Abstract
Objective
To study the value of T2-mapping and diffusion weighted imaging (DWI) in the diagnosis of early injury of knee cartilage.
Methods
Seventy-two subjects, including healthy group (n=30) and early cartilage injury group (n=42), were tested on MR scans with T2-mapping and DWI. T2 and apparent diffusion coefficient (ADC) values of cartilage were measured after being processed at the workstation, and the differences were statistically analyzed between the two groups.
Results
The mean T2 and ADC values of cartilage in early injury group and health group were respectively 51.58±4.15 ms and 1.78±0.35 ×10−3mm2/s, 39.54±4.02 ms and 1.44±0.17 ×10−3 mm2/s. There was significant difference between the values of T2 and ADC.
Conclusion
T2 and ADC values in early cartilage injury have obviously increased. T2-mapping and DWI have high clinical value in the diagnosis of early articular cartilage injury.
Keywords: Articular cartilage, magnetic resonance imaging, diffusion
INTRODUCTION
The knee is the largest and most complex joint, and articular cartilage is an important and indispensable structure of the knee in normal activities. Aging and various joint diseases may lead to cartilage degeneration or injury and then affecting normal movements of the knee joint. It cannot receive the same attention and is often overlooked or delayed, resulting in irreversible changes[1], just because it does not directly endanger human life. In recent years, development of molecular biology, pharmacology and other related subjects make early treatment of cartilage lesions possible, which requires early diagnosis of cartilage lesions. Magnetic Resonance Imaging (MRI) has proven to be a useful non-invasive tool in imaging articular cartilage, but previous studies have been mostly focused on the shape and signal changes of cartilage[2–3]. 3.0 T MR provides high spatial resolution and signal-to-noise ratio. In addition to detecting structural changes, advanced MRI techniques have been shown to have the potential of probing biochemical changes in the cartilage. T2-mapping and diffusion weighted imaging (DWI) can make quantitative analysis of the cartilage and detect lesions of early cartilage damage. By comparing T2 relaxation time and apparent diffusion coefficient (ADC) between the early cartilage injury and normal cartilage, we evaluated the significance of T2-mapping and the DWI in the early diagnosis and monitoring of cartilage damage.
PATIENTS AND METHODS
Data collection
This study was approved by the institutional review board of People’s Hospital of Liaocheng. Written informed consent was obtained from all participants. Patients were divided into two groups. One group was healthy, including 30 patients (aged 19 to 32 years, 24.5 years on average, 18 males and 12 females) without knee joint discomfort, trauma or surgical history. The other group was with early cartilage injury, including 42 patients (aged 25 to 43 years, 36.8 years on average, 25 males and 17 females) with medial or lateral compartment pain and positive McMurray sign. A vertical or oblique line of high signal reaching the articular surface of the menisci and no obvious abnormal signal in articular cartilage were seen on MR images. All MR examinations were performed within 30 days after injury, with an average of 12 days.
Scanning equipment and parameters
All examinations were performed on 3.0T scanner (Philips Medical Systems, Netherlands) using an 8-channel SENSE knee coil. Conventional MR imaging sequences, including T1WI, T2WI and PDWI and T2-mapping and DWI were performed in the right knees of normal and early cartilage damage groups. T2-mapping and the DWI scanning were performed according to lesion location. T2-mapping was conducted by 6-echo SE sequence of one-time scan and its parameters were as follows: TR / TE = 2400 ms/15, 30, 45, 60, 75, 90 ms, slice thickness=3 mm, gap=0.3 mm, FOV=16cm × 16cm, matrix=320 × 512, NSA = 1, scanning time=5min54s. DWI was conducted by using echo-planar imaging (EPI) and its parameters were as follows: TR / TE = 2000 ms/70 ms, slice thickness=3 mm, gap=0.3 mm, FOV=16cm × 16 cm, NSA = 8, matrix=288 × 160, b value = 600 s/mm2, scan time=1min8s. Corresponding T2-mapping and the ADC map were obtained at post-processing workstation.
