Abstract
We report the case of a 35 year old African American female who developed hypertrophic olivary degeneration secondary to resection of a pontine cavernous malformation. The patient initially complained of headaches and diplopia. Unenhanced computed tomography (CT) and magnetic resonance images (MRI) of the brain revealed a left pontine cavernous malformation with scattered foci of recent and remote hemorrhage. The patient subsequently underwent surgical resection of the lesion. Follow up MRI 7 months post surgery demonstrated hypertrophy and T2 signal hyperintensity in the ipsilateral inferior olivary nucleus secondary to hypertrophic olivary degeneration. Familiarity with this diagnosis and its imaging characteristics is required of the radiologist to prevent erroneous diagnoses of other pathology.
Keywords: Magnetic resonance imaging, Computed Tomography, Hypertrophic olivary degeneration, cavernous malformation, Triangle of Guillain-Mollaret
CASE REPORT
A 35 year old African American female with a history of prior intracranial hemorrhage presented with headaches and acute 6th nerve palsy of three weeks duration. At the time of her first hemorrhage she had been diagnosed with a pontine cavernous malformation. She was managed conservatively at that time and had remained asymptomatic for a number of years until the present episode. Upon re-admission, an unenhanced CT of the brain showed a focal hyperdensity in the left pons causing mild effacement of the 4th ventricle (Figure 1). MRI demonstrated blood products of varying ages with foci of acute/subacute hemorrhage in the left dorsolateral pons (Figure 2A–C) compatible with a cavernous malformation (Zabramski Type 1). No enhancement was seen after administration of intravenous gadolinium, and no abnormality was seen in the medulla oblongata at initial imaging.
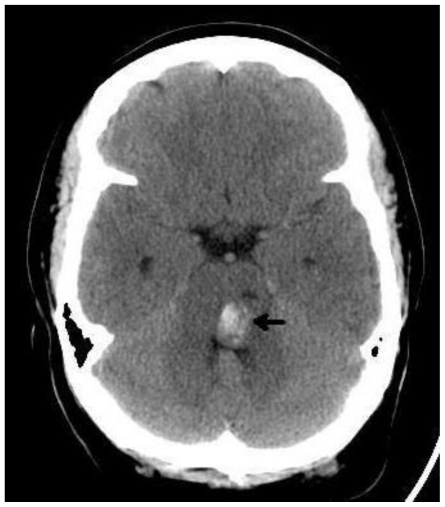
Figure 1.
35 year old female with a history of pontine cavernous malformation, now with headaches and 6th nerve palsy. Unenhanced axial CT (GE Lightspeed Pro16, 140 mA, 142 kV, 5mm slice thickness) demonstrates a focal hyperdensity in the left pons with mass effect on the 4th ventricle compatible with acute hemorrhage.
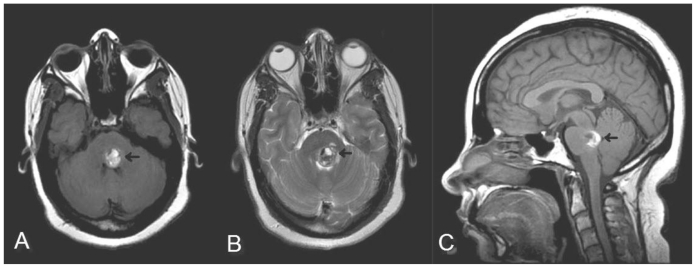
Figure 2.
35 year old female with a history of pontine cavernous malformation, now with headaches and 6th nerve palsy. Selected corresponding MR images (GE Excite 1.5T) including (A) axial noncontrast T1 (Spin Echo, TR 650, TE 13, NEX 1, flip angle 90), (B) T2 fast spin echo sequence (TR 3500, TE 129, NEX 1, Flip angle 90, echo train length 28) and (C) saggital noncontrast T1 (Spin Echo, TR 483, TE 13, NEX 1, flip angle 90, slice thickness 5mm) show focal hemorrhage into the left posterior pons. Blood products of varying ages are demonstrated by bight signal on T1 and T2 corresponding to subacute blood with foci of T1 and T2 low signal representing more acute hemorrhage.
