Abstract
Primary neoplasms of the petrous apex are rare and include eosinophilic granuloma, chondroma, chondrosarcoma, chordoma, and schwannoma. We report just the second published case of an intraosseous schwannoma of the petrous apex and are the first to describe the entity using magnetic resonance imaging. By studying the computed tomography and magnetic resonance imaging features of this rare tumor, it is possible to suggest the diagnosis preoperatively.
Keywords: Magnetic Resonance Imaging, Computed Tomography, petrous apex, temporal bone, intraosseous, schwannoma
CASE REPORT
A 48-year-old woman with a history of long-standing migraines presented with a 4-month history of new-onset headache and decreased sensation of the left face. There was no notable surgical, family, or social history. Physical exam revealed slightly diminished sensation in dermatomes corresponding to the left ophthalmic and maxillary divisions of the trigeminal nerve, but no other neurologic deficits.
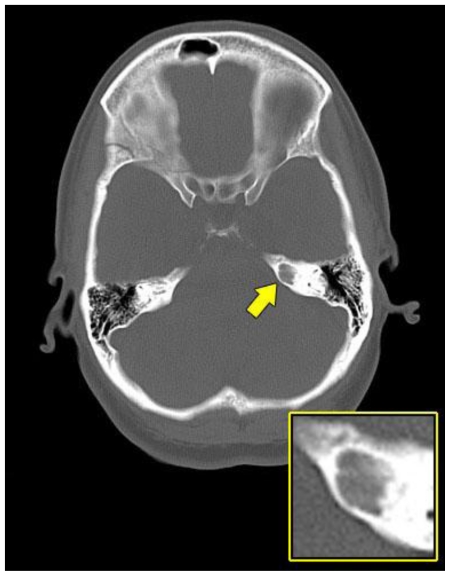
Computed tomography (CT) of the brain revealed a seven-millimeter (mm), well-defined, smoothly marginated lytic lesion of the left petrous bone. The lesion did not demonstrate an internal matrix and showed no associated extraosseous component (Fig. 1).
Figure 1.
A 48-year-old female with a left-sided intraosseous schwannoma of the petrous apex measuring 8.5 mm in the anteroposterior, 7 mm in the transverse, and 7 mm in the craniocaudal dimensions. Axial plain CT scan of the brain at the level of the petrous apex demonstrates a well-defined, smoothly marginated, lytic lesion with no internal matrix and no aggressive features located in the left petrous apex (arrow). (Protocol: 420 mAs, 140 kVp, 4.8 mm slice thickness).