Image post-processing and statistical analysis
ROI of normal group was selected and T2 and ADC values were measured in the medial tibial condyle, lateral tibial condyle, medial femoral condyle, lateral femoral condyle and patellar cartilage respectively. Each cartilage was measured from front to back; the patella was measured from the medial to the lateral. These were divided into three parts and measured at least 6 times and the average value was selected. T2 values were measured from the ROI of 42 knee joints corresponding to the articular cartilages near the meniscus tear, and the average values were calculated. Because the articular cartilage was thin, a small ROI was selected and T2 values were measured at an enlarged image in order to minimize random errors in measurement. The measurement results were indicated with the mean ± standard error. All data was analyzed statistically by SPSS12.0, and the comparison of two groups was tested by using two-sample t-test comparison. The difference (P <0.01) was significant.
RESULTS
30 patients of the normal group showed no abnormality in Conventional MR imaging. 42 patients underwent conventional MR examination, 10 cases in which the conventional images of showed significant abnormal cartilages were not included in the study. 32 patients with no cartilage abnormalities but different amounts of joint effusion were included in the research of early injury group. T2 values of the normal group and the cartilages of early cartilage injury group were shown in Table 1, and so were the ADC values in Table 2. The results showed that the average T2 values and ADC values of cartilage in early cartilage injury group were significantly higher than the normal group. The remarkable differences showed statistical significance (t values were −5.245, −4.438, P value<0.01). T2-mapping and ADC map of normal cartilage showed that cartilages were continuously intact and the signals were uniform (Figure 1). T2-mapping and ADC map from early cartilage damage group showed uneven thickness and the internal signals were mixed and uneven (Figure 2).
Table 1.
Normal group and the early cartilage injury group compared cartilage T2 values (x ± s; ms)
| Group | patella | *MCF | *LCF | *MCT | *LCT | average |
|---|---|---|---|---|---|---|
| Normal group, | 39.75±4.18 | 39.48±3.46 | 39.83±5.04 | 39.21±3.18 | 39.45±3.96 | 39.54±4.02 |
| Injury group | 51.32±3.46 | 53.28±4.72 | 52.46±4.18 | 49.36±3.98 | 51.47±4.26 | 51.58±4.15 |
MCF:medial-femoral-condyle
LCF:lateral condyle of femur
MCT: medial condyle of tibia
LCT:lateral condyle of tibia
Table 2.
Normal group and the early cartilage injury group compared cartilage ADC values (x ± s; × 10−3mm2/ s)
| Group | patella | *MCF | *LCF | *MCT | *LCT | average |
|---|---|---|---|---|---|---|
| Normal group | 1.44±0.16 | 1.43±0.18 | 1.45±0.16 | 1.44±.020 | 1.43±0.17 | 1.44±0.17 |
| Injury group | 1.78±0.32 | 1.76±0.41 | 1.80±0.47 | 1.88±0.36 | 1.80±0.31 | 1.78±0.35 |
MCF: medial-femoral-condyle
LCF: lateral condyle of femur
MCT: medial condyle of tibia
LCT:lateral condyle of tibia
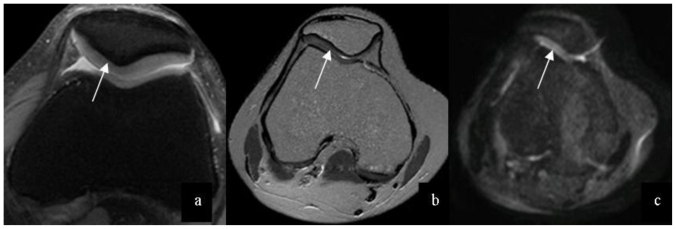
Figure 1.
27-year-old male patient with normal knee cartilage. Normal cartilage on PDW-SPAIR (a) (3.0 Tesla, TR/TE=415msec/8msec, Slice Thickness=3mm), T2 mapping (b) (3.0 Tesla, TR/TE=2400 msec/15, 30,45,60,75,90msec, Slice Thickness = 3mm) and DWI image (c) (3.0 Tesla, TR/TE = 2000 msec/70 msec, Slice Thickness = 3mm) were expressed as a continuous cartilage and uniform signal (arrows).
