Abstract
Pseudoaneurysms are common vascular abnormalities due to disruption of the vessel wall. Pseudoaneurysm with arteriovenous fistula is a rare presentation. Complications associated with them develop unpredictably and carry a high mortality rate. Traditionally pseudoaneurysms have been treated surgically. However, with the advent of new interventional techniques, management using endovascular approach have gained popularity in treating pseudoaneurysms. Here, we present two cases of large pseudoaneurysms with arteriovenous fistula treated by percutaneous stent graft. Present studies on pseudoaneurysms are all either iatrogenic or secondary to nephrologic dialysis treatment and only few present studies exist describing such large post-traumatic femoral pseudoaneurysms with arteriovenous fistulas were treated successfully by stent grafting through femoral approach, with good patency at 6 months follow up.
Keywords: Arteriovenous fistula, pseuroaneurysm, stenting, stent graft
CASE REPORT
CASE REPORT 1
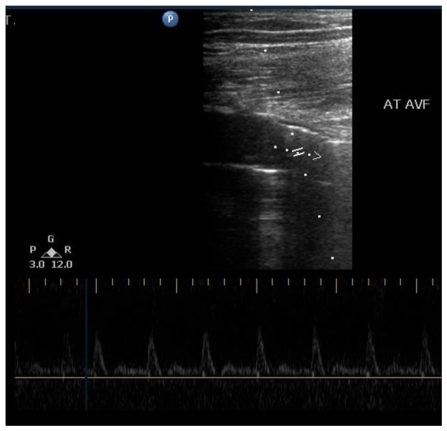
A 56 year old male presented with a large tender pulsatile mass in the left thigh after a stab injury by a knife 6 months back. There was a palpable thrill and a bruit heard over the mass. Ultrasound examination revealed a large, pulsatile bilobed, anechoic saccular structure (Fig 1) arising from the distal superficial femoral artery (SFA). Colour Doppler showed turbulent flow within the saccular structure with a wide necked communication with the SFA (superficial femoral artery), findings consistent with the diagnosis of pseudoaneurysm (Fig 1). There was also a communication visualized between the cystic lesion and the SFV (superficial femoral vein) with arterialization of the normal venous flow within the vein.
Figure 1.
56 year old male with distal left superficial femoral artery arteriovenous fistula. B-Mode Ultrasound Image (Ultrasound machine: Philips HD11XE; Probe: 12 to 3 MHz Broadband linear array transducer) transverse section left lower thigh shows a large bilobed cystic anechoic lesion, Duplex Doppler study transverse section left lower thigh shows colour aliasing (arterial as well as venous) in the cystic sac suggestive of large pseudoaneurysm and spectral doppler study showing turbulent flow (PSV of 380 cm/sec) at the site of arteriovenous fistula.
On examination, the patient appeared healthy. He had a pulse rate of 88/minute and was a known hypertensive on regular treatment. His BP was 150/100 mm Hg. Laboratory investigations including blood sugar, complete haemogram, renal and liver function tests were within normal limits. He had normal prothrombin time and partial thromboplastin time with an INR of 1.32 (normal range − 0.8 to 1.4).
Lower limb digital subtraction (DS) angiography was carried out which revealed a well defined bilobed aneurysm of approximate size 5.3 × 2.5 × 5.1 cm arising from the distal SFA with dilated early draining superficial femoral vein suggestive of a high flow arteriovenous fistula (Fig 2). The diagnosis of pseudoaneurysm with arteriovenous fistula formation was straightforward and no other differential diagnoses were considered.
Figure 2.
56 year old male with distal left superficial femoral artery arteriovenous fistula. Digital Subtraction Angiogram of left lower limb shows arterio-venous fistula with early draining superficial femoral vein with bilobed pseudoaneurysm. 80 kV, 320 mA, 2.56 mAs and 4/sec frame rate.
The right femoral artery was punctured using modified Seldinger’s technique and using a 10 F introducer sheath (William Cook Europe, Bjaeverskov, Denmark) a 0.035 inch hydrophilic guide wire (Terumo, Tokyo, Japan) was passed through it. Next, a 4F angio catheter (William Cook Europe, Bjaeverskov, Denmark) was placed via the right main femoral artery into the left common iliac artery. Next, under road-map guidance, the site of the fistula was negotiated using terumo guidewire and exchanged with a 0.035 inch 260 cm stiff guide wire (Amplatz Super Stiff, Boston Scientific Corporation, Miami, FL, USA). Heparin was then administered with an initial bolus dose of 5,000 units followed by 1,000 units/hour infusion. After performing the necessary vessel diameter measurements with digital calibration, a peripheral polytetrafluoroethylene (PTFE) covered self expandable stent (Peripheral Stent Graft, Jomed GmbH, Rangendingen, Germany) of 12 mm diameter and 40 mm length was passed over stiff guidewire and deployed at the site with roadmap guidance. Check angiogram showed a constriction waist at the site of stent deployment; hence post-stenting balloon angioplasty was done using balloon of size 12 mm × 40 mm. It was placed across the AVF and dilated to achieve proximal and distal graft vessel apposition to seal the entrance into the pseudoaneurysm.