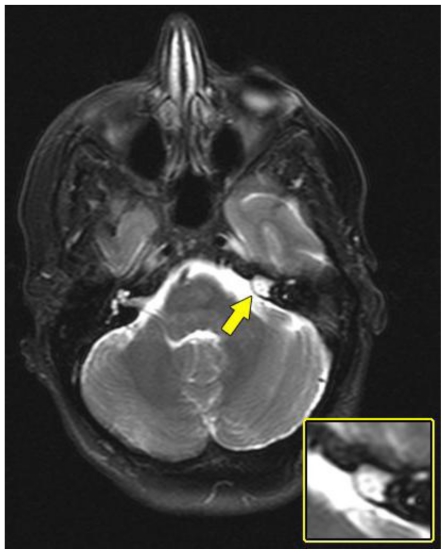
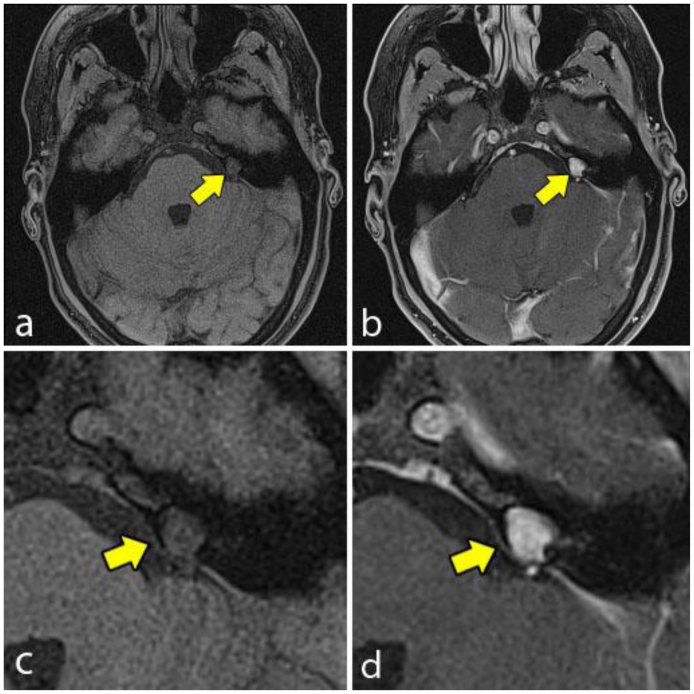
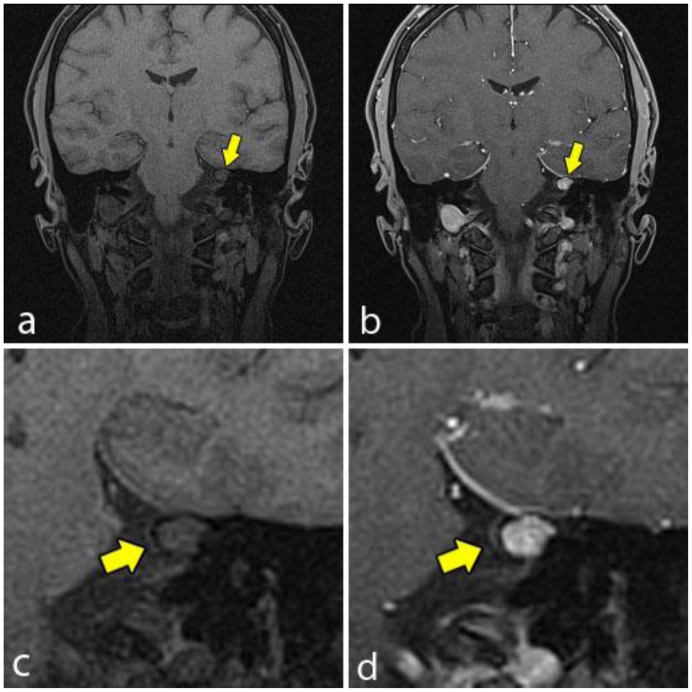
Magnetic resonance imaging (MRI) was obtained for further characterization and demonstrated a lesion within the medial aspect of the left petrous apex just superior to the porous acousticus. The lesion was hyperintense on T2 weighted (T2W) sequence (Fig. 2), isointense to brain on T1 weighted (T1W) sequence (Fig. 3a and 4a) and demonstrated solid enhancement after intravenous administration of gadolinium (Fig. 3b and 4b). It measured approximately 8 mm in the anteroposterior, 7 mm in the transverse and 7 mm in the craniocaudal dimensions. The lesion was mildly expansile, causing a thinning of the adjacent cortical bone, but it was well circumscribed with no evidence of cortical breakthrough. Furthermore, there was no significant extraosseous extension and no association with the cranial nerves or vasculature. No high signal was seen on diffusion-weighted images (DWI) (Fig. 5).
Figure 2.
A 48-year-old female with a left-sided intraosseous schwannoma of the petrous apex measuring 8.5 mm in the anteroposterior, 7 mm in the transverse, and 7 mm in the craniocaudal dimensions. Axial T2W fast spin echo fat saturation MRI of the brain at the level of the petrous apex demonstrates homogenous high signal and intraosseous location of the petrous apex lesion (arrow). (Protocol: Magnet strength 1.5 Tesla, TR 5310, TE 111, without contrast, 5 mm slice thickness).
