Abstract
We present a case in which the undiagnosed condition of sarcoidosis complicated the staging of bilateral, subtype-discordant renal cell carcinoma. Initially thought to have metastatic renal cell carcinoma based on computed tomography imaging and referred for immunotherapy, a positron emission tomography/computed tomography scan demonstrated different levels of radiotracer activity in the primary site and the presumed pulmonary metastatic sites. The patient underwent bilateral partial nephrectomies and was ultimately diagnosed with stage T1 bilateral renal cell carcinoma and sarcoidosis. This case highlights the need to consider concurrent medical conditions that can lead to false positive results when evaluating for metastatic disease with imaging studies as well as the importance of evaluating the levels of radiotracer activity between different sites.
Keywords: renal cell carcinoma, bilateral, sarcoidosis, positron emission tomography, metastatic, computed tomography
CASE REPORT
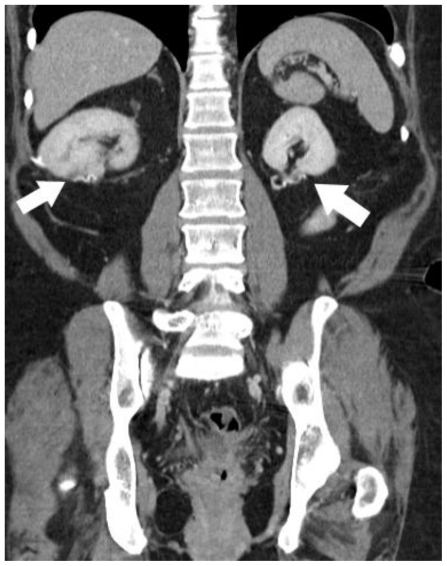
A 48 year-old Caucasian female presented to an urgent care clinic with a three-day history of left lower quadrant abdominal pain, which was diagnosed as diverticulitis. Her past medical history was significant for hypertension, chronic back pain, and uterine fibroids for which she had undergone an abdominal hysterectomy. She denied any family history of malignancy. A computed tomography (CT) scan at the time of presentation incidentally demonstrated solid renal masses in both kidneys, measuring 4 × 4 × 5 centimeters on the right and 3 × 3 × 3.5 centimeters on the left as well as bilateral pulmonary nodules (figures 1 and 2). Her creatinine was 0.6. A CT-guided needle biopsy of the right lower pole renal mass demonstrated renal cell carcinoma (RCC) (figure 3). A biopsy of the pulmonary nodules was attempted but was unsuccessful in obtaining tissue adequate for diagnosis.
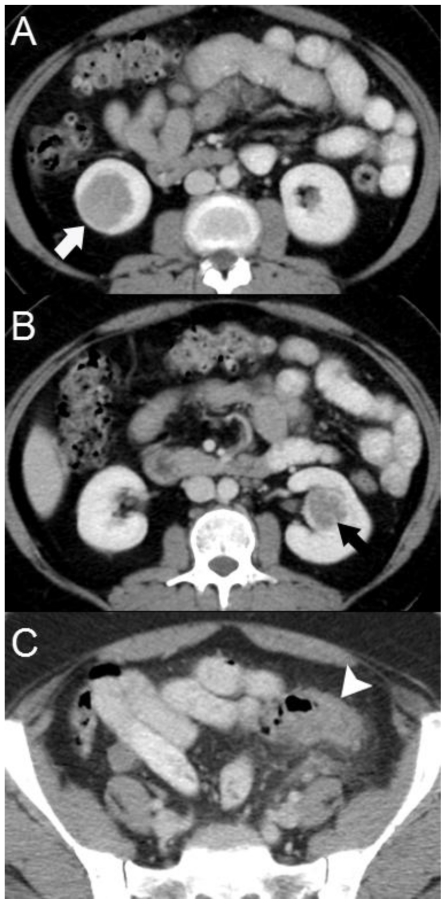
Figure 1.
