Abstract
Mullerian duct abnormalities are congenital malformations that are easily missed and can lead to incorrect diagnosis and unnecessary operative procedures. In this case, a young female presented with cyclic pelvic pain that continued after previous surgical resection of an ovarian cyst. Further investigation with clinical examinations and multimodality imaging demonstrated ipsilateral renal agenesis and a Class III Mullerian duct anomaly (MDA) requiring a second operative procedure. It is believed that this case is a variant of the described obstructed hemi-vagina with ipsilateral renal agenesis (OVIRA) anomaly as pathologically there was ipsilateral renal agenesis and complete vaginal agenesis in our case. It is imperative to have a high clinical suspicion of mullerian duct abnormalities when encountering a patient with other urogenital anomalies. This will decrease the amount of misdiagnoses, guide appropriate surgical intervention, and decrease the risk of future reproductive complications.
Keywords: didelphys, uterine, unilateral, vaginal agenesis, ipsilateral renal agenesis
CASE REPORT
A 13-year-old pre-pubertal female was seen in the pediatric emergency room at our institution for a complaint of lower abdominal pain for two days. This pain was first worked up at another local hospital over 6 months ago, and the patient was told that it was due to a ruptured ovarian cyst, which was surgically excised along with her appendix. The patient has since had dysmenorrhea requiring increasing pain control. On presentation the patient appeared uncomfortable, but in no visible distress. On physical exam, she had abdominal tenderness in the left lower quadrant, but no distention, or palpable organomegaly. Urinalysis, complete blood count (CBC), complete metabolic panel (CMP), and urine pregnancy test were all negative.
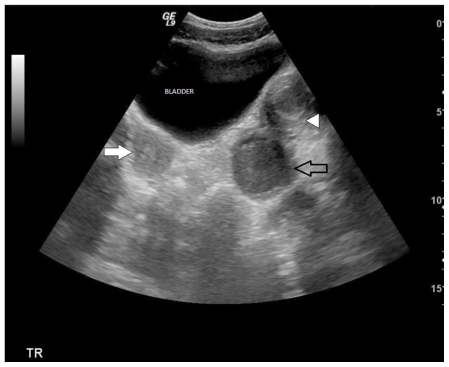
Transabdominal ultrasound demonstrated widely divergent uterine corpi, raising the index of suspicion for uterine duplication anomaly. A normal appearing uterus and cervix were identified on the right (figure 1). On the left, the endometrial cavity was markedly distended with complex fluid, consistent with hematometra (figure 1). A complex cyst superior to the urinary bladder was initially presumed to be a complex left ovarian cyst. Renal evaluation revealed an absent left kidney corroborating the diagnosis of uterine duplication anomaly, most likely uterine didelphys. Transvaginal imaging could not be performed and the patient was referred to MRI for more precise imaging of the left lower uterine segment and cervix, which were not clearly identified on transabdominal ultrasound.
Figure 1.
13 year old female with uterus didelphys, unilateral distal vaginal agenesis, and ipsilateral renal agenesis. Transabdominal ultrasound. Transverse sections demonstrated widely divergent uterine corpi, raising the index of suspicion for uterine duplication anomaly. Normal appearing uterus on the right (filled arrow). Distended endometrial cavity identified on the left (open arrow). Complex cyst superior the urinary bladder was initially presumed to be a complex left ovarian cyst (arrow head). (Protocol: 3.5 MHz curvilinear transducer (Phillips).
