Abstract
Angiomyolipomas are the most common mesenchymal renal neoplasms. Two types have been described: (i) sporadic angiomyolipoma and (ii) angiomyolipoma associated with tuberous sclerosis. Giant aneurysm formation is usually noted in angiomyolipomas associated with tuberous sclerosis and is rare in sporadic variety. Tumor diameter and aneurysm diameter have been used as predictors of rupture. We report a rare case of aneurysm formation in a sporadic angiomyolipoma.
Keywords: Angiomyolipoma, Aneurysm, Computed Tomography, Tuberous Sclerosis complex
CASE REPORT
A 42 year old female patient was referred for transabdominal ultrasonography for right flank pain and two episodes of hematuria. The urine microscopic examination showed normochromic normocytic red blood cells.
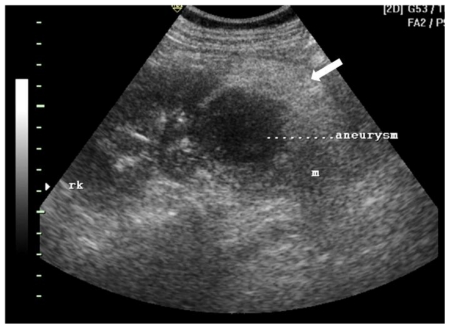
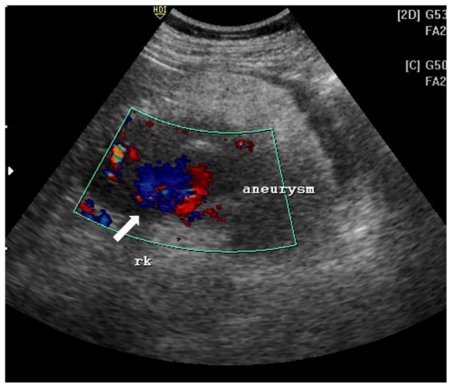
Ultrasonography revealed a 8 × 7 × 8.5 cm heteroechoic mass in the lower pole of the right kidney. The mass had central anechoic region and a thick hyperechoic periphery (Fig. 1a). On color doppler, the central anechoic area showed “swirling” flow pattern due to blood flow both towards and away from the probe (Fig. 1b).
Figure 1a.
42 yr female with aneurysm formation in right renal angiomyolipoma: Transabdominal ultrasonography using 3.5 MHz electrical curvilinear array probe (Philips HDI 4000) of right lumbar region shows 8 × 7 × 8.5 cm heteroechoic mass in lower pole. The mass (m) shows central anechoic region (marked aneurysm) and a thick hyperechoic periphery (arrow)
Figure 1b.
42 yr female with aneurysm formation in right renal angiomyolipoma:
Transabdominal ultrasonography using 3.5 MHz electrical curvilinear array probe (Philips HDI 4000) of right lumbar region with application of color Doppler shows “swirling” flow pattern within the anechoic region of the mass (arrow).