Computed Tomography. 48 year-old female with bilateral renal cell carcinoma, sarcoidosis and diverticulitis. Axial images of a contrast-enhanced CT of the abdomen and pelvis demonstrate endophytic, enhancing renal masses without evidence of extracapsular extension or renal vein involvement in the lower pole of the right kidney (1a, white arrow) and the left interpolar kidney (1b, black arrow). Thickening of the sigmoid colon wall & diverticulosis is evident in the pelvis (1c, arrowhead). (CT, mA 380, kV 120, slice thickness 5 mm, 150 ml Isovue-300 as IV contrast and 900 ml of barium suspension as oral contrast)
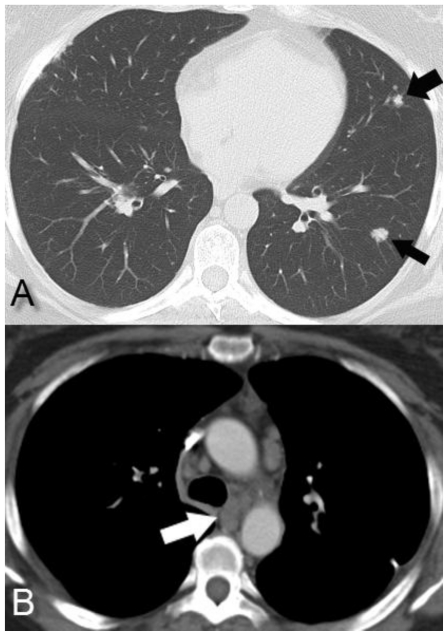
Figure 2.
Computed Tomography. 48 year-old female with bilateral renal cell carcinoma, sarcoidosis and diverticulitis. Axial contrast-enhanced CT of the chest demonstrates bilateral pulmonary nodules (2a, black arrows) and mediastinal lymphadenopathy (2b, white arrow) suspicious for metastatic disease. (CT, mA 200, kV 120, slice thickness 5 mm, 80 ml Isovue-370 as IV contrast)
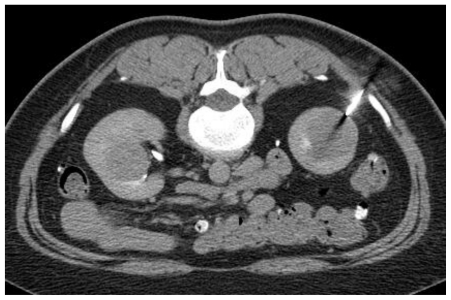
Figure 3.
Computed Tomography. 48 year-old female with bilateral renal cell carcinoma (RCC), sarcoidosis and diverticulitis. Percutaneous renal biopsy with an 18-gauge core biopsy needle was performed on the mass in the lower pole of the right kidney in the prone position. Pathology demonstrated RCC. (CT, mA 170, kV 120, slice thickness 5 mm, 50 ml Isovue-370 as IV contrast)
After referral to our tertiary referral center for consideration of interleukin-2 (IL-2) immunotherapy in the setting of presumed metastatic RCC, a fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT scan was obtained. This demonstrated increased FDG activity in nodal stations from the mediastinum to the pelvis (maximum standardized uptake value [maxSUV] range of 6.1 to 9.3), bilateral pulmonary nodules (maxSUV 3.2), and multiple bony sites (maxSUV range of 8.8 to 12.6) (figure 4, 5). However, neither renal mass demonstrated significant metabolic activity. As a point of reference, the blood pool activity was 2.83.