Figure 3.
A 48-year-old female with a left-sided intraosseous schwannoma of the petrous apex measuring 8.5 mm in the anteroposterior, 7 mm in the transverse, and 7 mm in the craniocaudal dimensions. Axial T1W high resolution fat saturation MRI of the brain pre (3a, 3c) and post (3b, 3d) gadolinium administration at the level of the petrous apex. The pre contrast image (3a, 3c) demonstrates the left petrous apex lesion (arrow), which is isointense to brain. The post contrast image (3b, 3d) demonstrates solid enhancement of the same lesion. (Protocol: 3a, 3c; Magnet strength 1.5 Tesla, TR 5.6, TE 2.8, without contrast, 1 mm slice thickness. 3b, 3d; Magnet strength 1.5 Tesla, TR 5.6, TE 2.8, with 14 mL Multihance gadolinium, 1 mm slice thickness).
Figure 4.
A 48-year-old female with a left-sided intraosseous schwannoma of the petrous apex measuring 8.5 mm in the anteroposterior, 7 mm in the transverse, and 7 mm in the craniocaudal dimensions. Coronal T1W high resolution fat saturation MRI pre (4a, 4c) and post (4b, 4d) gadolinium administration. The pre contrast image (4a, 4c) demonstrates the left petrous apex lesion (arrow), which is isointense to brain and demonstrates no involvement of the cranial nerves. The post contrast image (4b, 4d) demonstrates solid enhancement of the same lesion. (Protocol: 4a, 4c; Magnet strength 1.5 Tesla, TR 5.37, TE 2.54, 1 mm slice thickness. 4b, 4d; Magnet strength 1.5 Tesla, TR 5.37, TE 2.54, with 14 mL of Multihance gadolinium, 1 mm slice thickness).
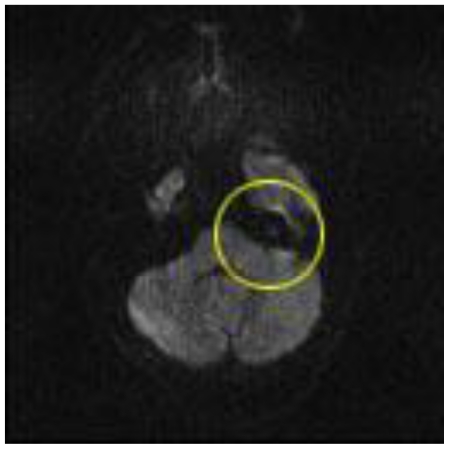
Figure 5.
A 48-year-old female with a left-sided intraosseous schwannoma of the petrous apex measuring 8.5 mm in the anteroposterior, 7 mm in the transverse, and 7 mm in the craniocaudal dimensions. DWI shows no area of high signal corresponding to petrous apex lesion (circle). (Protocol: Magnet strength 1.5, TR 4200, TE 111, without contrast, 5 mm slice thickness).
Based on location, morphology, and signal characteristics the preoperative differential included mucocele, chondrosarcoma, or cholesteatoma (epidermoid). The patient underwent gross total resection of the tumor. The procedure involved a left temporal craniotomy with subtemporal extradural approach and partial Kawase approach. Intraoperative monitoring for facial nerve and bilateral brainstem evoked potentials was used with no changes noted.
Tissue samples were sent for frozen section analysis and were positive for progesterone receptor. The preliminary pathological diagnosis was meningioma. Final pathological examination demonstrated a benign mesenchymal lesion most consistent with schwannoma (Fig. 6 and 7; see discussion below).
