (e) is consistent with nonocclusive portal vein thrombus.
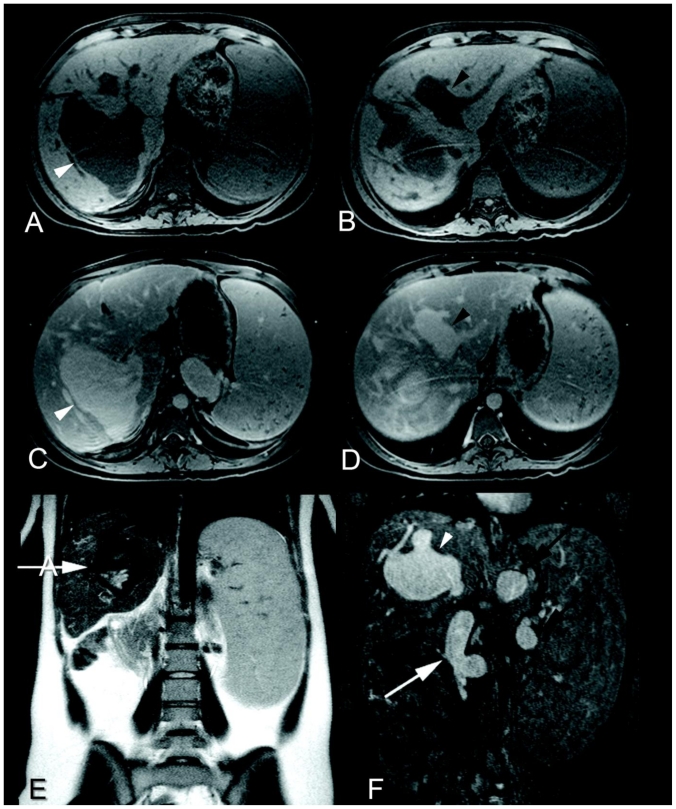
Figure 3.
23 year-old woman status post orthotopic liver transplantation presents with portal venous system aneurysms. Axial fat-saturated T1-weighted imaging without (a and b) and with contrast (c and d) of the liver during the portal venous phase (Siemens Sonata 1.5 Tesla magnet, 3D gradient-recalled-echo sequence, TR/TE 4.8/2.3 msec, 3 mm slice thickness, 15 mL Magnevist®, Bayer). There is 8.9 × 7.5 × 4.9 cm saccular dilatation of the right intrahepatic portal vein (a and c, white arrowheads) and fusiform dilatation of the left intrahepatic portal vein (b and d, black arrowheads), as well as splenomegaly. Coronal T2-weighted imaging (Siemens Sonata 1.5 Tesla magnet, single-shot turbo-spin-echo sequence, TR/TE 6150/128 msec, 5 mm slice thickness) (e) shows a flow void within a dilated intrahepatic portal vein (white arrow). Five minute delayed contrast- enhanced coronal fat-saturated T1-weighted imaging from a MRA/MRV (f) (Siemens Sonata 1.5 Tesla magnet, fast-low-angle- shot sequence, TR/TE 5.9/1.9 msec, 1.5 mm slice thickness, 25 mL Magnevist®, Bayer) clearly illustrates communication of the enhancing structure with the intrahepatic portal vein (f, white arrowhead). The splenic and superior mesenteric veins are also dilated (f, black arrows and white arrow, respectively).
CASE 3
A 51 year-old man presented to the emergency department with decreased visual acuity and a blood pressure of 232/115. The patient’s only significant past medical history was long standing hypertension controlled by a single antihypertensive medication. Pertinent family history included a sister with pheochromocytoma. Besides vision changes and elevated blood pressure, his review of systems and physical examination were unremarkable. The patient had an elevated creatinine. Laboratory analyses, including cardiac enzymes, were otherwise normal. Magnetic resonance imaging (MRI) of the brain demonstrated a right temporal-parietal infarct (Figure 4). Given the patient’s hypertensive crisis, secondary end-organ damage, and family history, a pheochromocytoma was considered. Due to the patient’s mild renal insufficiency, a MRI was performed.