Surgical resection was undertaken due to the patient’s recurrent symptomatic hemorrhages and pathology was consistent with a pontine cavernous malformation. Routine follow up MRI of the brain 7 months after discharge showed post operative changes with prominent T2 signal hypointensity in the area of prior hemorrhage related to hemosiderin and ferritin deposition(Figure 3A). However, new focal abnormality was seen in the brainstem. T1 sequences demonstrating swelling (Figure 3B) and T2 images signal hyperintensity (Figure 3C) in the ipsilateral medulla oblongata corresponding to the location of the inferior olivary nucleus. These changes are most obvious on FLAIR (Figure 4). There was no corresponding enhancement with gadolinium, and diffusion weighted sequences showed no restriction. The patient had no clinically observable palatal tremor and her diplopia had improved. MR imaging was performed on two different 1.5T MR scanners (Symphony-Siemens, Medical Solutions USA, Malvern PA, USA and Signa Excite--General Electric, Medical Systems, Milwaukee, WI, USA).
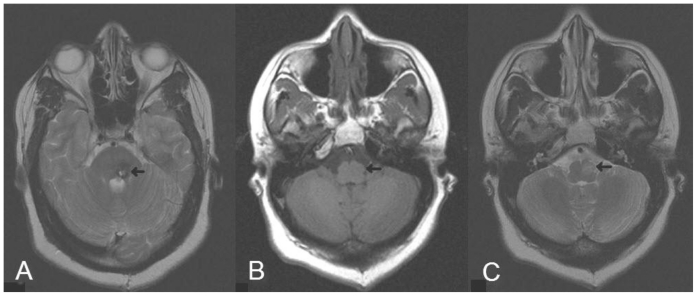
Figure 3.
35 year old female now status post resection of her pontine cavernous malformation with MR (Siemens Symphony 1.5T) obtained 7 months post-operatively. (A) Axial T2 fast spin echo (TR 4340, TE 91, NEX 1, Flip angle 150, Echo train length 13, slice thickness 5mm) at the level of the middle cerebellar peduncle shows chronic blood products and gliosis at the site of the resected cavernous malformation. (B) Axial spin echo T1 noncontrast image (TR 389, TE 8, NEX 1, Flip angle 90) and (C) axial T2 (TR 4340, TE 91, NEX 1, Flip angle 150, Echo train length 13, slice thickness 5mm) at the level of the internal auditory canals shows hypertrophy and T2 signal hyperintensity of the left inferior olive.
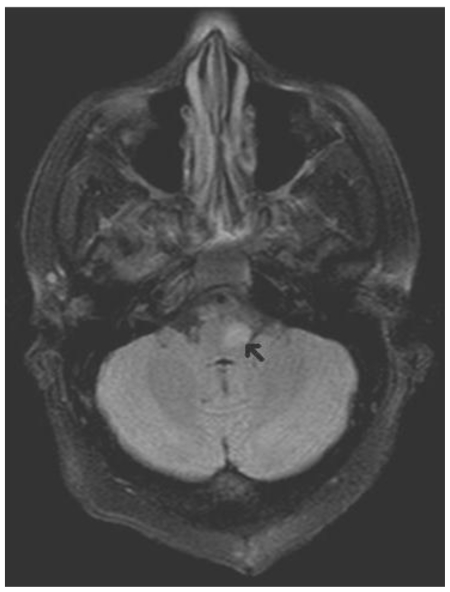
Figure 4.
35 year old female now status post resection of pontine cavernous malformation with MR (Siemens Symphony 1.5T) obtained 7 months post-operatively. Axial FLAIR image (TR 7500, TE 93, IT 2500, NEX 1, Flip angle 150, Echo train length 15) best demonstrates the T2 signal changes with marked signal hyperintensity of the left inferior olivary nucleus.
DISCUSSION
Hypertrophic olivary degeneration (HOD) is a rare form of neuronal degeneration that occurs secondary to any injury that disrupts the afferent fibers to the inferior olive within the dentato-rubro-olivary tract (Triangle of Guillain-Mollaret) [1]. Deafferentation causes transsynpatic degeneration and leads to hypertrophy of the inferior olive in a specific timeframe as demonstrated on MR imaging [2]. Classically, disruption of the triangle of Guillain and Mollaret results in clinical palatal myoclonus or other dentatorubral tremor [3].