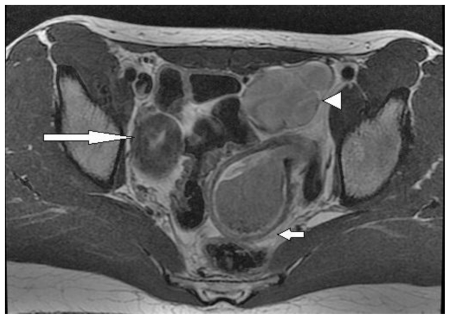
Magnetic resonance imaging (MRI) of the pelvis utilizing multiplanar T2 and T1 sequences demonstrated widely divergent right uterus and left lower uterine segments with the uterine volume of the right horn significantly smaller than the left (figure 2). The left lower uterine horn appeared to be distended with heterogeneous T2/high T1 signal fluid, suggesting the presence of subacute blood products and may possibly extend into the endocervix/vagina. The distended lower uterine segment appeared to correspond with the cystic structure demonstrated on ultrasound, and was contiguous with a distended left uterine body/fundus seen more superiorly. A distended tubular high T2/high T1 signal structure was noted to extend to the left ovary which is seen superior to the distended left uterus, compatible with hematosalpinx (figure 2). Both ovaries appeared within normal limits and the remaining pelvic structures were unremarkable. Limited coronal imaging of the lower abdomen confirmed absence of the left kidney as noted on prior ultrasound. Collectively these findings suggested hematometra on the left with evidence of a Class III Mullerian duct anomaly, such as didelphys uterus.
Figure 2.
13 year old female with uterus didelphys, unilateral distal vaginal agenesis, and ipsilateral renal agenesis. Axial T2 noncontrast demonstrates a normal right uterine horn (long arrow). Distended lower uterine segment with blood products (short arrow). Distended tubular structure noted extending to the expected left adnexa superior to the distended left uterus, compatible with hematosalpinx (arrow head). (Protocol: 1.5 Tesla MRI (GE Signa Excite), TR/TE: 2800/80, 5mm slice thickness, non-contrast).
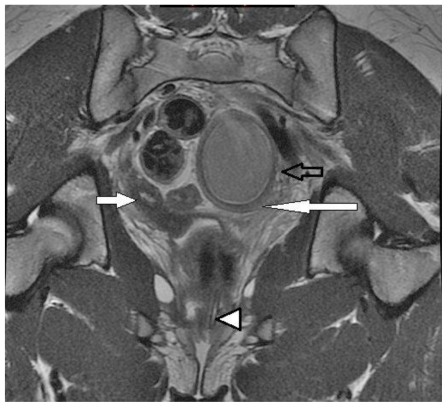
After the initial MRI, the patient had a physical exam under sedation demonstrating a single cervical opening tilting in the direction of the expected right uterine horn. Two days later, an additional MRI of the pelvis was performed to further assess the patency of the uterine segments and correlate with physical exam findings. Multiplanar non-fat saturated FSE T2 sequences were obtained upon administration of sterile saline plus sterile ultrasound gel into the vaginal fornix via a small sterile catheter. High T2 signal was seen in the vaginal fornix and extended into a normal appearing cervix and uterine horn, confirming patency on the right and corresponding to physical exam findings performed by the surgeon (figure 3).
Figure 3.
13 year old female with uterus didelphys, unilateral distal vaginal agenesis, and ipsilateral renal agenesis. Coronal long axis non-fat saturated FSE T2. After administration of sterile saline plus sterile ultrasound gel into the vaginal fornix via a small sterile catheter (arrowhead) high T2 signal extended from the vaginal fornix into a normal appearing cervix and uterine horn confirming patency on the right (filled arrow) and corresponding with a prior physical exam performed by the surgeon. Note blind-ending uterus (long arrow). Please note the lower uterine segment (open arrow). (Protocol: 1.5 Tesla MRI (GE Signa Excite), TR/TE: 4000/110, 5mm slice thickness, non-contrast).
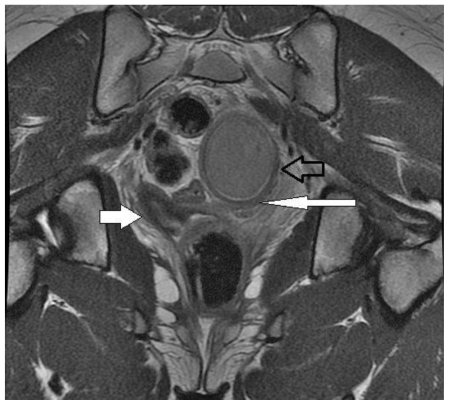
The left uterine horn and distended, blind-ending left lower uterine segment ended in intermediate signal tissue at the superior aspect of what appeared to be the vagina as noted on prior MRI (figure 4). There was no vaginal opening or other external connection to these structures.
Figure 4.