A post stenting check angiogram confirmed complete occlusion of the pseudoaneurysm and AVF with good patency of the newly placed stent graft (Fig 3). The angio sheath was removed and haemostasis was achieved using prolonged manual compression for about half an hour. There were no complications and the patient was discharged home after overnight bed rest. Heparin infusion was stopped after 4 hours and the patient was put on clopidogrel (before angiography, loading dose of 300 mg followed by 75 mg/day per orally) for 6 months and 75 mg/day aspirin for lifetime.
Figure 3.
56 year old male with distal superficial femoral artery arteriovenous fistula. Post-stenting Digital Subtraction angiogram of left lower limb shows exclusion of arterio-venous fistula and pseudoaneurysm from the circulation with good distal run off. 80 kV, 320 mA, 2.56 mAs and 4/sec frame rate.
Post-stenting follow up ultrasound and Doppler examination showed complete closure of fistula at 6 months. (Fig 4)
Figure 4.
56 year old male with distal left superficial femoral artery arteriovenous fistula. Post stenting Ultrasound (Ultrasound machine: Philips HD11XE; Probe: 12 to 3 MHz Broadband linear array transducer) and Spectral Doppler study in longitudinal profile showing normal arterial waveform in the SFA at the site of fistula.
CASE REPORT 2
A 36 year old male presented with a tense swelling and pain in left lower limb (Fig 5) since 1 Month. On clinical examination, patient had a pulsatile swelling in the pelvis (Fig 5) and palpitations since 5 years. Patient gave history of trauma to left thigh by a sharp object 10 years back. His 2D Echo showed Mild LV Hypertrophy and mild changes of congestive cardiac failure.
Figure 5.
36 year old male with distal left mid-thigh femoral arteriovenous fistula. Clinical photograph of the patient showing tense swelling involving left lower limb and photograph of pelvis showing site of pulsatile swelling (arrow).
Doppler study showed a large pseudoaneurysm in the pelvis and flow aliasing at the site of superficial femoral AVF (arteriovenous fistula) and MRI (magnetic resonance imaging) coronal images showed dilated left common iliac, external iliac (more than the calibre of abdominal aorta), common femoral artery, pelvic venous aneurysm (Fig 6), and the site of AVF (Fig 6). DS angiogram showed excessive tortuosity of the left common iliac, external iliac (Fig 7) and common femoral arteries, a giant (12 cm diameter) pelvic venous pseudoaneurysm (Fig 8) and arteriovenous fistula in left thigh between left SFA & SFV with left SFA pseudoaneurysm at fistula site (Fig 9). The diagnosis of pseudoaneurysm with arteriovenous fistula formation was straightforward and no other differential diagnoses were considered.
Figure 6.
36 year old male with distal left mid-thigh femoral arteriovenous fistula. Magnetic Resonance Angiography (Philips MR Achieva 1.5 T MRI machine) Phase Contrast Coronal image (venous phase) showing giant venous pseudoaneurysm in the pelvis (left image) and the tortuous iliac and femoral artery with superficial femoral artery arteriovenous fistula (right image).
Figure 7.
36 year old male with distal left mid-thigh femoral arteriovenous fistula. Digital Subtraction Angiogram of left lower limb shows excessive tortuosity of left common and external iliac arteries secondary to long standing arteriovenous fistula. 80 kV, 320 mA, 2.56 mAs and 4/sec frame rate.
Figure 8.
36 year old male with distal left mid-thigh femoral arteriovenous fistula. Digital Subtraction Angiogram of left lower limb (venous phase) shows the giant pelvic venous aneurysm. 80 kV, 320 mA, 2.56 mAs and 4/sec frame rate.
Figure 9.
36 year old male with distal left mid-thigh femoral arteriovenous fistula. Digital Subtraction Angiogram of left lower limb shows arteriovenous fistula with early draining veins and pseudoaneurysm formation in the region of the mid thigh. 80 kV, 320 mA, 2.56 mAs and 4/sec frame rate.