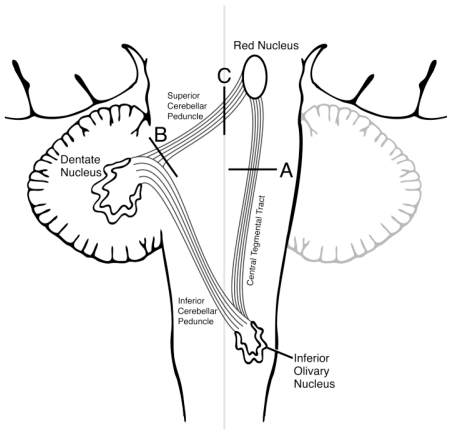
The triangle of nerve tracts involved in the genesis of HOD was first described by Guillain and Mollaret in 1931[1]. These dentato-rubro-olivary fibers connect the ipislateral red nucleus and inferior olive with the contralateral dentate nucleus. Efferent fibers from the dentate nucleus course in the superior cerebellar peduncle and connect to the contralateral red nucleus after decussating in the brachium conjuntivum [4]. The red nucleus in turn sends efferent fibers through the central tegmental tract to the ipsilateral inferior olivary nucleus. The inferior olive is connected to the contralateral cerebellum via the inferior cerebellar peduncle thus forming a triangle (Figure 5). Typically lesions disrupting the superior cerebellar peduncle or central tegmental tracts lead to HOD because de-afferentiating the inferior olivary nucleus is the key phenomenon [3, 5]. Site of the inciting lesion localizes the subsequent HOD. If the lesion affects the red nucleus or central tegmental tract, HOD is ipsilateral [6, 7]. Alternatively if the lesion is in the superior cerebellar peduncle or dentate nucleus, HOD occurs in the contralateral inferior olive [8, 9]. If a lesion involves the brachium conjunctivum (decussation of the superior cerebellar peduncle) or interrupts both the central tegmental tract and superior cerebellar peduncle, bilateral HOD occurs [10, 5]. Lesions involving the inferior cerebellar peduncle do not typically cause HOD, however, some patients with HOD demonstrate contralateral cerebellar atrophy presumably mediated by the olivo-dentate fibers [8]. Rare cases of bilateral HOD have been described with lesions affecting a single cerebellar hemisphere; the reason for which is not clear [4]. Our patient’s cavernous malformation in the dorsolateral pons affected the central tegmental tracts and thus produced ipsilateral HOD.
Figure 5.
Graphic representation of the dentato-rubro-olivary tracts (Triangle of Guillain-Mollaret). Various typical lesion locations are labeled and would be expected to cause (A) ipsilateral, (B) contralateral, or (C) bilateral HOD respectively.
Hypertrophic olivary degeneration is quite unusual since enlargement rather than atrophy occurs. Enlargement is due to a phenomenon called trans-synaptic (or trans-neuronal) degeneration. Histopathological analysis of autopsy specimens has demonstrated an unusual pattern of neuronal cytoplasmic vacuolization, hypertrophy of astrocytes, increased gliosis and demyelination of the olivary nucleus leading to macroscopic hypertrophy [11].
Clinical symptoms classically include palatal tremor, and can include ocular myoclonus and dentatorubral tremors. These abnormal involuntary motor movements are thought to arise from failure of inhibition of the inferior olive as many of the supplying nerve fibers from the dentate nucleus are primarily inhibitory or GABAergic [9]. Although a classic finding, palatal tremors are not always present in patients with HOD seen on imaging, but the reverse is not true and HOD is essentially always found in patients with palatal myoclonus [3, 6, 9]. Palatal myoclonus is reported to typically occur 10–11 months subsequent to the anatomic insult [12], and thus our 7 month post-operative patient may still develop this symptom. When these tremors occur, they are difficult to treat and rarely resolve [12, 7] but successful management of symptoms with benzodiazepines and carbamazepine has been reported [5].
A variety of insults can lead to HOD, including hemorrhage, infarct, trauma, surgery, or tumor [5, 7], and HOD has been described in both adults and children [6]. In our case, it is not clear if HOD occurred secondary to the initial brainstem hemorrhage or secondary to surgical intervention. Given the patient’s history of previous hemorrhage without HOD, we favor surgery as the causative insult.