13 year old female with uterus didelphys, unilateral distal vaginal agenesis, and ipsilateral renal agenesis. Coronal long axis non-fat saturated FSE T2. Left uterine horn and distended, blind-ending left lower uterine segment ended in intermediate signal tissue at the superior aspect of what appeared to be the vagina as noted on prior MRI (open arrow). Normal right uterine horn (filled arrow) and blind-ending uterus (long arrow). (Protocol: 1.5 Tesla MRI (GE Signa Excite), TR/TE: 4000/110, 5mm slice thickness, non-contrast).
On the basis of the above clinical and radiologic findings, the patient was taken to surgery. The patient underwent a left total hysterectomy and left salpingectomy and upon further intraoperative evaluation a normal right uterus, fallopian tube, ovary and corpus luteum were demonstrated. The left uterus was distended with mixed blood products narrowing into what appeared to be a markedly dilated blind-ending structure and fallopian tube filled with blood. It was unclear whether this blind-ending structure contained vagina or uterus from gross evaluation. The omentum, fallopian tube, and dilated uterine structure were dissected from the peritoneum sparing the mesosalpinx and a normal left ovary. No significant blood loss occurred and the patient did not require additional blood products.
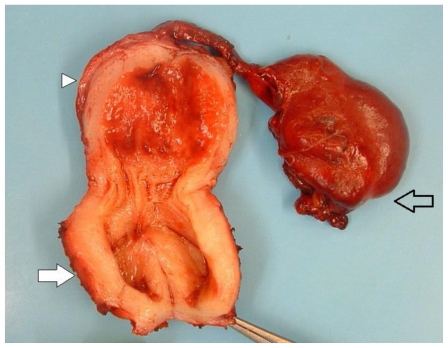
The specimen, labeled left vagina, uterus, and fallopian tube was sent to pathology for frozen section. It consisted of an 8 × 2.5 × 2.5 cm uterus with cervix, fallopian tube, and what appeared to be vagina weighing 78 grams. Upon sectioning, it contained red bloody fluid with a smooth endometrial cavity. The left fallopian tube measured 8 × 3.5 × 2 cm and was severely dilated near the fimbriated end, which measured 3 × 2.5 × 2 cm and demonstrated multiple cysts containing bloody material (figure 5).
Figure 5.
13 year old female with uterus didelphys, unilateral distal vaginal agenesis, and ipsilateral renal agenesis. Pathological specimen. Left uterine horn (arrowhead) and distended, blind-ending left lower uterine segment (filled arrow). Hematosalpinx (open arrow).
No squamous epithelium, which is normally found in transformation zone, exocervix, and vagina, was identified (figure 6). A blind-ending distended uterus with endocervix and hematosalpinx were identified. Cysts at the fimbriated end of the fallopian tube were believed to represent mesothelial cysts and consistent with paratubal adenomatoid tumors, benign entities. No rudimentary hemivagina, transverse, or vertical septum was identified. The patient had an unremarkable post-operative course and was discharged two days later.
Figure 6.
13 year old female with uterus didelphys, unilateral distal vaginal agenesis, and ipsilateral renal agenesis. Uterine didelphys diagram. Normal right ovary, fallopian tube, uterus, cervix, and vagina. Left uerine horn with normal left ovary, left hematosalpinx, hematometra, and blind-ending left uterine segment (open arrow). Note there is no left vagina or exocervix.
DISCUSSION
In our case, the patient had a Class III Mullerian duct that demonstrated some features that were similar to obstructed hemi-vagina with ipsilateral renal agenesis (OVIRA) anomaly, which presents with a didelphys uterus, absence of the ipsilateral kidney, and a hemivagina. The cause of OVIRA can be related to damage of the caudal portion of the Wolffian duct. It is believed that insult occurs from embryonic arrest at 8 weeks of gestation that simultaneously affects the adjacent Mullerian and metanephric ducts. The defect may also occur as early as the 4th week of gestation and can affect both the mesonephric ducts and ureteral buds. The mesonephric duct, which is maldeveloped, does not allow crossover of the mullerian duct and consequent fusion. This results in a didelphys uterus and obstruction of the ipsilateral horn and the vagina [10]. Additionally, patients with uterus didelphys may present with a unilateral hemivaginal septum causing obstruction with hematometrocolpos [5]. While a complete or partial vaginal septum is associated with this anomaly in 75% of cases there was no vaginal septum in this case. Although left renal agenesis is present in our case, it is unique from the classically described OVIRA anomaly in that there is no vaginal tissue and proves to lie on a spectrum of this class of anomalies [6, 7]. This was confirmed by the fact that there was no squamous cell epithelium to suggest the presence of vaginal tissue and only endocervix existed.