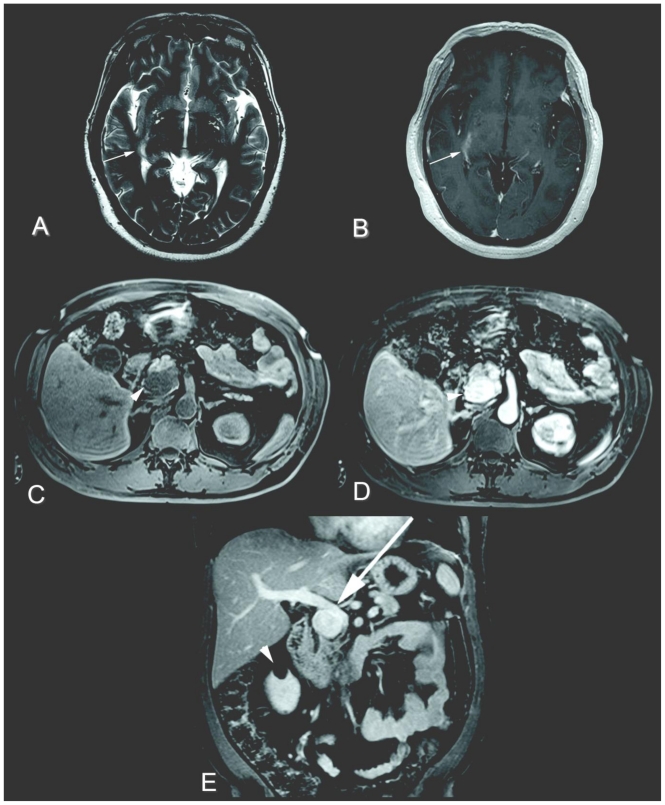
Figure 4.
51 year-old man with a right temporal-parietal infarct and extrahepatic portal vein aneurysm. Axial T2-weighted imaging (a) (Siemens Sonata 1.5 Tesla magnet, fast-spin-echo sequence, TR/TE 6150/128 msec, 5 mm slice thickness) and axial contrast-enhanced T1-weighted imaging (b) (Siemens Sonata 1.5 Tesla magnet, gradient-recalled-echo sequence, TR/TE 588/5 msec, 5 mm slice thickness, 12 mL Magnevist®, Bayer) of the brain. There is a subtle area of increased T2-weighted signal (a, white arrow) with corresponding enhancement (b, white arrow) within the right temporal-parietal region consistent with an infarct. Axial fat-saturated T1-weighted imaging without contrast (c), and axial and coronal fat-saturated T1-weighted imaging with contrast of the abdomen during portal venous phase (d and e) (1.5 Tesla magnet, 3D gradient-recalled-echo sequence, TR/TE 4.8/2.3 msec, 3 mm slice thickness, 12 mL Magnevist®, Bayer). There is a 3.5 × 2.3 × 3.3 cm mass in the region of the pancreatic uncinate process which is hypointense on T1-weighted imaging and enhances (c and d, white arrowheads), eventually found to represent an extrahepatic portal vein aneurysm on endoscopic ultrasound (not shown). Possible mass effect on the portal vein secondary to the aneurysm is suggested (e, white arrow). A right renal cyst is incidentally noted (e, white arrowhead).
Contrast-enhanced MRI of the abdomen and pelvis demonstrated a hyperenhancing lesion centered in the region of the pancreatic uncinate process (Figure 4) No para-aortic masses were noted and the adrenal glands were normal. The differential diagnosis suggested for this hypervascular pancreatic mass was neuroendocrine tumor, metastatic disease or portal vein aneurysm. The latter two differentials were favored less as the patient did not have a known primary malignancy, the liver was not cirrhotic-appearing, nor were there stigmata of portal hypertension. An iodine-131-meta-iodobenzylguanidine (MIBG) scan was negative for pheochromocytoma (not shown). A transabdominal ultrasound with Doppler was suggested for further characterization, specifically to assess for internal vascular flow within this lesion which would be consistent with an aneurysm. However, the gastroenterology service was consulted and elected to perform an endoscopic ultrasound. The endoscopic ultrasound (not shown) demonstrated fusiform dilation of the portal vein at the level of the portal confluence, reportedly measuring 3.5 × 3 cm. The remaining visualized portal vein, splenic vein and superior mesenteric vein were normal caliber, without thrombus (not shown).
The pateint’s remaining workup for secondary hypertension, including a MRA for renal artery stenosis and urine catacholamines/metanephrines, was negative. The patient was therefore thought to have uncontrolled primary hypertension, and his medical regiment and risk factors addressed accordingly.