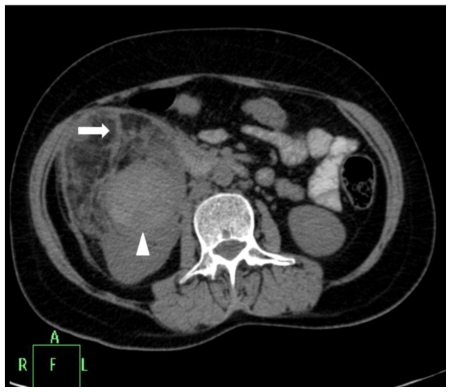
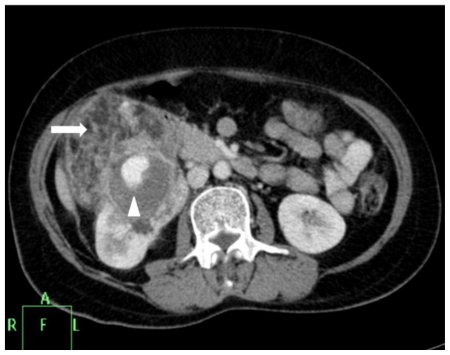
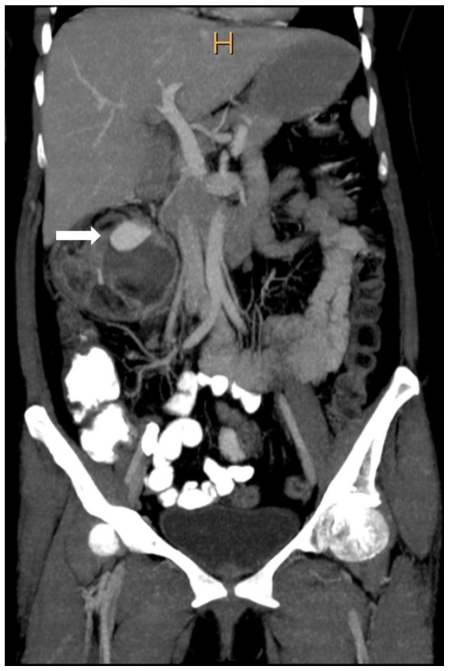
Contrast enhanced CT of abdomen was done for better lesion characterization using Philips Brilliance 40 slice scanner. Images were obtained in both nephrographic (80–120 secs) and excretory (3–5 mins) phase. The scans revealed a right renal mass measuring 9 × 8.4 × 8.5 cm with areas of intermixed soft tissue and fat attenuation (HU= −45 −80) along with thick enhancing septae (Fig. 2a). A well defined round area measuring 2.9 × 2 × 2.6 cm with enhancement similar to arterial enhancement was noted within the mass (Fig. 2b, Fig. 2c). Other abdominal structures were normal. Concern of tuberous sclerosis was raised and a CT head was performed to rule out calcified subependymal nodules and cortical tubers. External stigma of tuberous sclerosis like facial, periungual angiofibromas, Shagreen patches and ash-leaf macules were absent.
Figure 2a.
42 yr female with aneurysm formation in right renal angiomyolipoma: Plain axial CT scan (Philips 40 slice multidetector helical CT, at lumbar level) shows mixed density right renal mass with areas of fat attenuation (arrow). Few areas of hyperdensity were also noted within the mass (arrowhead). Parameters used: kVp - 120; mA - 306; slice thickness - 3mm.
Figure 2b.
42 yr female with aneurysm formation in right renal angiomyolipoma: Contrast enhanced axial CT (Philips 40 slice multidetector helical CT at same level in arterial phase using non-ionic iodinated contrast agent Iohexol 90 ml manually injected) shows moderate heterogenous enhancement of the mass along with septal enhancement (arrow head). Intense vascular equivalent enhancing area is seen within mass consistent with partially thrombosed aneurysm (arrow). Parameters used: kVp - 120; mA - 206; slice thickness - 2mm.
Figure 2c.
42 yr female with aneurysm formation in right renal angiomyolipoma: Coronal reformatted CT in maximum intensity projection (Philips 40 slice multidetector helical CT in arterial phase using non-ionic iodinated contrast agent Iohexol 90 ml manually injected) clearly demonstrates fatty right lower pole renal mass with partially thrombosed aneurysm (arrow). Parameters used: Parameters used: kVp - 120; mA - 206; Maximum Intensity Projection applied.
