Abstract
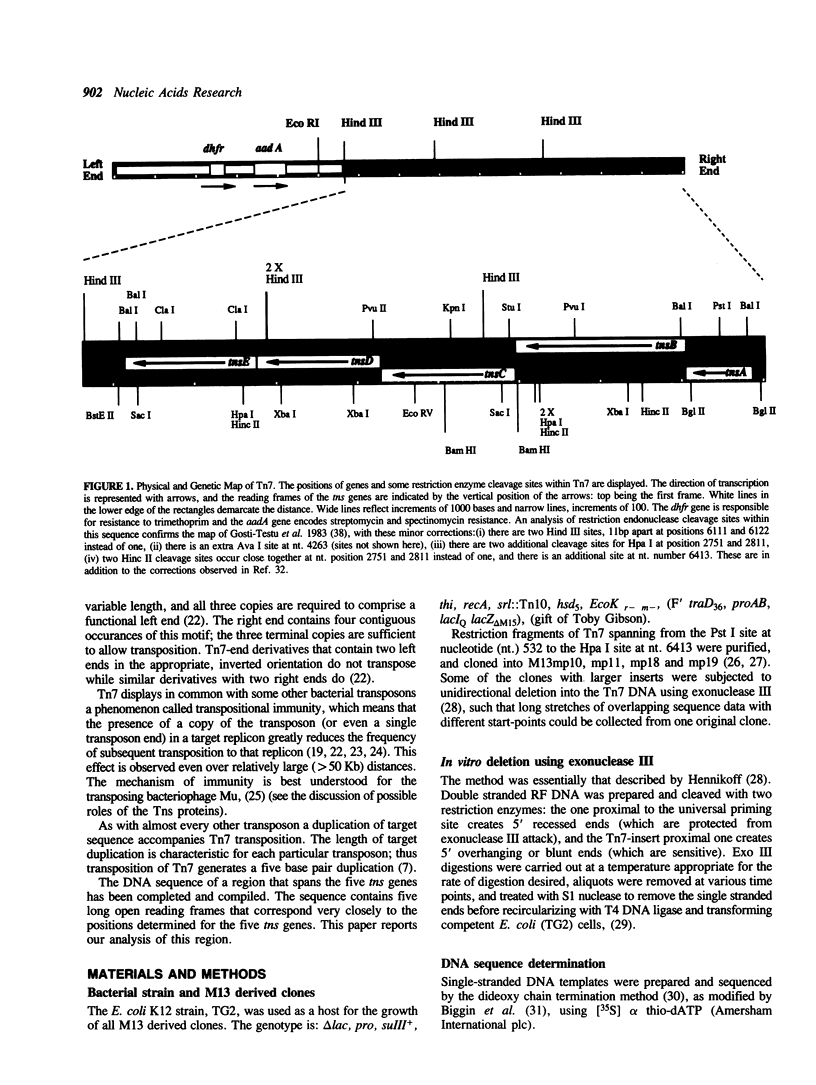
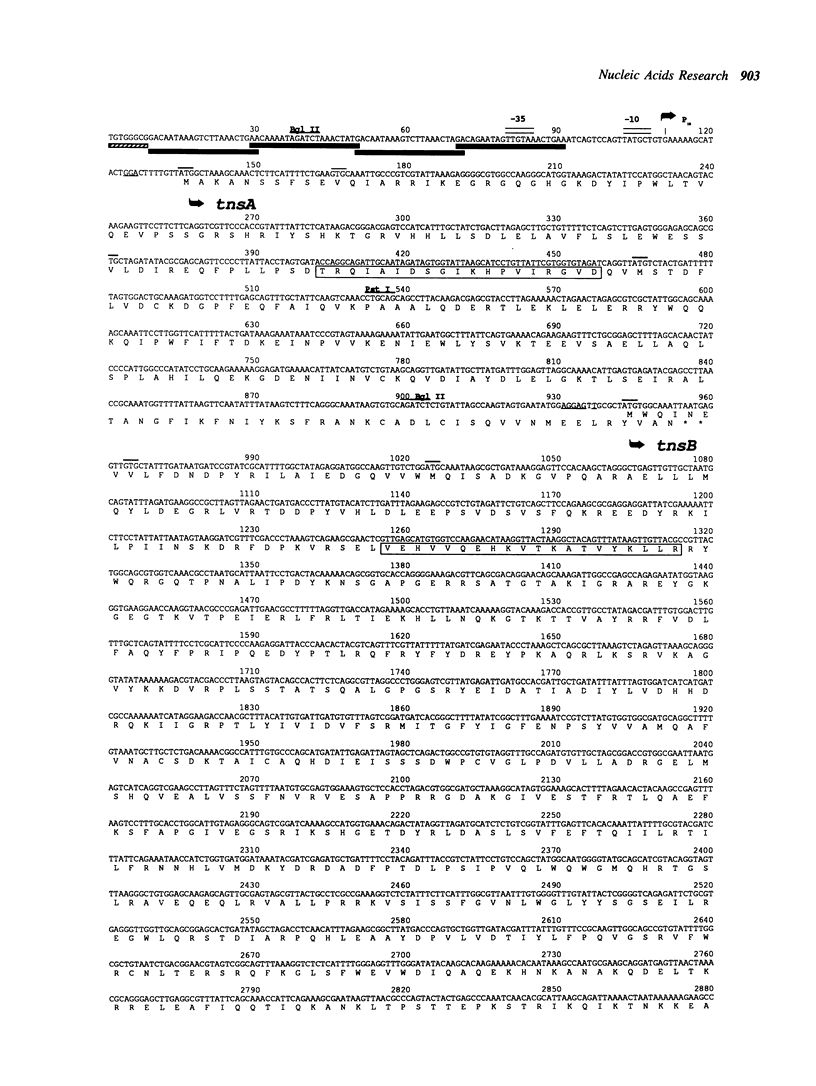
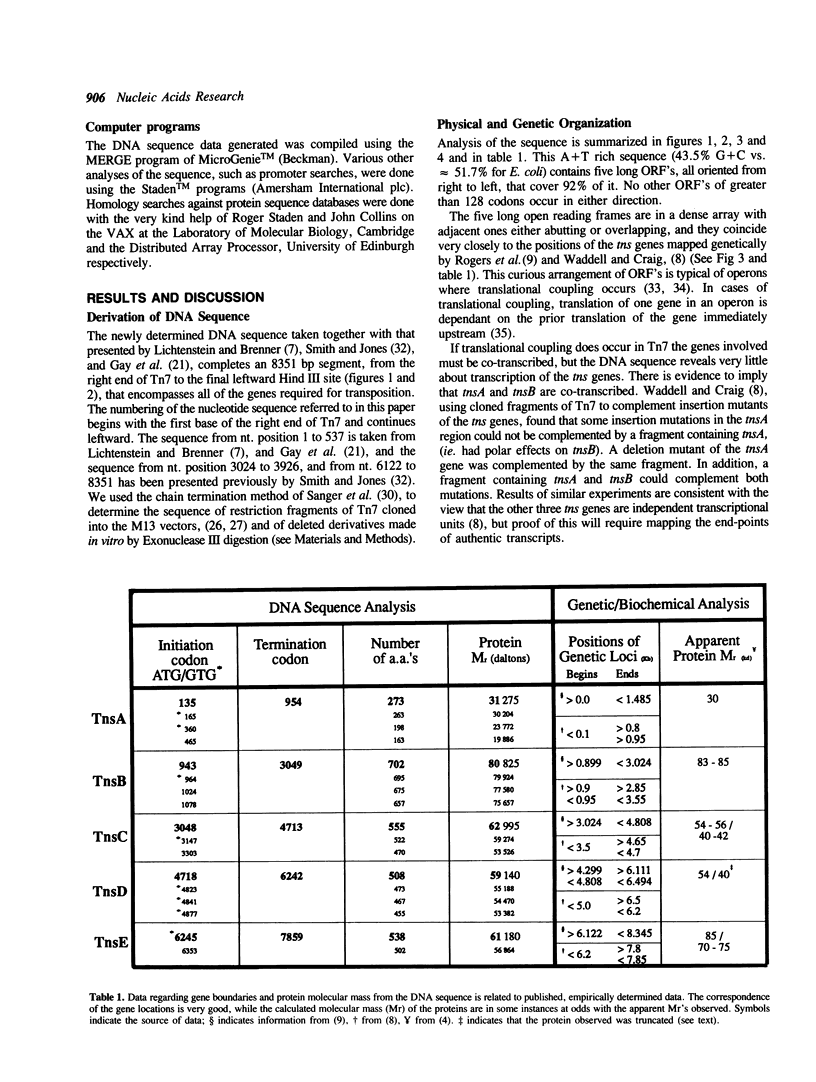
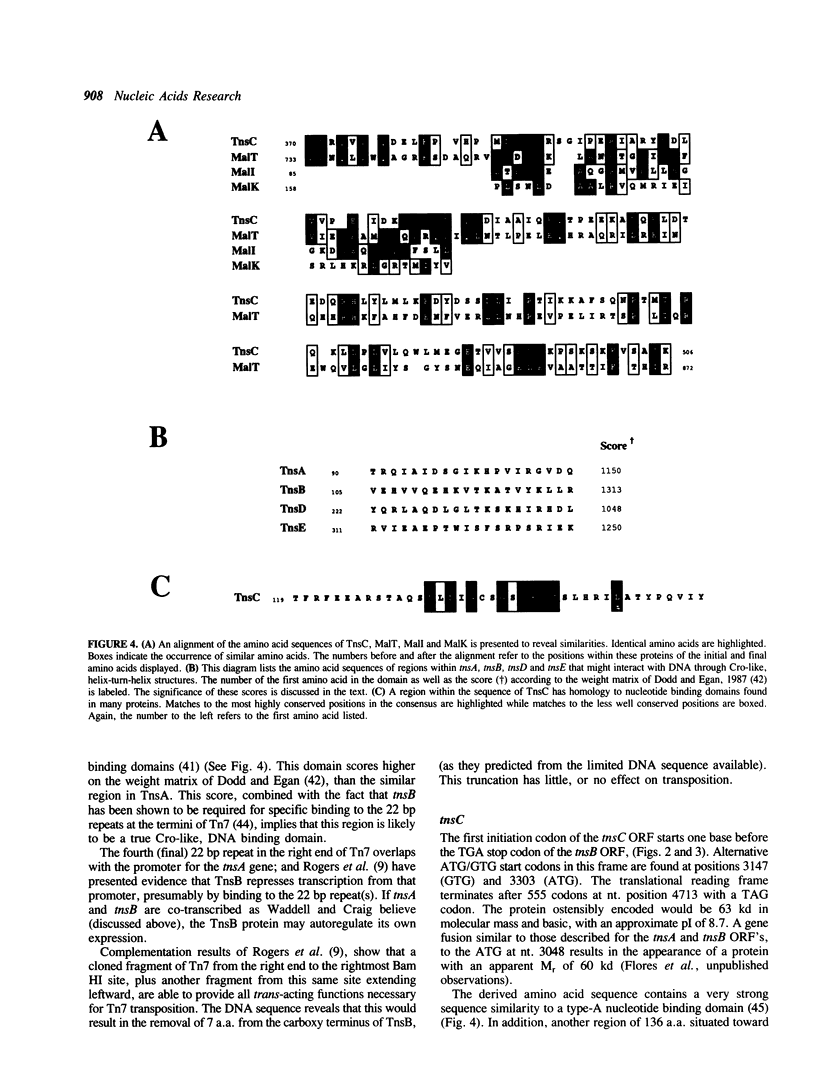
A region of DNA sequence of the bacterial transposon Tn7, which is required for transposition, has been determined. This DNA sequence completes an 8351 base pair (bp) region containing five long open reading frames (ORF's) that correspond to the genetically defined genes, tnsA, B, C, D and E, required for Tn7 transposition. All of the ORF's are oriented in the same direction, ie. inward from the element's right end. The genes are in a very compact arrangement with the presumed initiation codons never more than two bases beyond the preceding termination