CASE 4
A 61 year-old female with a history of long-standing liver cirrhosis, secondary to alcohol abuse and hepatitis C virus, had undergone radiofrequency ablation and chemoembolization for multifocal hepatocellular carcinoma. Review of systems and physical examination were normal. Abnormal laboratory values included elevated serum transaminases, thrombocytopenia, and elevated alpha fetoprotein. Surveillance CT (Figure 5) showed nodular arterial hypervascularity at the radiofrequency ablation site, suspicious for recurrent tumor. There was no biliary dilatation. Cirrhosis, splenomegaly, and focal dilatation at the portal-superior mesenteric venous confluence was noted (retrospectively present on multiple CT studies dating back 3 years) (Figure 5). The patient was admitted for liver transplant.
Figure 5.
61 year-old female with recurrent hepatocellular carcinoma and an extrahepatic portal vein aneurysm. Axial (a) contrast-enhanced CT imaging of the abdomen in the late arterial phase (Siemens Sensation 64 slice scanner, 200 mAs, 120 kV, 3 mm slice thickness, 100 mL Isoview 370). There is a 5 × 5 × 7 mm focus of nodular enhancement (a, black arrowhead) at the site of prior radiofrequency ablation consistent with tumor recurrence. Axial (b) and coronal (c) contrast-enhanced CT imaging of the abdomen in the portal venous phase (Siemens Sensation 64 slice scanner, 200 mA, 120 kV, 5 mm slice thickness, 100 mL Isoview 370®, Bracco Diagnostics Inc.). There is a 1.5 × 1.6 × 1.1 cm saccular dilatation of the portal-superior mesenteric venous confluence (b and c, black arrows), consistent with a portal vein aneurysm. Nodularty of the hepatic contour compatible with cirrhosis and mild splenomegaly are noted. Axial (d) contrast-enhanced CT imaging of the abdomen in the late arterial phase (Siemens Definition 20 slice scanner, 200 mAs, 120 kV, 3 mm slice thickness, 100 mL Isoview 370) 3 years prior demonstrates the portal vein aneurysm also present at that time and similar in size (d, white arrow).
DISCUSSION
Portal vein aneurysms are being discovered more frequently with the increase in abdominal imaging. There is a limited understanding of this entity as it is relatively uncommon, and often an incidental finding unrelated to the patient’s initial complaint [1,2]. One author approximates that there have only been 150 cases of portal vein aneurysms reported since the first reported case in 1956 [3,4]. In a retrospective study of 4,186 random patients, the prevalence of portal vein aneurysms was 0.43%, 72% of which were asymptomatic at presentation [5]. All of the patients presented above in our case series were asymptomatic at presentation with regard to their portal vein aneurysms.
Although rare, an aneurysm of the portal vein is the most common site of visceral venous aneurysms. There appears to be no gender predilection and the mean age of diagnosis is 53 years [5]. Formed by the confluence of the superior mesenteric and splenic veins, significant variation in portal vein diameter has been reported, ranging from 0.64 – 1.21 cm in patients without portal hypertension or cirrhosis at autopsy [6]. Maximum main portal vein diameter have been reported ≤1.5 cm in normal patients and ≤1.9 cm in cirrhotic patients [7], with a diameter of >2.0 cm considered aneurysmal [8]. Values for quantifying intrahepatic portal vein branches as aneurysmal are less universally agreed upon. Doust and Pearce consider an intrahepatic portal vein aneurysmal if its diameter measures greater than 0.7 cm in normal patients and 0.85 cm in cirrhotic patients [7].
Some portal vein aneurysms are thought to be congenital. Supporting this hypothesis is the in-utero diagnosis of portal vein aneurysms by sonography [9], as well as the occurrence of the entity in children or healthy young adults [10]. Since the portal vein develops from the vitelline and umbilical veins, it has been proposed that congenital aneurysms of the portal vein result from the absence of regression of the right primitive vitelline vein, forming a diverticulum from the vitelline vein remant. This remnant in turn enlarges overtime, resulting in a saccular portal vein aneurysm later in life with increasing portal vein pressures [11]. Inherent portal vein wall weakness secondary to developmentally defective segments have also been postulated as a congenital factor [12]. Other portal vein aneurysms appear to be acquired. Portal hypertension, related to chronic liver disease such as cirrhosis, is the most common reported etiology of portal vein aneurysms [8], as seen in cases 1 and 4. Since portal vein thrombosis is a common cause of portal hypertension, there is though to be an association between portal vein aneurysms and thrombosis [5,13]. However, aneurysms of the portal vein are also rare in cirrhotics. One study found portal vein aneurysms were associated with cirrhosis in 12% of patients and portal hypertension as the most common etiology in 32% of cases [5]. It has therefore been suggested that both developmental portal vein wall weakness and portal hypertension are needed for portal vein aneurysm development in the setting of cirrhosis [11]. Inflammatory processes, namely pancreatitis, trauma, and invasive malignancy are other reported causes of portal vein aneurysms [11,14].