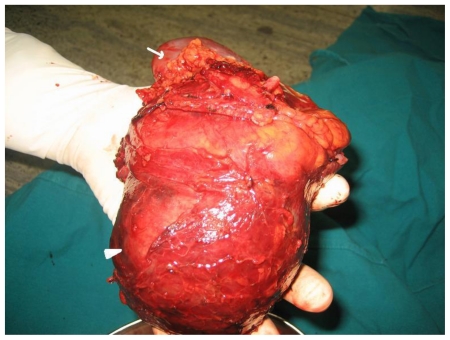
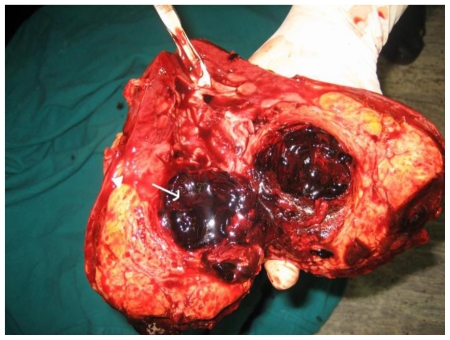
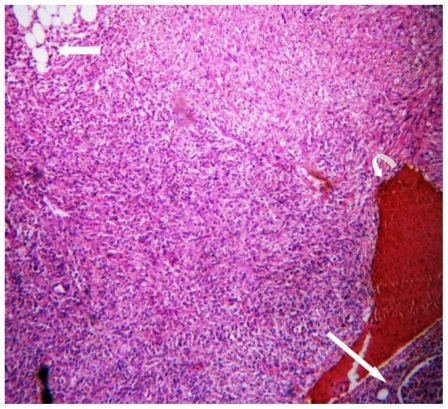
Based on the clinical and imaging findings, diagnosis of “Sporadic angiomyolipoma of the right kidney with giant aneurysm formation” was made. Right radical nephrectomy was performed in this patient using transperitoneal approach (Fig. 3a, Fig. 3b). The dissected specimen showed a large fat intermixed lower pole mass measuring 9.5 × 9 × 8.5 cm. Cut section showed a large aneurysm that corresponded to the imaging appearance. Histopathological specimen revealed admixture of mature adipose tissue, thick walled blood vessels lacking elastic tissue and smooth muscle cells (Fig. 3c, Fig. 3d). The post operative period was uneventful and renal function tests stayed within normal limits.
Figure 3a.
42 yr female with right renal angiomyolipoma: Post operative appearance of the radical nephrectomy specimen showing lower pole mass (arrowhead) and the normal renal parenchyma (arrow).
Figure 3b.
42 yr female with right renal angiomyolipoma: Cut section of the radical nephrectomy specimen showing fat containing 9.5 × 9 × 8 cm mass (arrowhead). Arrow points to aneurysm with thrombosed blood within.
Figure 3c.
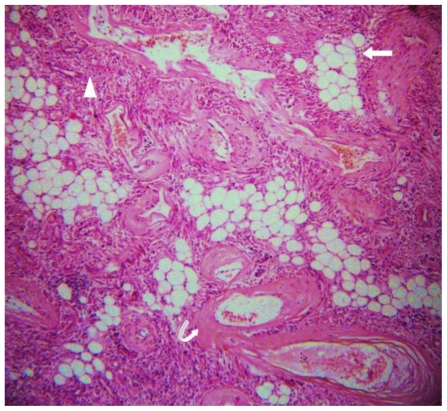
42 yr female with right renal angiomyolipoma: Histopathology with H & E staining (low magnification) showing mature adipose cells (arrow) and areas of hemorrhage (curved arrow). Long straight arrow shows the normal renal tissue.
Figure 3d.
42 yr female with right renal angiomyolipoma: Histopathology with H & E staining (high magnification) showing admixture of mature adipose tissue (arrow), thick walled blood vessels lacking elastic tissue (curved arrow) and smooth muscle cells (arrowhead).
DISCUSSION
Angiomyolipomas (AMLs) are the most common mesenchymal renal neoplasms, and neoplasms of perivascular epithelioid cells’. They are observed in 0.1 to 0.22 % of the general population and are four times more common in female [1]. Two types have been described: sporadic isolated angiomyolipoma and angiomyolipoma associated with tuberous sclerosis. Approximately 80–90% of renal AMLs occur sporadically [2].
Tuberous sclerosis complex (TSC) is a common neurocutaneous disease inherited in an autosomal dominant pattern, although spontaneous mutations are noted. TSC affects many organs, most commonly the brain, skin, eyes, heart, kidneys, and lungs. Renal manifestations of tuberous sclerosis complex include angiomyolipomas and renal cysts [3]. Angiomyolipomas are found in 70–80% of patients with TSC and 20% of individuals with angiomyolipoma have TSC.