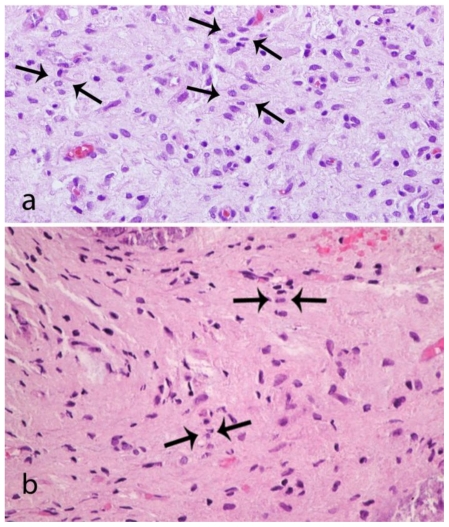
Figure 6.
A 48-year-old female with a left-sided intraosseous schwannoma of the petrous apex measuring 8.5 mm in the anteroposterior, 7 mm in the transverse, and 7 mm in the craniocaudal dimensions. Composite photomicrograph of two tissue sections (6a and 6b) demonstrating nuclei arranged in palisades (arrows). This is consistent with Antoni A tissue which is a characteristic feature of schwannomas. (Hematoxylin and eosin, original magnification 400x)
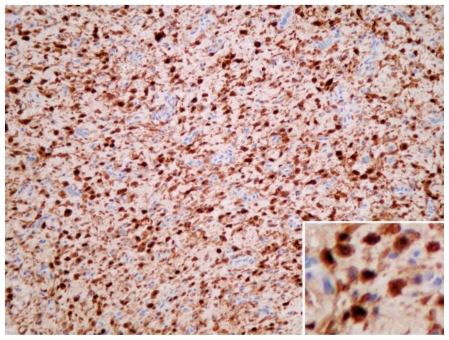
Figure 7.
A 48-year-old female with a left-sided intraosseous schwannoma of the petrous apex measuring 8.5 mm in the anteroposterior, 7 mm in the transverse, and 7 mm in the craniocaudal dimensions. Photomicrograph of tissue with dense brown nuclear staining for S-100, a neural crest marker, which is highly supportive of schwannoma. (Immunohistochemistry stain for S-100 antigen, original magnification 200x)
The patient did well postoperatively with no new neurologic deficits. She was discharged home with instructions for follow-up at a private clinic.
DISCUSSION
A variety of petrous apex lesions are described in the medical literature. These can be categorized as lesions originating from the temporal bone or those extending into the bone from adjacent tissues. Of those primarily arising within bone, most develop in the setting of a pneumatized apex, and include cholesterol granuloma, cholesteatoma, mucocele, and petrous apicitis [1].
Primary neoplasms of the petrous apex are rare and include eosinophilic granuloma, chondroma, chondrosarcoma, chordoma, and schwannoma [1,2]. Most neoplasms of the petrous apex arise from the direct extension of nearby primary tumors such as cranial nerve schwannomas, or as a result of metastatic spread from breast, lung, prostate and other cancers [1,3]. Most schwannomas affecting the petrous apex arise from the posterior fossa, jugular foramen or Meckel's cave [4].
We report a case of an intraosseous schwannoma of the petrous apex. A review of the literature revealed just one other such case reported by Solodnik et. al in 1986, which described the CT findings of an intraosseous petrous apex schwannoma [5]. This is the first report describing CT and MRI features of this entity.
Intraosseous schwannomas are rare, and compromise 0.2% of bone tumors, which are themselves quite rare [6]. They are generally solitary with no gender or ethnicity predilection. Up until 1995, only 80 cases had been described in the world literature, with most arising within the mandible or sacrum [6]. In 2000, a case report and literature review by Ersahin et. al revealed only 6 cases of intraosseous schwannoma of the skull, which includes the single known case of petrous apex schwannoma reported by Solodnik et. al [7].
In general, schwannomas arise almost exclusively from sensory nerves [8, 9]. Thus, the rarity of intraosseous schwannoma can, in part, be accounted for by the low density of sensory nerves within bone [9]. Schwannoma of bone is thought to originate from Schwann cells of the paravasal nerves, which travel with the nutrient arteries [10]. In their 1986 report on an intraosseous schwannoma of the petrous apex, Solodnik et. al postulated that the tumor arose from small sympathetic or parasympathetic fibers traversing the petrous apex [5]. Later, in 1995, Horn et. al suggested that the deep petrosal nerve was the nidus for development of two schwannomas arising from the carotid canal [2].