As seen in cases 3 and 4, aneurysms of the portal vein are most commonly extrahepatic, occurring frequently at sites of bifurcations/confluences [5,8,15]. Clinical symptoms are proportionally related to size, small aneurysms often asymptomatic. Some patients present with nonspecific epigastric pain, or gastrointestinal bleeding, in the clinical setting of portal hypertension. Mass effect resulting in compression of adjacent structures can result in jaundice and duodenal obstruction [5,16]. Because of low portal venous pressures, portal vein aneurysm rupture is rare, occurring up to 2.2% of patients in one study, and increases with portal hypertension [4, 17,18].
On grayscale ultrasound, the typical finding of a portal vein aneurysm is an anechoic mass near the portal hepatis [8]. As seen in case 2, this finding can mimic a cyst which is why color Doppler interrogation is imperative, demonstrating a non-pulsatile, monophasic waveform in the setting of portal vein aneurysm. If thrombosed, the aneurysm may appear echogenic mimicking a solid mass. Ultrasound is a favorable modality to follow and monitor the growth of portal vein aneurysms as it is a relatively inexpensive modality and is without patient radiation exposure. Ultrasound is also useful in differentiating a portal vein aneurysm from a hypervascular pancreatic mass, which can be seen with neuroendocrine tumors or metastatic disease. This was demonstrated in case 3. Portal vein calcifications are best seen with CT and rare, but when present imply thrombosis and portal hypertension [17]. Contrast-enhanced CT and MR are both useful in the setting of equivocal sonographic findings, in particular when differentiating slow flow from thrombosis. The prevalence of portal vein aneurysm thrombosis has been reported as 30%, and when present can cause symptoms such as nausea, abdominal pain, and fever [8,16]. These modalities are also useful in surgical planning given their multiplanar capabilities. The use of portal venography is often restricted to when interventional procedures are necessary [2,8].
The majority of portal vein aneurysms are managed conservatively. Patients with small, asymptomatic aneurysms in the absence of cirrhosis or portal hypertension are followed with imaging, documenting stability in aneurysm size, and monitored for new symptoms [8,11]. In the setting of acute portal vein thrombosis, anticoagulation therapy is recommended resulting in complete or partial recanalization in up to 80% of patients. Patients failing anticoagulation therapy, with extensive thrombus burden involving the splenic and superior mesenteric veins, or with symptoms related to aneurysm mass effect may be refered to percutaneous thrombolysis or thrombectomy [5,19]. If the portal vein aneurysm enlarges or recanalization procedures fail, porto-systemic bypass surgery or aneurysmorrhaphy can be performed. Portocaval, mesocaval, or splenorenal shunts decrease portal pressure and may prevent aneurysm size progression. In the absence of portal hypertension, aneurysmorrhaphy is preferred as it restores portal vein laminar flow while preserving normal hepatic flow, and decreases stasis and resultant thrombosis [8,11].
Portal vein aneurysm in the setting of liver transplantation is even rarer than portal vein aneurysms in native livers. To our knowledge, only one case has been previously reported in the literature [20]. Similar to our above reported case (case 2), the portal vein aneurysm also arose from the donor portal vein and was discovered during surveillance. The patient in that reported case also developed portal vein thrombus and had splenomegaly, but eventually progressed to collateral vessel formation and renal and hepatic failure, resulting in death. In that case report, the authors felt that an intrahepatic portal vein aneurysm after liver transplantation is likely associated with recurrence of portal hypertension, and that intervention, including retransplantation, may be warranted. The referring clinicians in our reported case of portal vein aneurysm after liver transplantation also felt that recurrent portal hypertension was the culprit of both the portal vein aneurysm and splenomegaly, and the patient was referred for splenectomy. It has been hypothesized that splenomegaly results in increased flow within the portal vein, and therefore increased pressure leading to portal vein aneurysm[21]. There are also other reports describing resultant regression of portal venous system aneurysm size, but in non-transplant patients, after appropriate therapy causing decreased splenic size or splenectomy [5,21,22].