Vascular Surgeon had refused open surgery in this patient and hence was referred to us.
The right femoral artery was punctured using the modified Seldingers technique and using a 5F introducer sheath (William Cook Europe, Bjaeverskov, Denmark) a 0.035 inch hydrophilic guide wire (Terumo, Tokyo, Japan) was passed through it. Next, a 5F pigtail angiography catheter (William Cook Europe, Bjaeverskov, Denmark) was placed via the right main femoral artery into the abdominal aorta. Under road-map guidance, left common femoral was punctured using 18 gauge needle anterogradely and the guidewire was introduced into the left SFA, 10F angio sheath was placed over the guidewire, the site of the fistula was negotiated using terumo guidewire and it was exchanged with a 0.035 inch 140 cm stiff guide wire using angiographic catheter (Fig 10).
Figure 10.
36 year old male with distal left mid-thigh femoral arteriovenous fistula. Digital Subtraction Angiogram (Road-map) of left lower limb shows arteriovenous fistula with negotiation of the fistulous site using 0.035″ Terumo guidewire. 80 kV, 320 mA, 2.56 mAs and 4/sec frame rate.
The largest covered stent available at that point of time was 12 × 60 mm (12 mm diameter and 60 mm length). Although there was a potential problem of stent being undersized with chances of technical procedure failure, stent grafting was undertaken as there was no avenue for open surgery. After performing the necessary vessel diameter measurements with digital calibration, a peripheral PTFE covered self expandable stent (of 12 mm diameter and 60 mm length) was passed over the stiff guidewire and the stent deployed. Check angiogram showed a constriction waist at the site of fistula; hence post-stenting balloon angioplasty was done using a balloon of size 12 mm × 60 mm. It was placed across the AVF and dilated to achieve proximal and distal graft vessel apposition to seal the site of entry into the pseudoaneurysm.
A post dilatation check angiogram confirmed complete occlusion of the pseudoaneurysm and AVF with good patency through the newly placed stent graft (Fig 11).The angio sheath was removed and haemostasis was achieved by using prolonged manual compression. The patient was put on low molecular weight heparin for three days overlapped with warfarin for six months with the target INR of 2–3. There were no complications and the patient was discharged four days after admission. Post-stenting follow up ultrasound and doppler (Fig 12) at six months showed complete closure of fistula with significant relief from the pain, palpitations and a noticeable reduction in the swelling of the left lower limb and the giant pelvic venous aneurysm (Fig 13).
Figure 11.
36 year old male with distal left mid-thigh femoral arteriovenous fistula. Post Stent-graft check Digital Subtraction Angiogram of left lower limb shows exclusion of arteriovenous fistula & pseudoaneurysm from the arterial circulation. 80 kV, 320 mA, 2.56 mAs and 4/sec frame rate.
Figure 12.
36 year old male with distal left mid-thigh femoral arteriovenous fistula. Post stenting ultrasound, colour and spectral doppler study (Ultrasound machine: Philips HD11XE; Probe: 12 to 3 MHz Broadband linear array transducer) in longitudinal profile showing normal arterial and venous color flow and spectral waveform in superficial femoral artery and superficial femoral vein at the site of fistula.
Figure 13.
36 year old male with distal left mid-thigh femoral arteriovenous fistula. Post stent-graft Clinical photograph shows non-visible swelling in the pelvis.
DISCUSSION
Any penetrating trauma to the vessel wall, whether iatrogenic, or accidental can lead to damage of the arterial wall with subsequent formation of a pseudoaneurysm and if there is a communication with the adjacent vein, an arteriovenous fistula.
The diagnosis of an AVF in the extremities can be made with history and physical examination [1]. While examining clinically, loud bruit in auscultation and thrill on palpation are highly suggestive. Dilation and prominent pulsations can be seen in the artery proximal to the fistula, as well as in the surrounding venous structures. Ischemia and skin ulceration can occur due to the blood steal through the fistula and venous hypertension [1]. Bacterial endocarditis may also occur due to abnormal venous drainage.
Although colour doppler sonography and nowadays, CT and MR imaging play an essential role in the diagnosis of extremity AVFs, in most cases conventional angiography is still required for accurate lesion localization and tailoring of the surgical or endovascular treatment [2].
In the treatment of extremity AVFs, surgical procedures such as partial resection, ligation, and primary repair were frequently used options [3]. In cases with a delayed diagnosis of an AVF, significant enlargement of the surrounding venous structures and nerve damage can potentially cause difficulties for a surgical approach in treatment [4]. A simple perforation of an enlarged venous structure with high flow, which has a relatively thin wall, may lead to massive hemorrhage during surgery [2]. Furthermore, peri-surgical mortality and morbidity rates are high in patients with severe trauma and multi-system dysfunction.