Based on histology two distinct subtypes of AMLs are described: classic triphasic and monotypic epithelioid AML [4]. Classical AMLs are benign and composed of a proliferation of blood vessels, smooth muscle and adipose tissue in variable proportions. Epithelioid AMLs are composed of pure epithelioid cells with characteristic absence of adipose cells and abnormal blood vessels [4]. These tumors are said to have malignant potential however, the percentage of cases of renal malignancy due to epithelioid AML is not known [5].
In most cases the diagnosis of classic AML is easily made in presence of detectable amount of fat which appears hyperechoic on sonography equal to renal sinus fat or producing negatively attenuating areas on CT or T1 hyperintensity with signal loss on fat saturated sequences on MR imaging [6, 7]. Small quantities of fat can be detected with confidence using opposed phase MR imaging and frequency-selective fat-suppressed T1-weighted imaging showing signal drop [2]. On opposed phase imaging characteristic sharp black line occurs at the fat-water interface referred to as the “India ink artifact” [8]. The frequency of AMLs with minimal fat content has been reported in as 4.5% [2]. Calcification is very rarely seen in AML with only four cases being reported in literature [6]. Presence of calcification however should suggest an alternate diagnosis especially renal cell carcinoma.
Blood vessels in AML frequently have an angiomatous arrangement, wherein the vessels are tortuous and thick walled. The characteristic absence of elastic tissue in the tumor vessels predisposes the patient to small, saccular aneurysms formation and spontaneous hemorrhage [9, 10]. Hemorrhage can be intraparenchymal, intraperitoneal or retroperitoneal and can be life threatening.
TSC-associated angiomyolipoma tends to be larger, multiple and more likely to cause spontaneous haemorrhage than sporadic forms of angiomyolipoma [11].
In a study by Yamakado et al, incidence of aneurysm formation in AML was 76%. The mean diameter range of aneurysms in his study was 2–7 mm [12]. Large aneurysms are rarely seen in imaging and to our knowledge three cases have been reported two in association with tuberous sclerosis complex [13, 14] and one sporadic [15].
Tumor diameter and aneurysm diameter have been used as a criterion for predictors of rupture and hence prophylactic treatment. Yamakado et al, reported significant differences in mean tumor size (11.4 cm− 5.5 vs 5.0 cm− 3.1) and mean aneurysm size (13.3 mm− 6.2 vs 2.4 mm− 2.9) between the ruptured and unruptured tumor groups. Grossly, tumor size of 4 cm or larger and aneurysm size of 5 mm or larger were used as predictors of rupture with sensitivity and specificity, respectively, being 100% and 38% with the former and 100% and 86% with the latter criterion [12].
Due to the tendency of progressive tumor growth and aneurysmal rupture, Oesterling et al have proposed the following treatment protocol based on size and symptoms of AML. Patients may be followed conservatively with yearly CT scans for those with isolated AML < 4 cm in diameter and 6 monthly scans for those with lesions > 4 cm for assessment of growth. Patients with tuberous sclerosis complex and AML < 4 cm in diameter should be followed by a semiannual CT scan [16].
As a benign lesion that is usually asymptomatic, angiomyolipoma may often not require intervention [11]. Indications for intervention include suspicion of malignancy, spontaneous haemorrhage causing significant symptoms, pain, haematuria and risk of rupture or other complication as the formation of an intrarenal aneurysm [12]. Most of symptomatic angiomyolipoma can be managed by nephron-sparing approaches, including angiographic embolization or partial nephrectomy; nevertheless some selected patients may require complete nephrectomy [11].
TEACHING POINT
Diagnosis of renal angiomyolipoma must prompt us to rule out tuberous sclerosis complex. Aneurysm formation in sporadic tumors is a rare phenomenon. Tumor size of 4 cm or larger and aneurysm size of 5 mm or larger are predictors of rupture.
Table 1.