TEACHING POINT
Because portal vein aneurysms can mimic solid, cystic and hypervascular abdominal masses, it is important that the radiologist is familiar with their multi-modality appearances. These aneurysms can cause symptoms related thrombosis or mass effect, which is an indication for therapy.
Table 1.
Summary table of portal vein aneurysms
| Etiology | Developmental portal vein wall weakness and portal hypertension. Can also be acquired via inflammatory conditions. |
| Prevalence | 0.43% |
| Gender ratio | Male = Female |
| Age predilection | 50–60 years |
| Risk factors | Chronic liver disease (cirrhosis) and portal vein thrombosis. |
| Treatment | Conservative treatment, anticoagulation, porto-systemic bypass surgery, and aneurysmorrhaphy, depending on signs and symptoms. |
| Prognosis | Good |
| Imaging findings | Ultrasound- anechoic mass with monophasic internal flow with pulsed Doppler. Can appear as solid mass if aneurysm is thrombosed. CT/MR- mass which enhances during portal venous phase imaging and is contiguous with the portal venous system. Look for flow void on MRI |
Table 2.
Differential diagnoses of portal vein aneurysms
| Portal vein aneurysm | Hyperenhancing mass | Hepatic/Pancreatitic Cyst | |
|---|---|---|---|
| Ultrasound | Anechoic mass with monophasic internal flow with pulsed Doppler. | Complex mass, not anechoic. May have internal color flow but should demonstrate arterial, rather than monophasic, blood flow. | Anechoic mass without internal color flow. |
| CT | Mass which enhances during portal venous phase imaging and is contiguous with the portal venous system. | Usually demonstrate avid arterial enhancement. Separate from portal vein. | Nonenhancing and fluid in attenuation. Separate from portal vein. |
| MRI-T2 | Mass which contains a flow void. | Usually hyperintense | Hyperintense (isointense to cerebral spinal fluid) |
| MRI-T1W post contrast | Mass which enhances during portal venous phase imaging and is contiguous with the portal venous system. | Usually demonstrate avid arterial enhancement. Separate from portal vein. | Nonenhancing and hypointense. Separate from portal vein. |
| Scintigraphy | Normal | Neuroendocrine tumors would demononstate uptake on an octreotride scan. Pheochromocytoma would uptake MIBG. | Normal |
ABBREVIATIONS
- COPD
chronic obstructive pulmonary disease
- CT
computed tomogram
- MRA/MRV
Magnetic resonance angiography/venography
- MRI
Magnetic resonance imaging
- MIBG
iodine-131-meta-iodobenzylguanidine
REFERENCES
- 1.Brock PA, Jordan PH, Barth MH, et al. Portal vein aneurysm: a rare but important vascular condition. Surgery. 1997;121:105–108. doi: 10.1016/s0039-6060(97)90190-2. [DOI] [PubMed] [Google Scholar]
- 2.Ozbek SS, Killi MR, Pourbagher MA, et al. Portal venous system aneurysms: report of five cases. J Ultrasound Med. 1999;18:417–422. doi: 10.7863/jum.1999.18.6.417. [DOI] [PubMed] [Google Scholar]
- 3.Mucenic M. Review. Gastroenterol Hepatol. 2007;3:299–300. [PMC free article] [PubMed] [Google Scholar]
- 4.Barzilai R, Kleckner MS. Hemocholecyst following ruptured aneurysm of portal vein. AMA Arch Surg. 1956;72:725–727. doi: 10.1001/archsurg.1956.01270220173023. [DOI] [PubMed] [Google Scholar]
- 5.Koc Z, Oguzkurt L, Ulusan S. Portal venous system aneurysms: imaging, clinical findings, and a possible new etiologic factor. Am J Roentgenol. 2007;189:1023–30. doi: 10.2214/AJR.07.2121. [DOI] [PubMed] [Google Scholar]