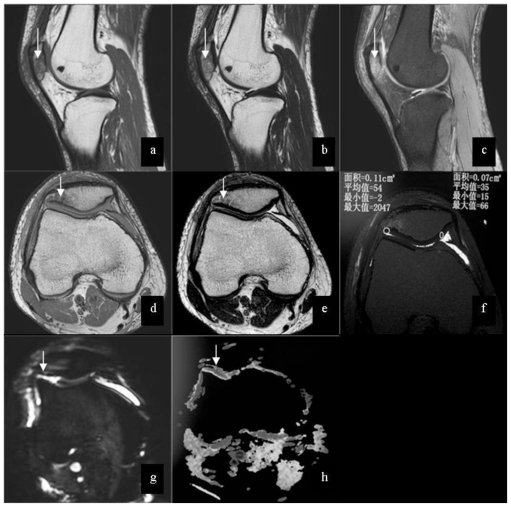
Figure 2.
25-year-old male patient with injury of right knee. Images shows low signal (arrow) on T1WI (3.0 Tesla, TR/TE = 2165 msec/ 30msec, Slice Thickness = 3mm) (a), mixed signal (arrow) on T2WI(3.0 Tesla, TR/TE = 2231 msec/ 100msec, Slice Thickness = 3mm) (b) and high signal(arrow) on PDW-SPAIR(3.0 Tesla, TR/TE = 415msec/ 8msec, Slice Thickness =3 mm) (c) in the bone marrow of patella. The axial images show low signal (arrow) on T1WI (d) and mixed signal (arrow) on T2WI (e) in the bone marrow of patella, and no abnormal signal in the cartilage of patellar is shown. On T2 map (f) (3.0 Tesla, TR/TE=2400 msec/15, 30,45,60,75,90msec, Slice Thickness = 3mm), the mean value of the right part of cartilage is 54, and the mean value of the left part of cartilage is 35. There are slightly high signal (arrow) on DWI (g) (3.0 Tesla, TR/TE=2000 msec/70 msec, slice thickness=3 mm, b =600 s/mm2 ) and high signal (arrow) on ADC(h) compared with normal cartilage.
DISCUSSION
Joint cartilage, mainly composed of the extra cellular matrix and cartilage cells, are hyaline cartilage, in which there are no vessels, nerves and lymph tissues, and its nutrition mainly relies on synovial fluid. Extra cellular matrix is mainly composed of water, collagen and protein glycogen polymer. Water, decreasing from the surface to the deep, takes up 80% of the cartilage weight. When articular surface is bearing weight, the water can be squeezed out from the cartilage matrix to reduce friction to the articular surface with its lubricating effect. Collagen is mainly composed of type II collagen, accounting for about 60% of the extracellular matrix. The stratification of hyaline cartilage is closely related to the arrangement of collagen fibers. According to the arrangement of the collagen fibers, the cartilage is divided into four layers: surface, intermediate layers, deep layers and calcified layers. Surface collagen fibers parallel to the cartilage surface, also known as parallel layers, account for 3–12% of full-thickness cartilage. Collagen fibers, without cartilage cells in it, are woven into a mesh to prevent the loss of proteoglycan and other internal macromolecules. The middle layer is known as the transition layer, in which the small cartilage cells are distributed in space frame of the collagen fibers. The deep layer, taking up more than 50% of full-thickness, is also known as radiation layer, in which the fibers are perpendicular to the cartilage surface and the cartilage cells are relatively large. The calcified layer bears less cartilage cells and very few collagen fibers are attached to the cartilage. The part between the radiation layer and calcified layer is called the tide line. The water of cartilage gradually decreased from the radiation to the surface layer and increased again from the calcified layer. Protein polysaccharide polymer weighs about 1 / 3 in soft joints, and the high water content in articular cartilage is mainly due to its large number of negative charge and donnan osmotic pressure[4]. The grading of cartilage injury by Recht[5] is as follows: grade 0, normal; grade 1, focal low signal in cartilage, the surface of cartilage is smooth; grade 2, the focal defect has a depth of less than 50% of the total cartilage thickness; grade 3, the focal defect has a depth of more than 50% of the total cartilage thickness; grade 4, the full cartilage were damaged, and the bone surface under cartilage is exposed.