Differential diagnoses of angiomyolipoma
| MASSES | US | CT | MRI | |
|---|---|---|---|---|
| T1W | T2W | |||
| Angiomyolipoma | Hyperechoic; can be multiple in tuberous sclerosis complex; | Pure fat/intermixed soft tissue with moderate enhancement; no calcification | Iso to Hyperintense with suppression on fat saturated images. | Hyperintense. in out phase imaging for microscopic fat shows signal drop. |
| Lipoma/liposarcoma | Hyperechoic; Lipomas are well encapsulated with pure fat content; intermixed soft tissue seen in liposarcoma; | Lipomas are well encapsulated with pure fat content; intermixed soft tissue with enhancement seen in liposarcoma; +/− calcification; | hyperintense with suppression on fat saturated sequences; hypointense if myxoid/soft tissue component dominates; +/− enhancement | Hyperintense |
Renal cell carcinoma
|
Hypo/iso/hyper echoic on US; | Hypodense soft tissue attenuating; presence of calcification; contrast enhancement less than renal parenchyma; | Hypointense; enhancement less than renal parenchyma | Hyperintense |
| Oncocytomas | Hypo; variable | +/− calcification; show typical spoke wheel pattern of contrast enhancement | Hypo/iso intense | Iso to hyperintense with hypointense central scar. “Spoke wheel” pattern of contrast enhancement. |
| Xanthogranulomatou s pyelonephritis (chronic granulomatous inflammation) | Large renal calculus in >90% cases with hydronephrosis and peripheral hyperechogenicity | Large renal calculus with hydronephrosis and peripheral fat attenuation. If microscopic fat then low attenuating. Usually non functioning kidney. | ‘Great mimicker of malignancy’ peripheral fat hyperintense with suppression on fat saturated sequence. Usually no contrast enhancement | Hyperintense. Calculus is signal void in all sequences |
Table 2.
Summary table for angiomyolipoma
| Etiology | Neoplasms of “perivascular epithelioid cells” |
| Incidence | 0.1 to 0.22 % in general population 20% in tuberous sclerosis complex |
| Gender ratio | F: M = 4:1 |
| Age predilection | Middle aged female |
| Imaging features | US: hyperechoic mass. “to and fro” color pattern on Doppler in aneurysm. CT: mass with fat attenuation. Calcification is absent. Aneurysm seen as a vascular enhancing mass with or without thrombosis in arterial phase MRI: bright mass on T1, T2 with drop in signal on fat suppressed sequences. |
| Complications | Aneurysm formation, rupture, hemodynamic instability, secondary infection |
| Predictors of aneurysm rupture | Aneurysm formation common in Tuberous sclerosis. Mean tumor diameter: 11.4 cm Mean aneurysm diameter: 13.3 mm |
| Risk factors for tumor rupture | Pregnancy, large tumor and aneurysm size (see above). |
| Prognosis | Tumor recurrence very rare in sporadic cases. In TSC, contra lateral tumor recurrence reported. |
| Treatment options | Symptomatic rupture: embolisation/partial/radical nephrectomy. Small/asymptomatic aneurysm: embolisation. |
ABBREVIATIONS
- AML
Angiomyolipoma
- CT
Computed Tomography
- HU
Hounsfield Unit
- MIP
Maximum Intensity Projection
- MRI
Magnetic Resonance Imaging
- TSC
Tuberous Sclerosis Complex
- US
Ultrasonography
REFERENCES
- 1.Fujii Y, Ajima J, Oka K, et al. Benign renal tumors detected among healthy adults by abdominal ultrasonography. Eur Urol. 1995;27:124–127. doi: 10.1159/000475142. [DOI] [PubMed] [Google Scholar]