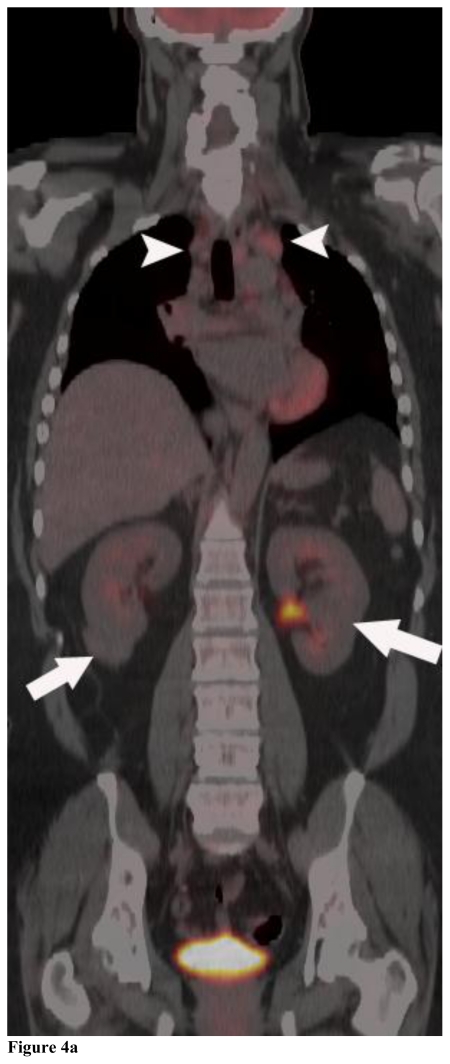
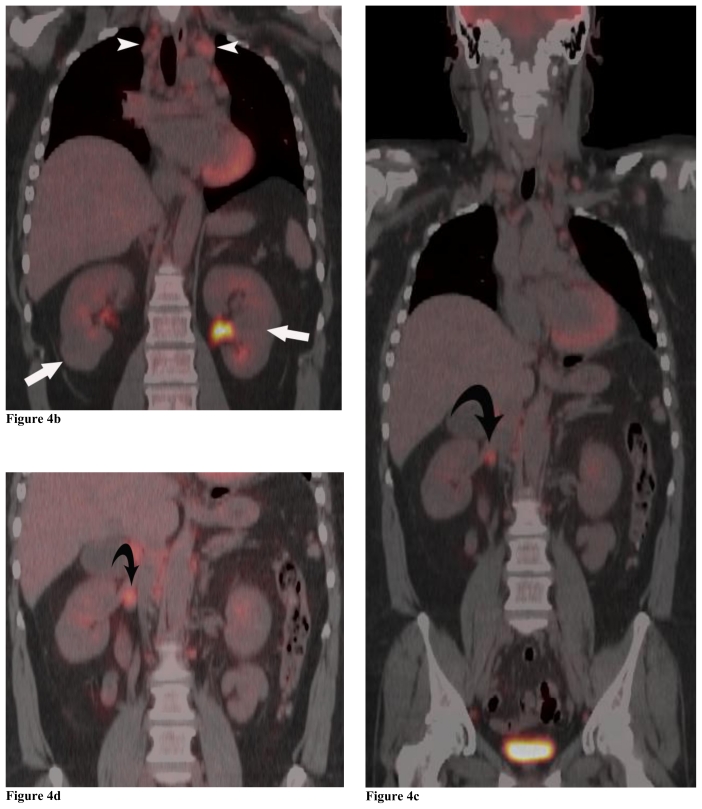
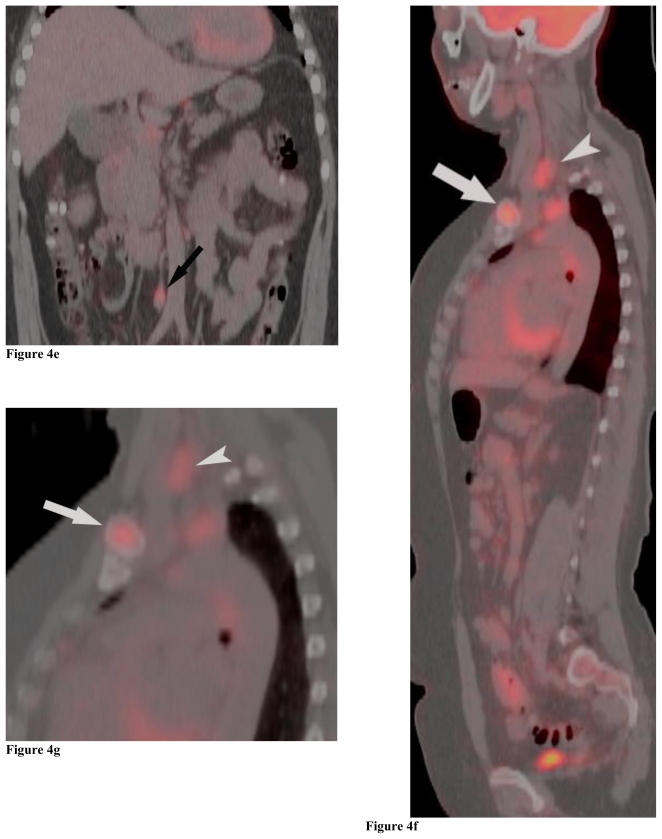
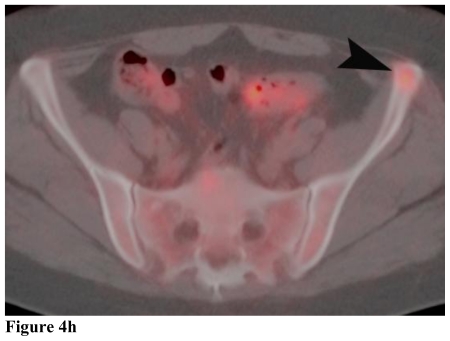
Figure 4.