In our case, the patient had no major neurologic deficits, but a slight decrease in unilateral sensation of the face. This finding may be incidental, as the imaging and operative findings revealed no relation of the tumor to the trigeminal nerve or any of its divisions. The greater and lesser petrosal nerves were unlikely candidates, as operative findings showed no relation between these nerves and the tumor. Furthermore, there was no preoperative loss of tearing, salivation or other deficit to support involvement of either of these nerves. This fits previous reports of intraosseous schwannoma, which note that these tumors are generally asymptomatic and discovered incidentally [11].
Diagnostic imaging with CT and MRI is highly useful in characterization of petrous apex lesions. CT accurately defines the anatomic extent of the lesions and proximity to or invasion of bony structures such as the internal auditory canal or carotid canal. In conjunction with patient history and CT findings, MRI signal characteristic and enhancement pattern often provides definitive diagnosis prior to histological examination [1, 12, 13]. In 2007, Isaacson et al detailed the CT and MRI characteristics of various petrous apex lesions based on literature review [1, 12, 14, 15]. On MRI, petrous apex schwannomas are T1W isointense, enhancing, with high or low T2W signal. CT demonstrates an expansile, minimally erosive, isodense lesion [5]. The lesion in our case reflected the typical CT and MRI findings of schwannoma, as well as the described cases of intraosseous schwannoma. Nevertheless, it was not considered in the differential due to the rarity of this entity in that location.
Instead, the preoperative differential diagnosis included mucocele, chondrosarcoma, or cholesteatoma. Mucocele is similar to schwannoma in that it is hyperintense on T2W images, but it shows only peripheral enhancement and demonstrates variable T1W signal depending on protein content. Unlike schwannoma, mucocele generally shows destroyed septae on CT. This feature is likely nonspecific, however, as a schwannoma arising within a pneumatized apex (like mucocele) could theoretically mimic this finding [1]. Like schwannoma, chondrosarcoma is isointense on T1W images, hyperintense on T2W images and enhances. On CT, however, it can be seen infiltrating bone with eroded fragments left behind [1]. In addition to its destructive nature, chondrosarcoma can also demonstrate a mineralized chondroid matrix with a classical "ring-and-arc" appearance. This feature is helpful when present, but is seen only in about 50% of cases [16]. Lastly cholesteatoma is hypointense on T1W images, hyperintense in T2W images, demonstrates no enhancement, and shows restricted diffusion on DWI. On CT, smooth erosion of bone can be seen [1]. Each of these lesions has a feature, which distinguishes it from schwannoma. Thus, despite its rarity, intraosseous schwannoma should have been considered preoperatively.
In 2006, Ilgenfritz et al. noted that all reported cases of intraosseous schwannoma in the literature have been diagnosed postoperatively based on pathology findings [11]. This was also true in our case as schwannoma was not included in the preoperative differential. In our case, the histology showed nuclei in palisades (Fig. 6). This was consistent with Antoni A tissue which is typically seen in schwannomas [17]. There was no cystic degenerative tissue to suggest Antoni B tissue. This comports with the MRI findings, which failed to demonstrate the heterogenous signal typically seen in schwannomas that display Antoni B tissue [17].
Immunohistochemistry demonstrated strong nuclear staining for S-100 (Fig. 7) and weak staining for progesterone receptor. While progesterone receptor positivity is seen in 70% of meningiomas [18], it can also be seen in schwannomas [19]. For example, in a 2008 retrospective study of 59 archival acoustic neuroma specimens, Cafer et al demonstrated progesterone positivity in all samples. Information is limited on this topic, however, because progesterone staining is not commonly performed on schwannoma tissue [19]. Staining for epithelial membrane antigen (EMA), which is almost always positive in meningiomas, was negative. Caldesmon, a smooth muscle marker, was also negative. S-100 antigen is found in neural crest tumors such as schwannoma, and is highly expressed in Antoni A areas [18]. Thus, histological architecture along with immunohistochemistry led to the final diagnosis of intraosseous schwannoma.