The self-repair capacity of articular cartilage is very limited. Before the chondral defect the internal matrix has altered obviously, including the water molecules increase, proteoglycan decrease and collagen fiber disintegration. The first two stages are recoverable and reversible, but irreversible changes will occur if the collagen fiber network collapses, and there would be cartilage damage. Therefore, the use of new imaging methods in early diagnosis of cartilage lesions becomes increasingly important. T2-mapping and DWI are newly physiological cartilage magnetic resonance imaging technology, which can be used to evaluate changes in articular cartilage matrix components and provide cartilage metabolism and biochemical information [6]. The value of T2 mapping of cartilage is determined by the T2 relaxing time, and ADC value is determined by the diffusion of water molecular. T2-mapping is a multi-echo spin-echo sequence (SE) technology. Through the workstation, T2 relaxation time of tissue can be obtained by measuring the intensity of ROI, which can reflect the tissue’s specific attenuation nature by describing the transverse magnetization of the tissue. The value is obtained by measuring the MR signal intensity of different echo time and then calculated by the equation [3]: SIi, j (t) = SI0i, j o exp (− t / T2i, j), where SI0i,j is the pixel intensity at t= 0 and T2i,j is the T2 time constant of pixel i,j. A magnitude image is generated from the pixel SI0i,j data, and a T2 map is generated from the T2i,j data. Articular cartilage has been reported abroad[7,8] that the relationship between T2 values and the arrangement of cartilage collagen orientation and collagen, proteoglycan and water content, which can quantitatively reflect the changes in cartilage composition. Mosher [9,10] said that the cartilage T2 values are mainly determined by collagen content and arrangement of the fibers. From the cartilage surface to the deepest levels, T2 values are gradually decreasing, which is mainly due to the different layers of collagen fibers and different content. Watrin [10] proved, through human and animal experiments, that there is a direct relation between the cartilage T2 values and cartilage proteoglycan, water content. T2 values in cartilage have an inverse proportion to the distribution of proteoglycan, while are proportional to water distribution. In short, there is a relevant relationship between the increase of cartilage T2 relaxation time and cartilage ultrastructure damage, so the T2-mapping imaging technology for clinical application is of high value. By using signal attenuation of water molecules resulting from the phase inconsistencies caused by Brownian motion in the gradient field, DWI reflects this characteristic of the tissue. Since the diffusivity of water molecules is very sensitive to hydration of the material, the self-diffusion of water provides a possible way for the determination of water content within the cartilage [11]. ADC value increases after the reduction of cartilage matrix macromolecules.
In this study, the cartilage T2 values and ADC values of the cartilage injury group were both higher than that in the normal group. The matrix of articular cartilage is mainly composed of water, large molecules of collagens and proteoglycans. Most of collagens are type II collagens. They form the network by crossing each other and the network form cartilage stent with a large number of sugar folded protein molecules embedded in the collagen net. The negatively charged anions SO32-and COO- in glycoproteins are mutually exclusive and attract cations (mainly Na +), resulting in osmotic pressure, so that the water is pulled into the cartilage. The water in cartilage is limited within the gap between the collagen mesh. At the early stage, the type II collagens at the surface of articular cartilage degenerates, which increases the surface friction of joints and water permeability. The rapidly flowing of water in cartilage under pressure, the rack damage and loss of proteoglycan will all seriously weaken the role of cartilage in the hydraulic system and weaken its load capacity. Breakage of collagen makes proteoglycan scattered and more anions exposed, thus the water content of cartilage increase. With the further loss of proteoglycan, the remaining proteoglycan will have greater room for maneuver, and this will also increase the water content. Therefore, the decrease of articular cartilage collagen and proteoglycan at early stage and the increase of water content cause the increase of T2 values and ADC values in cartilage.
T2 values and ADC values of early stages cartilage injury were significantly higher, so a check can be carried out to find the changes in articular cartilage in early injury without significant morphological changes by measuring T2 relaxation time and the ADC values and provide an important reference in early diagnosis and guide the early clinical treatment, intervention, and to prevent irreversible changes in cartilage. Therefore, T2-mapping and DWI provides a more reliable and new method of quantitative measurement in the early diagnosis of traumatic articular cartilage. It is of high clinical value. The limitation of this study is that the number of samples is small, so a multi-center, large sample study is needed.