Positron Emission Tomography/Computed Tomography. 48 year-old female with bilateral renal cell carcinoma, sarcoidosis and diverticulitis. Coronal images demonstrate bilateral renal masses and mediastinal lymphadenopathy (4a, 4b). Note the lack of increased metabolic activity in the renal masses (4a, 4b; white arrows) whereas the mediastinal lymphadenopathy demonstrates a maximum standardized uptake value (maxSUV) of 8.3 in the left hilar region and 6.1 in the right hilar region (4a, 4b; white arrowheads). Different levels of metabolic activity are also noted between the right renal mass and a paracaval lymph node (4c, 4d; curved black arrow) and an aortocaval lymph node (4e, black arrow), which had a maxSUV of 9.1. Sagittal images demonstrate increased metabolic activity in the left clavicular head with a maxSUV of 12.6 (4f, 4g; grey arrow) and a lymph node posterior to the thyroid with a maxSUV of 7.7 (4f, 4g; grey arrowhead). Increased metabolic activity in the left iliac wing is demonstrated on the axial image with a maxSUV of 8.8 (4h, black arrowhead). (PET, 15.4 mCi 18-F FDG injected as IV radiotracer 84 minutes prior to scan. CT, mA 200, kV 120, slice thickness 5 mm)
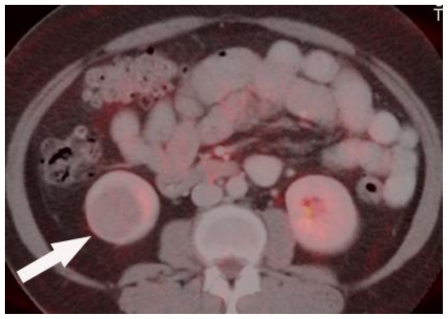
Figure 5.
Positron Emission Tomography/Computed Tomography. 48 year-old female with bilateral renal cell carcinoma, sarcoidosis and diverticulitis. Fused PET/CT axial image demonstrates the right lower pole hypometabolic renal mass. (FDG-PET using 7.5 mCi 18-F FDG followed by 15 mCi water bolus fused with CT, mA 380, kV 120, slice thickness 5 mm, 150 ml Isovue-300 as IV contrast and 900 ml of barium suspension as oral contrast)
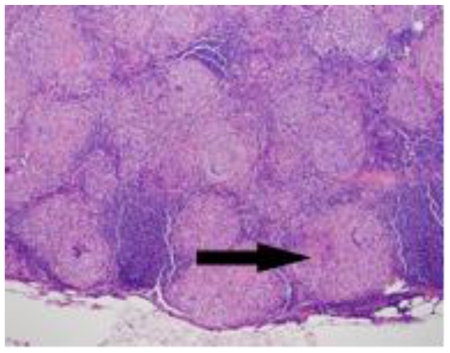
The difference in metabolic activity between the primary site of disease and the presumed metastatic disease suggested the possibility of two separate disease processes. Because of this possibility as well as the lack of a pathologic diagnosis of metastatic disease, the patient underwent an open right partial nephrectomy and right paracaval lymph node dissection through a flank incision. The excision plane of the partial nephrectomy entered the collecting system, which was closed with a bolster. There were no post-operative complications and her creatinine was 0.6 one month after surgery. Pathology of the renal mass demonstrated a stage T1b, chromophobe cell renal carcinoma, which distorted the adjacent calyx but did not invade the calyceal urothelium (figure 6). Pathologic margins were negative. The excised lymph node contained multiple non-caseating granulomata (figure 7).