The signal changes and swelling seen on MRI occur in a specific sequence. T2 hyperintensity in the inferior olivary nucleus can be seen as early as 1–2 months after ictus and has been reported to persist indefinitely [2]. The inferior olive may be isointense on T1 imaging, as in our case, or in some instances slightly hyperintense [6]. Hypertrophy of the inferior olive is a delayed finding seen beginning 4–6 months after the event and resolving usually within the first year and always by 3–4 years [12, 2]. Atrophy of the inferior olive can be seen years after the event [2]. These imaging findings have been described as three distinct stages (Table 1). Stage one showing T2 hyperintensity without hypertrophy, stage two both hypertrophy and high signal, and stage three resolution of hypertrophy (with or without atrophy) with persistent signal abnormality [2]. According to this classification, our patient would fall within the second stage of HOD evolution.
Table 1.
Stages of hypertrophic olivary degeneration
| Stage 1 | T2 signal hyperintensity without hypertrophy |
| Stage 2 | T2 signal hyperintensity with hypertrophy |
| Stage 3 | Resolution of hypertrophy with or without atrophy and persistent signal abnormality |
Focal signal changes seen in the inferior medulla are not pathognomonic for HOD. Infarction, demyelinating disease, malignancy-such as primary brainstem astrocytoma or metastasis, infections-such as TB or AIDS related syndromes, and inflammatory processes such as sarcoidosis, could all produce similar signal changes [12]. Absence of enhancement helps distinguish HOD from tumor, or inflammation/infection. Precise localization of the signal changes to the inferior olivary nucleus is also very helpful in making the diagnosis, but again not specific as similar changes have been reported to occur in Wernicke-Korsakoff syndrome [12]. Enlargement of the inferior olive helps narrow the differential, since a chronic infarction or demyelinating process would be expected to produce atrophy. Identifying an inciting lesion appropriately located to interrupt the dentate-rubro-olivary tracts with the correct timing of the signal change and swelling is the key to diagnosis.
TEACHING POINT
Hypertrophic olivary degeneration is a rare and potentially confusing imaging finding with unusual histological features causing macroscopic enlargement rather than atrophy of the inferior olive. Palatal myoclonus or other movement disorders may not always occur; however, the possibility of developing HOD and its clinical sequela is a relevant concern for clinicians and patients when surgery is contemplated. Knowledge of the condition and its MR characteristics on the part of the radiologist can prevent erroneous diagnoses of more sinister pathology.
Table 2.
Summary table for hypertrophic olivary degeneration
| Etiology | Transsynaptic neuronal degeneration secondary to any disruption of the dentato-rubro-olivary tracts. |
| Incidence | Rare. |
| Gender and age predilection | No known age or gender distribution. HOD has been described in both adults and children. |
| Risk factors | Any insult to the brain stem and cerebellum involving the relevant anatomical tracts can potentially cause HOD, including hemorrhage, infarct, trauma, surgery, or tumor. |
| Treatment | Successful symptomatic management of typical tremors has been reported with benzodiazepines and carbamazepine. |
| Prognosis | Guarded as symptoms are likely irreversible. |
| Findings at imaging | Enlargement and T2/FLAIR signal hyperintensity of the inferior olivary nucleus without enhancement in the setting of a prior inciting lesion appropriately located to interrupt the dentate-rubro-olivary tracts. The correct timing of the signal change and swelling is the key to diagnosis |
Table 3.