Recent improvements in endovascular techniques have created significant and effective alternatives to surgical treatment [5–7]. Metallic coils and covered stent implantations are being utilized frequently for endovascular treatment of AVFs. With the significant improvement in stent technology in the last decade, utilization of covered stents for traumatic AVFs and pseudo aneurysms is becoming quite popular [5, 6, 8–10]. Using covered stents for treating AVFs is technically easy and it has been reported to have a high technical success rate and a low complication rate in various arteries in different series of literature [2, 6, 10].
In a superficial or endoluminally inaccessible artery, management of pseudoaneurysms will include options like direct coil embolization, thrombin injection or direct glue injection. In an endoluminally accessible artery, the choice depends on whether the artery is expendable or not. If expendable, the artery can be embolized, however, in additon, distal embolization might be needed if there is retrograde filling into the sac. In an inexpendable artery, options available are aneurysm embolization if neck is narrow or stent graft placement if wide necked.
Endoluminal management serves to exclude a pseudoaneurysm from the circulation. Selecting the optimal method depends on the size of the pseudoaneurysmal neck and the expendability of the donor artery [11]. Exclusion methods fall into two broad categories: embolization and stent placement [11]. A pseudoaneurysm arising from an inexpendable artery must be excluded from the circulation while preserving the circulation in the involved vessel. The width of the pseudoaneurysmal neck relative to the diameter of the donor artery is the determining factor for the selection of method of therapy.
If the neck is narrow, the pseudoaneurysm may be embolized with catheter-directed delivery of coils (the preferred embolization material) into the sac itself made of either stainless steel or platinum [12]. Polyester fibers are incorporated into the body of the coil to increase its thrombogenicity which conform to the shape of and fill the pseudoaneurysmal sac. A disadvantage of using coils as an embolization material is the potential for recanalization of the embolized sac if the coils are not tightly packed.
The use of endoluminal coils has to be avoided in treating AVFs for the same reason that the coils could easily track through the high flow fistula and enter the venous circulation. Stent-graft placement requires a higher profile and a stiffer delivery system than does catheter-directed coil embolization. As a result, the arterial anatomy and the caliber of the arteries leading to and at the pseudoaneurysm site should be favorable (ie, reduced arterial tortuosity and large diameter arteries). An additional reason for placing stent-grafts only in larger arteries is that in small arteries they pose a higher risk of thrombosis or significant vascular stenosis [13].
TEACHING POINT
Traumatic pseudoaneurysms with high flow AVFs cause hyperdynamic circulation, cardiac overload and may lead to death of the patient apart from causing hyperperfusion of the affected limb, pain and development of venous aneurysms distant to the site of actual fistula. Not only the AVFs but also pseudoaneurysms at the site of fistula can be treated effectively by excluding them from circulation by deploying a stent graft across the fistulous opening.
Table 1.
Summary table for pseudoaneurysm
| Aetiology | Trauma, catheter intervention, post-surgical, autoimmune disorders |
| Available surgical techniques | Primary repair, graft interposition, bypass, ligation, patch angioplasty |
| Available interventional techniques | Stent grafting, coiling, thrombin injection, ultrasound guided compression, |
| Complications | Infection, embolism, deep vein thrombosis, mortality, amputation |
Table 2.
Treatment options for pseudoaneurysm
| Feature | Management |
|---|---|
| Superficial artery/Endoluminally inaccessible | Neck compression by ultrasound Direct percutaneous management
|
| Endoluminally accessible (In-expendable donor artery) |
|
| Endoluminally accessible (Expendable donor artery) |
|
Table 3.
Endovascular Stent Graft for Post-traumatic Superficial Femoral Artery Pseudoaneurysms with Arteriovenous Fistula - Summary table of 2 cases
| Title | Traumatic pseudoaneurysms and Arteriovenous fistula |
| No of cases | 2 |
| Device used for endovascular treatment | Stent graft (covered stent) |
| Mechanism | Exclusion from circulation |
| Technical success | 100% |
| Complications | None |
| Period of follow up | 6 months |
| Clinical improvement | Very good in both cases |
Table 4.