Mullerian duct anomalies have been separated into many classification systems over the years, but perhaps the most well known separates these anomalies into classes that demonstrate similar clinical manifestations, treatment, and prognosis. Although prevalence has ranged from 0.16–10% overall in various studies, one study employing a Medline search showed the prevalence of uterine anomalies subtypes in the general population was approximately 0.50%. In this study the prevalence of subtypes throughout the studied cases was 7% for arcuate, 34% for septate, 39% for bicornuate, 11% for didelphic, 5% for unicornuate, and 4% for hypoplastic/aplastic/solid and other forms [9].
According to the American Fertile Society classification system, there is a spectrum of Mullerian duct anomalies and they each present with unique imaging features. Class I anomalies consist of segmental agenesis and a spectrum of uterovaginal hypoplasia. On MR imaging of Class I anomalies there can be the absence or anomalies of the uterus and upper vagina, and varying differences of lower vaginal development. These anomalies can be accurately seen on a combination of sagittal and axial imaging. Normal ovaries are present. Class II anomalies include unicornuate uteri, which includes both partial or complete hypoplasia. T2-weighted MR imaging of Class II anomalies reveals a unicornuate uterus, which is curved and elongated with narrowing of the fundal portion off midline. The rudimentary horn displays lower signal intensity. Class III includes a spectrum known as uterus didelphys which duplication of the uterus is a result of complete nonfusion of the mullerian ducts. On T2-weighted MR imagining, two separate normal-sized uterine horns and cervices are visualized. The two uterine horns are usually widely separated and the endometrial and myometrial widths are preserved. Class IV anomalies only demonstrate partial nonfusion of the superior segments of the uterovaginal canal and are known as bicornuate uteri. MRI demonstrates that in the external fundal myometrium there are uterine horns separated by an intervening cleft longer than 1 cm. There is normal zonal anatomy visualized in each horn and a dividing septum composed of myometrium. Class V anomalies are unique in that they represent disorders in resorption of the uterovaginal septum and include septate uteri. On T2-weighted MR images taken parallel to the long axis of the uterus there is a convex, flat, or concave (<1cm) outer uterine contour and fibrous septa are visualized. Class VI anomalies demonstrate partial resorption of the uterovaginal septum and Class VII anomalies result from exposure to diethylstilbestrol (DES) in utero and reveal a classic “t-shaped” configuration of the uteri [1, 7]. Variations and sub-organizations of these classes have been offered throughout the literature, but these subtypes serve to classify the primary uterine defect.
Clinically, the spectrum of class III anomalies usually present due to symptoms from obstruction. Almost uniformly this presentation occurs at menarche when the hemiuterus or hemivagina becomes symptomatic and the patient experiences cyclical dysmenorrhea. This results in retrograde menstrual flow and may present as a unilateral abdomino-pelvic mass that terminates in a bluish bulge in lateral vaginal wall on physical examination. The nonobstructive, asymptomatic additional uterus is usually found when the patient becomes symptomatic during a pelvic examination and imaging is required or on another incidental imaging exam [4]. Complications associated with this disorder are endometriosis, pyosalphinx, pyocolpos and pelvic adhesions. Intraoperatively a didelphys uterus with hematometrocolpos may be mistaken for a ruptured ovarian cyst if a thorough evaluation is not performed [2, 3]. Additionally, while women with this anomaly are at an increased risk for unfavorable obstetric outcome, attempts should be made to preserve the affected uterus if possible because successful pregnancy could occur on either side [8].
TEACHING POINT
When encountering a patient with urogenital anomalies it is imperative to consider mullerian duct anomalies as well. It is important to consider variants of commonly known conditions especially when a patient is presenting with lower abdominal pain. This will decrease the amount of misdiagnoses, guide appropriate surgical intervention, and decrease the risk of future reproductive complications.