Figure 6.
48 year-old female with bilateral renal cell carcinoma, sarcoidosis and diverticulitis. Hematoxylin & eosin stain of the right partial nephrectomy surgical specimen demonstrates polygonal cells with abundant granular eosinophilic cytoplasm (white arrow) and pleomorphic nuclei (black arrow) consistent with chromophobe cell carcinoma, 40x magnification.
Figure 7.
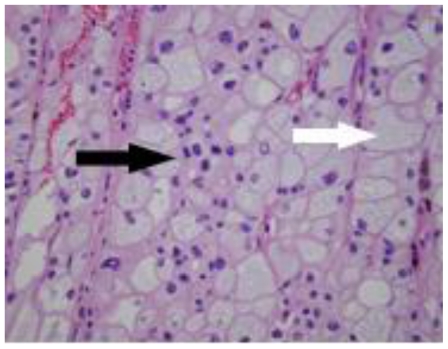
48 year-old female with bilateral renal cell carcinoma, sarcoidosis and diverticulitis. Hematoxylin & eosin stain of the right paracaval lymphadenectomy surgical specimen demonstrates non-caseating granulomata (arrow) without evidence of malignancy, 10x magnification.
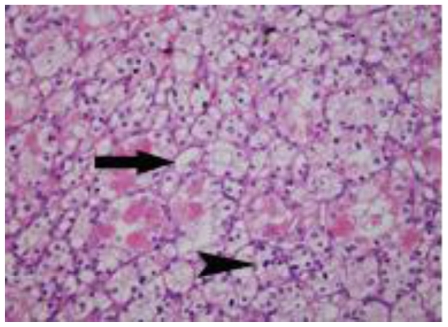
Three months later, an open left heminephrectomy was performed through a flank incision for a stage T1a, clear cell renal cell carcinoma (figure 8). The defect in the collecting system was closed primarily using a bolster, and, again, the pathology specimen demonstrated negative surgical margins and a tumor localized to the renal cortex. Her post-operative course was uneventful. Finally, a biopsy of several lung nodules was performed at an outside hospital; the biopsy demonstrated non-caseating granulomata and she was diagnosed with sarcoidosis.
Figure 8.
48 year-old female with bilateral renal cell carcinoma, sarcoidosis and diverticulitis. Hematoxylin & eosin stain of the left partial nephrectomy surgical specimen demonstrates cells with clear cytoplasm (arrow) and slightly irregular nuclei (arrowhead) consistent with clear cell carcinoma, 40x magnification.
A repeat CT scan of the chest, abdomen, and pelvis performed 12 months after the last surgery demonstrated stable pulmonary nodules and lymphadenopathy. Postoperative changes were seen in the kidneys, without evidence of residual or recurrent RCC (figure 9). Her creatinine was 1.22.
Figure 9.
Computed Tomography. 48 year-old female with bilateral renal cell carcinoma, sarcoidosis and diverticulitis. Coronal images obtained 1 year after the second partial nephrectomy demonstrate post-operative changes in both kidneys (arrows) with no evidence of recurrence and decreased retroperitoneal lymphadenopathy. (CT, mA 290, kV 120, slice thickness 5 mm with 3 mm coronal reconstruction, 140 ml of Isovue-300 as IV contrast and 900 ml of barium suspension as oral contrast)
DISCUSSION
RCC accounts for 2–3% of all adult malignant tumors [1]. Currently, over half of all RCC’s are diagnosed incidentally on imaging studies obtained for unrelated reasons [1]. Two to four percent of non-familial RCC will have bilateral involvement [1]. In patients with subtype-concordant, bilateral RCC, cancer specific and distant metastasis-free survival is similar to that in patients with unilateral RCC; however, less is known regarding subtype-discordant RCC [2].
Metastatic disease is present in about one third of patients with RCC at the time of diagnosis [1]. Despite therapeutic advances such as immunotherapy and tyrosine kinase inhibitors, long-term survival in patients with metastatic RCC is relatively rare [1].