- 6.Douglass BE, Baggenstoss AA, Hollingshead WH. The anatomy of the portal vein and its tributaries. Surg Gynecol Obstet. 1950;9:562–576. [PubMed] [Google Scholar]
- 7.Doust B, Pearce J. Gray-scale ultrasonic properties of the normal and inflamed pancreas. Radiology. 1976;20:653–657. doi: 10.1148/120.3.653. [DOI] [PubMed] [Google Scholar]
- 8.Lopez-Machado E, Mallorquin-Jimenez F, Medina-Benitez A, et al. Aneurysms of the portal venous system: ultrasonography and CT findings. Eur J Radiol. 1998;26:210–214. doi: 10.1016/s0720-048x(96)01146-1. [DOI] [PubMed] [Google Scholar]
- 9.Gallagher DM, Leiman S, Hux CH. In utero diagnosis of a portal vein aneurysm. J Ultrasound Med. 1993;21:147–151. doi: 10.1002/jcu.1870210214. [DOI] [PubMed] [Google Scholar]
- 10.Lee HC, Yang YC, Shih SL, et al. Aneurysmal dilatation of the portal vein. J Pediatr Gastroenterol Nutr. 1989;8:387–389. doi: 10.1097/00005176-198904000-00021. [DOI] [PubMed] [Google Scholar]
- 11.Lau H, Chew DK, Belkin M. Extrahepatic portal vein aneurysm: a case report and review of the literature. Cardiovasc Surg. 2002;10:58–61. doi: 10.1016/s0967-2109(01)00104-1. [DOI] [PubMed] [Google Scholar]
- 12.Tolgonay G, Ozbek SS, Oniz H, et al. Regression of splenic vein aneurysm following resolution of splenomegaly. J Clin Ultrasound. 1998;26:98–102. doi: 10.1002/(sici)1097-0096(199802)26:2<98::aid-jcu9>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 13.Wang JT, Zhao HY, Liu YL. Portal vein thrombosis. Hepatobiliary Pancreat Dis Int. 2005;4:515–518. [PubMed] [Google Scholar]
- 14.Yang DM, Yoon MH, Kim HS, et al. CT findings of portal vein aneurysm caused by gastric adenocarcinoma invading the portal vein. Br J Radiol. 2001;74:654–656. doi: 10.1259/bjr.74.883.740654. [DOI] [PubMed] [Google Scholar]
- 15.Fulcher A, Turner M. Aneurysms of the portal vein and superior mesenteric vein. Abdom Imaging. 1997;22:287–292. doi: 10.1007/s002619900191. [DOI] [PubMed] [Google Scholar]
- 16.Gallego C, Velasco M, Marcuello P, et al. Congenital and acquired anomalies of the portal venous system. RadioGraphics. 2002;22:141–159. doi: 10.1148/radiographics.22.1.g02ja08141. [DOI] [PubMed] [Google Scholar]
- 17.Verma V, Cronin DC, Dachman AH. Portal and mesenteric venous calcification in patients with advanced cirrhosis. Am J Roentgenol. 2001;176:489–492. doi: 10.2214/ajr.176.2.1760489. [DOI] [PubMed] [Google Scholar]
- 18.Sfyroeras G, Antoniou G, Drakou AC, et al. Visceral Venous Aneurysms: Clinical Presentation, Natural History and Their Management: A Systematic Review. Eur J Vasc Endovasc Surg. 2009;38:498–505. doi: 10.1016/j.ejvs.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 19.Wang JT, Zhao HY, Liu YL. Portal vein thrombosis. Hepatobiliary Pancreat Dis Int. 2005;4:515–518. [PubMed] [Google Scholar]
- 20.Ferraz-Neto BH, Sakabe D, Buttros DAB, et al. Portal vein aneurysm as late complication of liver transplantation: a case report. Transplant Proc. 2004;36:970–971. doi: 10.1016/j.transproceed.2004.03.105. [DOI] [PubMed] [Google Scholar]
- 21.Tolgonay G, Ozbek SS, Oniz H, et al. Regression of splenic vein aneurysm following resolution of splenomegaly. J Clin Ultrasound. 1998;26:98–102. doi: 10.1002/(sici)1097-0096(199802)26:2<98::aid-jcu9>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 22.Mucenic M, Rocha S, Laudanna A, et al. Treatment by splenectomy of a portal vein aneurysm in hepatosplenic schistosomiasis. Rev Inst Med Trop Sao Paulo. 2002;44:261–4. doi: 10.1590/s0036-46652002000500005. [DOI] [PubMed] [Google Scholar]