TEACHING POINT
Intraosseous schwannoma of the petrous apex is a rare entity, and thus may be neglected when considering the differential diagnosis of a newly discovered petrous apex lesion. While diagnosis of intraosseous schwannoma has traditionally been made by histological examination after surgery, an awareness of its appearance on CT (expansile, isodense, and minimally erosive) and MRI (T1 W isointense, T2W hypo or hyperintense, and homogenously enhancing) makes it possible to suggest this diagnosis preoperatively.
Table 1.
Summary table for intraosseous schwannoma of the petrous apex
| Etiology | Hypothesized to originate from the Schwann cells of paravasal nerves, which travel with nutrient arterioles in bone. |
| Incidence | Intraosseous schwannoma in any location accounts for 0.2% of bone tumors. There are only two reported cases of intraosseous schwannoma of the petrous apex. |
| Gender ratio | 1:1 |
| Age predilection | Known cases include a 48 year old female and a 59 year old male. |
| Risk factors | None known |
| Treatment | Surgical excision |
| Prognosis | Benign tumor with low recurrence risk |
| Computed Tomography | Expansile, minimally erosive, isodense lesion |
| Magnetic Resonance Imaging | Expansile with hyper or hypo intense T2 weighted signal, isointense T1 weighted signal (to brain), with solid enhancement after intravenous administration of gadolinium. |
Table 2.
Differential diagnosis table for intraosseous schwannoma of the petrous apex
| Petrous Apex Lesion | MRI T1 W | MRI T2W | Other MRI Features | CT |
|---|---|---|---|---|
| Schwannoma | Isointense to brain | Hypo or Hyperintense | Homogenous enhancement | Expansile, minimally erosive, isodense lesion |
| Mucocele | Isointense or hyperintense (depends on protein concentration) | Hyperintense | Peripheral enhancement | Destroyed septae |
| Chondrosarcoma | Isointense | Hyperintense | Avid enhancement | Destroys bone with eroded fragments, “Ring-and-arc” appearance present in half of cases |
| Cholesteatoma | Hypointense | Hyperintense | No enhancement, DWI shows restricted diffusion | Smooth erosion of bone |
ACKNOWLEDGEMENTS
Thanks to Dr. Charif Sidani for guidance and editing.
ABBREVIATIONS
- CT
Computed tomography
- DWI
Diffusion weighted images
- EMA
Epithelial membrane antigen
- H & E
Hematoxylin and Eosin
- mAs
Milli ampere second
- mm
Millimeter
- MRI
Magnetic resonance imaging
- T1W
T1 weighted
- T2W
T2 weighted
- TE
Echo Time
- TR
Repetition Time
REFERENCES
- 1.Isaacson B, Kutz WJ, Roland PS. Lesions of the Petrous Apex: Diagnosis and Management. Otolaryngol Clin North Am. 2007;40:479–519. doi: 10.1016/j.otc.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Horn KL, Hankinson HL, Nissen AJ, McDaniel SL. Primary Schwannoma of the Petrous Apex. Skull Base Surgery. 1995;5(4):261–268. doi: 10.1055/s-2008-1058924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gloria-Cruz TI, Schachern PA, Paparella MM, et al. Metastases to temporal bones from primary nonsystemic malignant neoplasms. Arch Otolaryngol Head Neck Surg. 2000;126:209–14. doi: 10.1001/archotol.126.2.209. [DOI] [PubMed] [Google Scholar]