The histologic subtypes of RCC include clear, papillary, chromophobe, and collecting duct carcinoma. The most common subtype is clear cell, making up 70–80% of all RCC’s [1]. Chromophobe cell carcinoma accounts for only 3–5% of all RCC’s and appears to carry a better prognosis than clear cell RCC with a five-year survival between 92–94% [1, 3, 4]. The pathologic stage of RCC at the time of presentation has been demonstrated to correlate most closely with survival rates [5].
In this case, the undiagnosed condition of sarcoidosis complicated the staging of bilateral RCC. Sarcoidosis is usually suspected based on the clinical presentation and radiographic evidence, such as mediastinal lymphadenopathy; the diagnosis is confirmed by the presence of non-caseating granulomata on biopsy specimens. Approximately 30–60% of cases, however, are diagnosed based on radiologic findings in an asymptomatic patient [6].
The precise role of FDG-PET in the diagnosis and staging of RCC remains somewhat elusive, with the highest utility in detecting visceral, lymph node and bony disease [7]. This case illustrates the fact that coexisting conditions can result in false positive results. The PET/CT for this patient showed different levels of radiotracer activity in the primary site and the presumed metastatic site. It was only after the functional information provided by the metabolic imaging was added to the diagnostic evaluation that additional testing became warranted.
Percutaneous renal biopsies are generally not used in the diagnostic workup of a suspected malignant renal mass because of the possibility of sampling error, difficulty distinguishing oncocytoma from RCC, and because biopsy results do not routinely give enough additional information beyond that provided by imaging studies to alter the course of management [8]. There are exceptions to this general rule; patients suspected of having a disease process that would be managed medically (lymphoma, renal abscess, or metastatic renal cell carcinoma) are good candidates for a percutaneous biopsy. Risks of a percutaneous renal biopsy include bleeding (which rarely requires aggressive intervention such as transfusion or embolization), infection, and arteriovenous fistulas. Tumor tract seeding has been reported but is very rare [8, 9]; the reported incidence of tumor tract seeding for percutaneous biopsy of abdominal tumors is less than 0.01% [10]. In this case, the indication for biopsy was to guide medical management in a patient with presumptive metastatic disease based on imaging.
In addition to the presence of sarcoidosis complicating the staging of this patient’s disease, the case is interesting because of the presence of two different subtypes of renal cell carcinoma. Bilateral, subtype-concordant RCC is likely the result of multiple de novo primary malignancy occurrences rather than metastasis to the contralateral kidney [2]. In this patient, the presence of bilateral, subtype-discordant renal cell carcinoma seems to support this theory.
TEACHING POINT
Undiagnosed coexisting conditions, such as sarcoidosis, can result in false positive findings on CT and PET/CT scans when evaluating patients with renal masses for metastatic disease. The levels of radiotracer activity at each site should be evaluated because significant differences in activity may suggest separate disease processes.
Table 1.
Summary table for Renal Cell Carcinoma
| Etiology [1] | Adenocarcinoma derived from renal tubular epithelial cells | |
| Incidence [1] | 31,000 cases/year | |
| Male:female ratio [1] | 3:2 | |
| Age predilection [1] | Sixth and seventh decades of life | |
| Risk factors [1] | Tobacco exposure, von Hippel-Lindau syndrome, hereditary papillary RCC syndrome, hereditary leiomyomatosis and renal cell cancer syndrome, Birt-Hogg-Dubé syndrome | |
| Treatment | Localized: Surgical excision is mainstay of treatment; however, thermal ablation and observation have recently become options for treatment. Metastatic: combination of cytoreductive nephrectomy with or without metastasectomy in reasonable surgical candidates, immunotherapy, and targeted therapy (ex. sorafenib, sunitinib, temsirolimus). |
|
| Prognosis [11] | Tumor stage | 5 year survival rates |
| I | 95% | |
| II | 88% | |
| III | 59% | |
| IV | 20% | |
| Findings on imaging | CT: Enhancing (>12–20 HU) mass with IV contrast, may have cystic component*, calcifications (30%), hemorrhage or necrosis [13] US: Solid or complex cystic mass with irregular borders, variable echogenicity but usually hyperechoic (rarely shadows) [14] MRI: Intermediate to high signal intensity, heterogeneous, enhancing mass on T1-weighted images with gadolinium [15] DMSA renal scan: decreased activity [1] Angiography: usually demonstrates neovascularity [1] |
|
10–15% of RCC contain a cystic component [9]. Bosniak I cysts (cysts with smooth walls without septations, calcifications, or enhancing component) are considered benign and Bosniak II cysts (cysts with very thin septa, fine calcifications, or hyperattenuating cysts) have an exceptionally low likelihood of being malignant [12]. The risk of sampling error is higher when a percutaneous biopsy is performed on a cystic lesions compared to a solid mass [9].