Table 1.
Summary table for uterus didelphys variations
| Etiology | Complete nonfusion of uterovaginal horns |
| Incidence | 1 per 1000 live births |
| Age Predilection | Uterus didelphys with obstruction in a patient presenting at menarche with cyclical dysmenorrhea and pelvic mass |
| Risk Factors | Genetics Majority of cases sporadic or multifactorial in origin May be caused by an autosomal dominant or recessive gene in certain cases Hepatocyte nuclear factor (HNF-1) β mutations reported in association with renal anomalies and diabetes |
| Anatomic Types | American Fertility Society classification Class I: Segmental agenesis, hypoplasia Occurs in a variety of forms Complete form most common (Mayer-Rokitansky-Kuster-Hauser syndrome) Class II: Unicornuate uterus May have rudimentary horn, which may or may not communicate with main uterine cavity Class III: Uterus didelphys Class IV: Bicornuate uterus Class V: Septate uterus Class VI: Arcuate uterus Class VII: DES exposure |
| Treatment | Expectant Metroplasty leaving duplicated cervix intact in selected patients with recurrent spontaneous abortions and premature deliveries Traditionally performed abdominally via a Pfannenstiel approach (e.g., Strassman metroplasty) Currently performed by a combined hysteroscopic and laparoscopic approach Benefits of metroplasty unclear Hysteroscopic resection of vaginal septum in patients with an obstructing vaginal septum |
| Prognosis | Generally good ≈ 25% of women with uterine anomalies have reproductive problems ≈ 3% of women with repeated pregnancy loss |
| Findings on Imaging |
MR Findings
|
Table 2.
Differential table for uterus didelphys variations
| Differential Diagnosis | MRI | Ultrasound |
|---|---|---|
| Uterine didelphys | MRI is not recommended in first trimester due to unknown effects on organogenesis Study of choice for evaluating non-pregnant patient Accuracy approaching 100% Image plane parallel to long axis of uterus Optimal assessment of fundal contour T2WI High signal endometrium Low signal junctional zone Intermediate signal myometrium T1WI occasionally useful High signal fat may outline low signal uterus |
Primary modality of evaluating uterine duplication in pregnancy Reported accuracy 90–92% 3D ultrasound provides improved spatial delineation Sensitivity 93%, specificity 100% Widely divergent uterus True orthogonal view along the long axis essential for diagnosis |
| Leiomyoma | Non-degenerated fibroid Intermediate signal T1WI Isointense to uterus Homogeneous, low signal intensity T2WI Degeneration causes variable signal Hemorrhagic T1WI: Diffuse high signal (early), high signal rim (late) T2WI: Variable, usually low signal intensity with low signal rim Cystic Low signal T1WI, high signal T2WI |
Typically well-circumscribed Homogeneous, hypoechoic Degenerated fibroids more heterogeneous and variable in appearance Borders often less well-defined Hyperechoic with hemorrhage Cystic, often with thick, irregular septations Calcified with dense shadowing Color Doppler Hypovascular compared to surrounding myometrium May see uterine vessels splayed around mass |
| Interstitial ectopic | Gestational sac off fundal portion of uterus Blood products will be of varying signal intensity depending on age |
Gestational sac high in uterus May have yolk sac, embryonic pole, cardiac activity Very vascular due to blood supply from ovarian and uterine arteries Eccentric location of gestational sac (40% sensitivity, 62% specificity) Sac does not communicate with endometrial cavity Less than 5 mm of myometrium around sac (40% sensitivity, 74% specificity) May see bulge in uterine contour Lack of double decidual sac sign Interstitial line sign Echogenic line extends up to middle of gestational sac or mass 80% sensitivity, 99% specificity |
| Bicornuate Bicollis Uterus | T1WI: Inferior portion of septum low signal intensity (SI) if fibrous T2WI Uterine horns separated by intervening cleft in external fundal myometrium > 1.0 cm Measured from apex of fundal cleft to the line connecting serosal contour of uterine horns Symmetric uterine horns, each with normal circumferential zonal anatomy Communication between endometrial or endocervical canal essential for diagnosis SI of the tissue separating the horns identical to myometrium on all sequences Low SI of inferior portion of septum if fibrous Accuracy of MR: 100% |
Grayscale ultrasound True orthogonal view along the long axis essential for diagnosis Large fundal cleft > 1 cm Fundal indentation of external contour below, or ≤ 5 mm above interostial line Widely divergent, symmetric, normal appearing echogenic endometrial complexes Endometrial complexes convergent at caudal extent Echogenicity of tissue separating horns identical to myometrium Pitfall: Extreme anteflexion or retroflexion and co-existing fundal leiomyomas causing convexity of fundal contour Accuracy of transvaginal ultrasound (TVS): 90–92% |
ACKNOWLEDGEMENTS
We would like to thank Dr. Valcek and the department of Surgery at Long Island College Hospital for their assistance and outstanding patient care in this interesting case. We would also like to thank Dr. Bardarov and the department of Pathology at Long Island College Hospital for their commitment to thoroughness.