Differential diagnosis table for hypertrophic olivary degeneration
| Diagnosis | T1 (non-contrast) | T1 (post contrast) | T2/FlAIR | Anatomical changes |
|---|---|---|---|---|
| Hypertrophic Olivary Degeneration | Isointense to slightly hyperintense | No enhancement | Hyperintense | Swelling of inferior olive. |
| Medullary Infarct | Iso to hypointense depending on age | None in acute or chronic infarcts. Variable with subacute. |
Hyperintense | Atrophy of involved brainstem |
| Demyelinating disease | Typically isointense | Variable with inhomogeneous enhancement often present. | Hyperintense | Atrophy of involved brainstem |
| Malignancy | Variable depending on cellularity and presence of hemorrhage. | Enhancement often present | Variable but often hyperintense | Enlargement can be seen. |
| Infection or inflammation (eg TB or sardcoidosis) | Hyper or hypointense | Enhancement often present | Usually hypersintense | Typically not localized to the inferior olive. |
ABBREVIATIONS
- CT
Computed tomography
- MRI
Magnetic resonance imaging
- HOD
Hypertrophic olivary degeneration
- FLAIR
Fluid attenuated inversion recovery
REFERENCES
- 1.Guillain G, Mollaret P. Deux de myoclonies synchrones et rhythmees velopharyngolaryngo-oculo-diaphragmatiques. Rev Neurol. 1931;2:545–566. [Google Scholar]
- 2.Goyal M, Versnick E, Tuite P, Cyr JS, Kucharczyk W, Montanera W, et al. Hypertrophic Olivary Degeneration: Metaanalysis of the Temporal Evolution of MR Findings. AJNR AM J Neuroradiol. 2000;21:1073–1077. [PMC free article] [PubMed] [Google Scholar]
- 3.Lapresle J. Rhythmic Palatal Myoclonus and the Dentato-Olivary Pathway. J Neurol. 1979;220:223–230. doi: 10.1007/BF00314146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conforto AB, Smid J, Marie SKN, Ciriaco JGM, Santoro PP, Leite CC, et al. Bilateral Olivary Hypertrophy After Unilateral Cerebellar Infarction. Arq Neuropsiqulatr. 2005;63(2-A):321–323. doi: 10.1590/s0004-282x2005000200022. [DOI] [PubMed] [Google Scholar]
- 5.Hornyak M, Osborn AG, Couldwell WT. Hypertrophic Olivary Degeneration after Surgical Removal of Cavernous Malformation of the Brain Stem: report of four cases and review of the literature. Acta Neurochir. 2008;150:149–156. doi: 10.1007/s00701-007-1470-0. [DOI] [PubMed] [Google Scholar]
- 6.Phatouros CC, McConachie NS. Hypertrophic olivary degeneration: case report in a child. Pediatr Radiol. 1998;28:830–831. doi: 10.1007/s002470050475. [DOI] [PubMed] [Google Scholar]
- 7.Macht S, Hanggi D, Turowski B. Hypertrophic Olivary Degeneration Folllowing Pontine Cavernoma Hemorrhage: A typical change Accompanying Lesions in the Guillain-Mollaret Triangle. Clin Neuroradiol. 2009;19:235–237. doi: 10.1007/s00062-009-9001-4. [DOI] [PubMed] [Google Scholar]
- 8.Choh NA, Choh SA, Jehangir M. Hypertrophic olivary degeneration: the forgotten triangle of Guillain and Mollaret. Neurol India. 2009;57:507–509. doi: 10.4103/0028-3886.55587. [DOI] [PubMed] [Google Scholar]
- 9.Akar S, Drappatz J, Hsu L, Blinder RA, Black PM, Kesari S. Hypertrophic Olivary degeneration after resection of a cerebellar tumor. J Neurooncol. 2008;87:341–345. doi: 10.1007/s11060-008-9523-7. [DOI] [PubMed] [Google Scholar]
- 10.Gerace C, Fele MR, Luna R, Piazza G. Neurologial Picture: Bilateral hypertrophic olivary degeneration. J Neurol Neurosurg Psychiatry. 2006;77:73. doi: 10.1136/jnnp.2005.069831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitajima M, Korogi Y, Shimomura O, Sakamoto Y, Hiria T, Miyayama H, et al. Hypertrophic olivary degeneration: MR imaging and pathologic findings. Radiology. 1994;192:539–534. doi: 10.1148/radiology.192.2.8029428. [DOI] [PubMed] [Google Scholar]
- 12.Salamon-Murayama N, Russell EJ, Rabin BM. Case 17: Hypertrophic Olivary Degeneration Secondary to Pontine Hemorrhage. Radiology. 1999;213:814–817. doi: 10.1148/radiology.213.3.r99dc43814. [DOI] [PubMed] [Google Scholar]