Table 2.
Differential diagnosis for solid renal mass
| Differential Diagnosis | CT | US [14] | MRI | Other [1] |
|---|---|---|---|---|
| RCC | Enhancing (>12–20 HU) mass with IV contrast, may have cystic component, calcifications (30%), hemorrhage or necrosis [13] | Solid or complex cystic mass with irregular borders, variable echogenicity but usually hyperechoic (rarely shadows) | Intermediate to high signal intensity, heterogeneous, enhancing mass on T1-weighted images with gadolinium [15] | DMSA renal scan: decreased activity. Angiography: Usually demonstrate neovascularity |
| Transitional cell carcinoma | Often ill-defined mass located centrally; radiolucent filling defect, obstruction or nonvisualization of the collecting system with IV contrast [1] | Usually hypoechoic, centrally-located mass with hydronephrosis or infundibular dilation if obstruction is present | Variable signal intensity, moderate enhancement with gadolinium [16] | |
| Sarcoma | Soft tissue mass arising from capsule or renal sinus, often quite large without lymphadenopathy; presence of fat suggests liposarcoma [1] | Soft tissue mass with variable echogenicity | Variable appearance | |
| Lymphoma | Multiple small renal masses (most common pattern), diffuse renal involvement, or direct invasion of lymphadenopathy into kidney. Usually hypoattenuating, occasionally hyperattenuating. [1] | Multiple small hypoechoic lesions (most common pattern), diffuse involvement results in homogenous hyperechoic appearance (“hepatization”) | Homogeneous small lesions, hypointense or isointense to normal parenchyma on T1W, hypointense on T2W; less pronounced enhancement than surrounding parenchyma [16] | Angiography: hypovascular pattern |
| Metastases | Multiple masses, moderate enhancement with IV contrast. [1] | Multiple lesions. Variable echogenicity (usually hypoechoic). | Multiple lesions, variable appearance depending on tissue composition of primary malignancy, usually hypointense. [16] | Angiography: hypovascular pattern |
| Oncocytoma | Central stellate scar [1] | Solid isoechoic mass possibly with central hypoechoic area representing central scar | Low intensity homogeneous mass on T1, central stellate scar (27%) [15] | Angiography: spoke-wheel pattern |
| Angiomyolipoma | Heterogeneous mass with areas of negative attenuation (below - 20 HU) without calcifications [1] | Well-circumscribed, highly echogenic mass with shadowing | Detection of fat on fat-suppressed images [15] | Angiography: possibly aneurysmal dilation |
| Pseudotumor | Renal segment that is isodense with surrounding parenchyma [1] | Normal echogenicity | Isointense to surrounding parenchyma, homogeneously enhancing segment on T1-weighted images [16] | DMSA renal scan: increased activity (normal uptake) |
ACKNOWLEDGEMENTS
Pathology images were provided by Drs. Wells Chandler and Deanna Fang of the University of Utah Pathology Department. PET/CT images provided by Dan Johnson of the University of Utah Radiology Department.