- 4.Jackler RK, Parker DA. Radiographic differential diagnosis of petrous apex lesions. Am J Otol. 1992;13:561–574. [PubMed] [Google Scholar]
- 5.Solodnik P, Som PM, Shugar JM. Intraosseous petrous apex neuroma: CT findings. J Comput Assist Tomogr. 1986;10:1027–1029. doi: 10.1097/00004728-198611000-00025. [DOI] [PubMed] [Google Scholar]
- 6.Sochart DH. Intraosseous schwannoma of the Calcaneum. The Foot. 1995;5:38–40. [Google Scholar]
- 7.Ersahin Y, Mutluer S, Damirtas E. Intraosseous neurinoma of the parietal bone. Child's Nerv Syst. 2000;16:181–183. doi: 10.1007/s003810050490. [DOI] [PubMed] [Google Scholar]
- 8.Goyal R, Saikia UN, Vashishta RK, Gulati G, Sharma RK. Intraosseous schwannoma of the frontal bone. Orthopedics. 2008 Mar;31(3):281. doi: 10.3928/01477447-20080301-16. [DOI] [PubMed] [Google Scholar]
- 9.De la Monte SM, Dorfman HD, Chandra R, Malawer M. Intraosseous schwannoma: histologic features, ultrastructure, and a review of the literature. Hum Pathol. 1984;15(6):551–558. doi: 10.1016/s0046-8177(84)80009-x. [DOI] [PubMed] [Google Scholar]
- 10.Flügel M, Lentzen B, Geldmacher J. Intraosseous Neurinoma Handchirurgie. 1977;9:3–5. [PubMed] [Google Scholar]
- 11.Ilgenfritz R, Jones K, Lueck N, Buckwalter J. Intraosseous neurilemmoma involving the distal tibia and fibula:A case report. The Iowa Orthopaedic Journal. 2006;26:138–143. [PMC free article] [PubMed] [Google Scholar]
- 12.Moore KR, Harnsberger HR. “Leave me alone” lesions of the petrous apex. AJNR Am J Neuroradiol. 1998;19:733–8. [PMC free article] [PubMed] [Google Scholar]
- 13.Chang P, Fagan PA, Atlas MD, et al. Imaging destructive lesions of the petrous apex. Laryngoscope. 1998;108:599–604. doi: 10.1097/00005537-199804000-00025. [DOI] [PubMed] [Google Scholar]
- 14.Sanna M, Zini C, Gamoletti R, et al. Petrous bone cholesteatoma. Skull Base Surgery. 1993;3:201–13. doi: 10.1055/s-2008-1060585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamakawa K, Shitara N, Genka S, et al. Clinical course and surgical prognosis of 33 cases of intracranial epidermoid tumors. Neurosurgery. 1989;24:568–73. doi: 10.1227/00006123-198904000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Murphey MD, Walker EA, Wilson AJ, Kransdorf MJ, Temple HT, Gannon FH. From the archives of the AFIP Imaging of primary chondrosarcoma: Radiologic-pathologic correlation. Radiographics. 2003;23:1245–1278. doi: 10.1148/rg.235035134. [DOI] [PubMed] [Google Scholar]
- 17.Wippold FJ, Lubner M, Perrin RJ, Laamle M, Perry A. Neuropathology for the neuroradiologist: Antoni A and Antoni B tissue patterns. Am J Neuroradiol. 2007;28:1633–38. doi: 10.3174/ajnr.A0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rockhill J, Mrugala M, Chamerlain MC. Intracranial meningiomas: an overview of diagnosis and treatment. Neurosurg focus. 2007;23(4):1–7. doi: 10.3171/FOC-07/10/E1. [DOI] [PubMed] [Google Scholar]
- 19.Cafer S, Bayramoglu I, Uzum N, Yilmaz M, Memis L, Uygur K. Expression and clinical significance of Ki-67, oestrogen and progesterone receptors in acoustic neuroma. The Journal of Laryngology & Otology. 2008;122:125–127. doi: 10.1017/S0022215107000229. [DOI] [PubMed] [Google Scholar]