- 2.Casper Keith A, Donnelly Lane F, Chen Bin, Bissler John J. Tuberous Sclerosis Complex: Renal imaging findings. Radiology. 2002;225:451–456. doi: 10.1148/radiol.2252011584. [DOI] [PubMed] [Google Scholar]
- 3.Evans JC, Curtis J. The radiological appearances of tuberous sclerosis. Br J Radiol. 2000;73:865, 91–98. doi: 10.1259/bjr.73.865.10721329. [DOI] [PubMed] [Google Scholar]
- 4.Pea M, Bonetti F, Martignoni G, et al. Apparent renal cell carcinomas in tuberous sclerosis are heterogeneous: the identification of malignant epithelioid angiomyolipoma. Am J Surg Pathol. 1998;22:80–187. doi: 10.1097/00000478-199802000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Bharwani N, Christmas TJ, Jameson C, Moat N, Sohaib SA. Epithelioid angiomyolipoma: imaging appearances. Br J Radiol. 2009;82:249–252. doi: 10.1259/bjr/27259024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merran S, Vieillefond A, Peyromaure M, Dupuy C. Renal angiomyolipoma with calcification: CT-pathology correlation. Br J Radiol. 2004;77:782–783. doi: 10.1259/bjr/33776173. [DOI] [PubMed] [Google Scholar]
- 7.Maizlin Z, Gottlieb P, Corat-Simon Y, Strauss S. Various Appearances of Multiple Angiomyolipomas in the Same Kidney in a Patient Without Tuberous Sclerosis. J Ultrasound Med. 2002;21:211–213. doi: 10.7863/jum.2002.21.2.211. [DOI] [PubMed] [Google Scholar]
- 8.Israel GM, Hindman N, Hecht E, Krinsky G. The Use of Opposed-Phase Chemical Shift MRI in the Diagnosis of Renal Angiomyolipomas. AJR Am J Roentgenol. 2005;184:1868–1872. doi: 10.2214/ajr.184.6.01841868. [DOI] [PubMed] [Google Scholar]
- 9.Gaikwad AB, Madathil MB, Kothari AS. Giant renal angiomyolipoma with fatal hemorrhage due to a large pseudoaneurysm. J Clin Ultrasound. 2007;36:174–176. doi: 10.1002/jcu.20387. [DOI] [PubMed] [Google Scholar]
- 10.Clark RE, Palubinskas AJ. The angiographic spectrum of renal Hamartoma. AJR Am J Roentgenol. 1972;114( 4):715. doi: 10.2214/ajr.114.4.715. [DOI] [PubMed] [Google Scholar]
- 11.Nelson CP, Sanda MG. Contemporary diagnosis and management of renal angiomyolipoma. J Urol. 2002;168:1315–1325. doi: 10.1016/S0022-5347(05)64440-0. [DOI] [PubMed] [Google Scholar]
- 12.Yamakado K, Tanaka N, Nakagawa T, Kobayashi S, Yanagawa M. Renal Angiomyolipoma: Relationships between Tumor size, Aneurysm Formation, and Rupture. Radiology. 2002;225:78–82. doi: 10.1148/radiol.2251011477. [DOI] [PubMed] [Google Scholar]
- 13.Kunzi T, Walther F, Marti HP, Frey F, Vogt B. Intrarenal arterial aneurysms with haematuria in a patient with tuberous sclerosis complex. Nephrol Dial Transplant. 2005;20:2268–2270. doi: 10.1093/ndt/gfh935. [DOI] [PubMed] [Google Scholar]
- 14.Choh NA, Choh SA, Yousuf R, Jehangir M. A giant aneurysm arising from renal angiomyolipoma in tuberous sclerosis. Arch Dis Child. 2009;94:47–48. doi: 10.1136/adc.2008.143610. [DOI] [PubMed] [Google Scholar]
- 15.Lapeyre M, Correas JM, Ortonne N, Balleyguier C, Helenon O. Color-Flow Doppler Sonography of Pseudoaneurysms in Patients with Bleeding Renal Angiomyolipoma. AJR Am J Roentgenol. 2002;179:145–147. doi: 10.2214/ajr.179.1.1790145. [DOI] [PubMed] [Google Scholar]
- 16.Oesterling JE, Fishman EK, Goldman SM, et al. The management of renal angiomyolipoma. J Urol. 1986;135:1121–1124. doi: 10.1016/s0022-5347(17)46013-7. [DOI] [PubMed] [Google Scholar]