ABBREVIATIONS
- OVIRA
Obstructed hemi-vagina with ipsilateral renal agenesis
- CMP
Comprehensive Metabolic Panel
- CBC
Complete Blood Count
- MRI
Magnetic Resonance Imaging
- DES
Diethylstilbestrol
- TR
Repetition time
- TE
Echo time
REFERENCES
- 1.Troiano RN, McCarthy SM. Müllerian duct anomalies: imaging and clinical issues. Radiology. 2004;233(1):19–34. doi: 10.1148/radiol.2331020777. [DOI] [PubMed] [Google Scholar]
- 2.Beatriz LP, Junqueira BP, Allen LM, Spitzer RF, Lucco KL, Babyn PS, Doria AS. Müllerian Duct Anomalies and Mimics in Children and Adolescents: Correlative Intraoperative Assessment with Clinical Imaging. Radiographics. 2009;29:1085–1103. doi: 10.1148/rg.294085737. [DOI] [PubMed] [Google Scholar]
- 3.Rock JA, Breech LL. Surgery for anomalies of mullerian ducts. In: Rock JA, Jones HW III, editors. TeLinde’s operative gynecology. 9th ed. New York, NY: Lippincott Williams & Wilkins; 2003. pp. 732–736. [Google Scholar]
- 4.Gray SW, Skandalakis JE, Broecker BH. Female reproductive system. In: Skandalakis JE, Gray SW, editors. Embryology for surgeons. 2nd ed. Baltimore, Md: Lippincott Williams & Wilkins; 1994. pp. 816–847. [Google Scholar]
- 5.Li S, Qayyum A, Coakley FV, Hricak H. Association of renal agenesis and mullerian duct anomalies. Journal of Computed Assisted Tomography. 2000;24( 6):829–834. doi: 10.1097/00004728-200011000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Orazi F, Lucchetti C, Schingo P, Marchetti P, Ferro F. Herlyn-Werner-Wunderlich syndrome: uterus didelphys, blind hemivagina and ipsilateral renal agenesis. Sonographic and MR findings in 11 cases. Pediatric Radiology. 2007;37:657–665. doi: 10.1007/s00247-007-0497-y. [DOI] [PubMed] [Google Scholar]
- 7.Chapter 106: Normal Development of the Urogenital System. 9th edition. Wein: Campbell-Walsh Urology. [Google Scholar]
- 8.Albert A, Paciuc J. Successful pregnancy following surgery in the obstructed uterus in a uterus didelphys with unilateral distal vaginal agenesis and ipsilateral renal agenesis: Case report and literature review. Journal of Pediatric Adolescent Gynecology. 2009;22( 5):159–162. doi: 10.1016/j.jpag.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Nahum G. Uterine anomalies. How common are they, and what is there distribution among subtypes? Journal of Reproductive Medicine. 1998 Oct;43(10):877–887. [PubMed] [Google Scholar]
- 10.Zurawin RK, Dietrich JE, Heard MD, Edwards CL. Didelphic Uterus and Obstructed Hemivagina with Renal Agenesis: Case Report and Review of the Literature. Journal of Pediatric Adolescent Gynecology. 2004;17:137–141. doi: 10.1016/j.jpag.2004.01.016. [DOI] [PubMed] [Google Scholar]