The project described was supported by Award Number P30CA042014 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
ABBREVIATIONS
- CT
Computed Tomography
- RCC
Renal Cell Carcinoma
- IL
Interleukin
- FDG
Fluorodeoxyglucose
- PET
Positron Emission Tomography
- IV
Intravenous
- SUV
Standardized Uptake Value
- HU
Hounsfield Units
REFERENCES
- 1.Campbell SC, Novick AC, Bukowski RM. Campbell-Walsh Urology. 9th ed. Philadelphia: Saunders Elsevier; 2007. Renal Tumors; pp. 1567–1637. [Google Scholar]
- 2.Blute ML, Itano NB, Cheville JC, Weaver AL, Lohse CM, Zincke H. The effect of bilaterality, pathological features and surgical outcome in nonhereditary renal cell carcinoma. J Urol. 2003 Apr;169(4):1276–81. doi: 10.1097/01.ju.0000051883.41237.43. [DOI] [PubMed] [Google Scholar]
- 3.Crotty TB, Farrow GM, Lieber MM. Chromophobe cell renal carcinoma: clinicopathological features of 50 cases. J Urol. 1995 Sep;154(3):964–7. doi: 10.1016/s0022-5347(01)66944-1. [DOI] [PubMed] [Google Scholar]
- 4.Thoenes W, Storkel S, Rumpelt HJ, Moll R, Baum HP, Werner S. Chromophobe cell renal carcinoma and its variants--a report on 32 cases. J Pathol. 1988 Aug;155(4):277–87. doi: 10.1002/path.1711550402. [DOI] [PubMed] [Google Scholar]
- 5.Kontak JA, Campbell SC. Prognostic factors in renal cell carcinoma. Urol Clin North Am. 2003 Aug;30(3):467–80. doi: 10.1016/s0094-0143(03)00020-x. [DOI] [PubMed] [Google Scholar]
- 6.Weinberger SE. Cecil Medicine. 23rd ed. Philadelphia: Saunders Elsevier; 2008. Sarcoidosis; pp. 667–672. [Google Scholar]
- 7.Lawrentschuk N, Davis ID, Bolton DM, Scott AM. Positron emission tomography (PET), immuno-PET and radioimmunotherapy in renal cell carcinoma: a developing diagnostic and therapeutic relationship. BJU Int. 2006 May;97(5):916–22. doi: 10.1111/j.1464-410X.2006.06125.x. [DOI] [PubMed] [Google Scholar]
- 8.Dechet CB, Zincke H, Sebo TJ, et al. Prospective analysis of computerized tomography and needle biopsy with permanent sectioning to determine the nature of solid renal masses in adults. J Urol. 2003 Jan;169(1):71–4. doi: 10.1016/S0022-5347(05)64038-4. [DOI] [PubMed] [Google Scholar]
- 9.Lane BR, Samplaski MK, Herts BR, Zhou M, Novick AC, Campbell SC. Renal mass biopsy-a renaissance? J Urol. 2008 Jan;179(1):20–27. doi: 10.1016/j.juro.2007.08.124. [DOI] [PubMed] [Google Scholar]
- 10.Smith EH. Complications of percutaneous abdominal fine-needle biopsy. Review Radiology. 1991 Jan;178(1):253–8. doi: 10.1148/radiology.178.1.1984314. [DOI] [PubMed] [Google Scholar]
- 11.Cohen HT, McGovern FJ. Renal cell carcinoma. N Engl J Med. 2005 Dec;353(23):2477–2490. doi: 10.1056/NEJMra043172. [DOI] [PubMed] [Google Scholar]
- 12.Silverman SG, Israel GM, Herts BR, Richie JP. Management of the incidental renal mass. Radiology. 2008 Oct;249(1):16–31. doi: 10.1148/radiol.2491070783. [DOI] [PubMed] [Google Scholar]
- 13.Sheth S, Scatarige JC, Horton KM, Corl FM, Fishman EK. Current concepts in the diagnosis and management of renal cell carcinoma: role of multidetector CT and three- dimensional CT. Radiographics. 2001 Oct;21:S237–S254. doi: 10.1148/radiographics.21.suppl_1.g01oc18s237. [DOI] [PubMed] [Google Scholar]
- 14.Bisset RAL, Khan AN. Differential Diagnosis in Abdominal Ultrasound. 3rd ed. New Delhi: Elsevier; 2008. Durr-e-Sabih Urinary Tract and Adrenal Glands; pp. 256–343. [Google Scholar]
- 15.Minor LD, Picken MM, Campbell SC. Benign Renal Tumors. AUA Update Series. 2003;22(22):170–175. [Google Scholar]
- 16.Kreft B. MR Imaging of the Body. New York: Thieme; 2009. Kidneys; pp. 279–292. [Google Scholar]