Imaging features and differential diagnoses for post traumatic pseudoaneurysm
| Investigation | Features of pseudoaneurysm | Differential diagnoses |
|---|---|---|
| X-ray | Soft tissue swelling, whole limb swelling. | Cellulitis, deep vein thrombosis, edema, soft tissue tumor. |
| Ultrasound | Hypoechoic cystic lesion in continuity with parent artery. | Acute hematoma. |
| Doppler | Yin yang pattern of flow within in continuity with parent artery. | None. |
| MRI | Cystic lesion in communication with parent artery showing flow related artefacts. | None. |
| Digital subtraction angiography | Contrast filling outpouching from the parent vessel. | None. |
ABBREVIATIONS
- AVF
arteriovenous fistula
- CT
Computed Tomography
- DS
Digital Subtraction
- MR
Magnetic Resonance
- MRI
Magnetic Resonance Imaging
- SFA
superficial femoral artery
- SFV
superficial femoral vein
REFERENCES
- 1.Stigall KE, Dorsey JS. Late complications of traumatic arteriovenous fistula. Case report and overview. Am Surg. 1989 Mar;55(3):180–3. [PubMed] [Google Scholar]
- 2.Parodi JC, Schonholz C, Ferreira LM, Bergan J. Endovascular stent-graft treatment of traumatic arterial lesions. Ann Vasc Surg. 1999 Mar;13(2):121–9. doi: 10.1007/s100169900230. [DOI] [PubMed] [Google Scholar]
- 3.Graham AN, Barros D’Sa AA. Missed arteriovenous fistulae and false aneurysms in penetrating lower limb trauma: relearning old lessons. Injury. 1991 May;22(3):179–82. doi: 10.1016/0020-1383(91)90035-d. [DOI] [PubMed] [Google Scholar]
- 4.Beregi JP, Prat A, Willoteaux S, Vasseur MA, Boularand V, Desmoucelle F. Covered stents in the treatment of peripheral arterial aneurysms: procedural results and midterm follow- up. Cardiovasc Intervent Radiol. 1999;22:13–19. doi: 10.1007/s002709900322. [DOI] [PubMed] [Google Scholar]
- 5.Cragg AH, Lund G, Rysavy JA, Salomonowitz E, Castaneda-Zuniga WR, Amplatz K. Percutaneous arterial grafting. Radiology. 1984 Jan;150(1):45–9. doi: 10.1148/radiology.150.1.6689786. [DOI] [PubMed] [Google Scholar]
- 6.Marin ML, Veith FJ, Panetta TF, et al. Percutaneous transfemoral insertion of a stented graft to repair a traumatic femoral arteriovenous fistula. J Vasc Surg. 1993 Aug;18(2):299–302. [PubMed] [Google Scholar]
- 7.Marin ML, Veith J, Panetta TF, et al. Transfemoral endoluminal stented graft repair of a popliteal artery aneurysm. J Vasc Surg. 1994 Apr;19(4):754–7. doi: 10.1016/s0741-5214(94)70052-4. [DOI] [PubMed] [Google Scholar]
- 8.Parodi JC. Endoluminal stent grafts: Overview. J Invasive Cardiol. 1997 Apr;9(3):227–229. [PubMed] [Google Scholar]
- 9.Marin ML, Veith FJ, Cynamon J, et al. Transfemoral endoluminal repair of a penetrating vascular injury. J Vasc Interv Radiol. 1994 Jul-Aug;5(4):592–4. doi: 10.1016/s1051-0443(94)71559-1. [DOI] [PubMed] [Google Scholar]
- 10.Baltacioglu F, Cimsit NC, Cil B, Cekirge S, Ispir S. Endovascular stent-graft applications in iatrogenic vascular injuries. Cardiovasc Intervent Radiol. 2003 Sep-Oct;26(5):434–9. doi: 10.1007/s00270-003-0038-5. [DOI] [PubMed] [Google Scholar]
- 11.Arata MA, Cope C. Principles used in the management of visceral aneurysms. Tech Vasc Intervent Radiol. 2000;3:124–129. Available at www.springerlink.com/content/mh4855839n84r328/ [Google Scholar]
- 12.Guillon R, Garcier JM, Abergel A, et al. Management of splenic artery aneurysms and false aneurysms with endovascular treatment in 12 patients. Cardiovasc Intervent Radiol. 2003 May-Jun;26(3):256–60. doi: 10.1007/s00270-003-1948-y. [DOI] [PubMed] [Google Scholar]
- 13.Saad NE, Saad WE, Davies MG, Waldman DL, Fultz PJ, Rubens DJ. Pseudoaneurysms and the role of minimally invasive techniques in their management. Radiographics. 2005 Oct;25(Suppl 1):S173–89. doi: 10.1148/rg.25si055503. [DOI] [PubMed] [Google Scholar]