CONCLUSION
T2 and ADC values of cartilage with early injury are increased. MR T2-mapping and DWI are valuable tools in the diagnosis of early articular cartilage injury.
TEACHING POINT
The early injury of cartilage can be detected by measuring T2 and ADC values, and reference information can be provided for early diagnosis.
ABBREVIATIONS
- MR
magnetic resonance
- DWI
diffusion weighted imaging
- ADC
apparent diffusion coefficient
- T1WI
T1 weighted imaging
- T2WI
T2 weighted imaging
- PDWI
proton density weighted imaging
- TR
time of repetition
- TE
time of echo
- EPI
echo-planar imaging
- FOV
field of View
- ROI
region of interest
REFERENCES
- 1.Felson DT. An update on the pathogenesis and epidemiology of osteoarthritis. Radiol Clin North Am. 2004;42:1–9. v. doi: 10.1016/S0033-8389(03)00161-1. [DOI] [PubMed] [Google Scholar]
- 2.Glaser C. New techniques of cartilage imaging: T2 relaxation time and diffusion weighted MR imaging. Radiol Clin North Am. 2005;43:641–653. doi: 10.1016/j.rcl.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Mosher TJ, Smith H, Dardzinski BJ, et al. MR imaging and T2 mapping of femoral cartilage: in vivo determination of the magic angle effect. American Journal of Roentgenology. 2001;177:665–669. doi: 10.2214/ajr.177.3.1770665. [DOI] [PubMed] [Google Scholar]
- 4.Nishii T, Sugano N, Ohzono K, et al. Significance of lesion size and location in the prediction of collapse of osteonecrosis of the femoral head: a new three-dimensional quantification usingmagnetic resonance imaging[J] JOrthop Res. 2002;20(1):130–136. doi: 10.1016/S0736-0266(01)00063-8. [DOI] [PubMed] [Google Scholar]
- 5.Recht MP, Bobic V, Burstein D, et al. Magnetic resonance of cartilage. Clin Orthop. 2001;391:379–396. doi: 10.1097/00003086-200110001-00035. [DOI] [PubMed] [Google Scholar]
- 6.Welschyz GH, Trattnigy S, Domayeryx S, et al. Multimodal approach in the use of clinical scoring, morphological MRI and biochemical T2-mapping and diffusion-weighted imaging in their ability to assess differences between cartilage repair tissue after microfracture therapy and matrix-associated autologous chondrocyte transplantation: a pilot study. Osteoarthritis and Cartilage. 2009;4:1–9. doi: 10.1016/j.joca.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 7.Stahl R, Blumenkrantz G, Carballido-Gamio J, et al. MRI-derived T2 relaxation times and cartilage morphometry of the tibio-femoral joint in subjects with and without osteoarthritis during a 1-year follow-up. Osteoarthritis Cartilage. 2007;15:1225–1234. doi: 10.1016/j.joca.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 8.Hannila I, Nieminen MT, Rauvala E, et al. Patellar cartilage lesions: comparison of magnetic resonance imaging and T2 relaxation-time mapping. Acta Radiol. 2007;48:444–448. doi: 10.1080/02841850701280817. [DOI] [PubMed] [Google Scholar]
- 9.Smith HE, Mosher TJ, Dardzinski BJ, et al. Spatial variation in cartilage T2 of the knee. J Magn Reson Imaging. 2001;14:50–55. doi: 10.1002/jmri.1150. [DOI] [PubMed] [Google Scholar]
- 10.Watrin A, Ruaud JP, Olivier PT, et al. T2 mapping of rat patellar cartilage. Radiology. 2001;219:395–402. doi: 10.1148/radiology.219.2.r01ma32395. [DOI] [PubMed] [Google Scholar]
- 11.Miller KL, Hargreaves BA, Gold GE, et al. Steady-State Diffusion-Weighted Imaging of In Vivo Knee Cartilage. Magnetic Resonance in Medicine. 2004;51:394–398. doi: 10.1002/mrm.10696. [DOI] [PubMed] [Google Scholar]