codon. Domains with similarity to the helix-turn-helix genre of Cro-like, sequence specific DNA binding sites occur within the deduced amino acid (a.a.) sequence of the TnsA, TnsB, TnsD and TnsE proteins. Translation of the tnsC ORF reveals strong homology to a consensus sequence for nucleotide binding sites as well as a region of similarity to a transcriptional activator (MalT). No striking a.a. sequence similarity to other DNA recombinases is observed. The possible roles of these proteins in Tn7 transposition is discussed in light of the analysis presented.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adzuma K., Mizuuchi K. Target immunity of Mu transposition reflects a differential distribution of Mu B protein. Cell. 1988 Apr 22;53(2):257–266. doi: 10.1016/0092-8674(88)90387-x. [DOI] [PubMed] [Google Scholar]
- Arciszewska L. K., Drake D., Craig N. L. Transposon Tn7. cis-Acting sequences in transposition and transposition immunity. J Mol Biol. 1989 May 5;207(1):35–52. doi: 10.1016/0022-2836(89)90439-7. [DOI] [PubMed] [Google Scholar]
- Barth P. T., Datta N., Hedges R. W., Grinter N. J. Transposition of a deoxyribonucleic acid sequence encoding trimethoprim and streptomycin resistances from R483 to other replicons. J Bacteriol. 1976 Mar;125(3):800–810. doi: 10.1128/jb.125.3.800-810.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth P. T., Grinter N. J., Bradley D. E. Conjugal transfer system of plasmid RP4: analysis by transposon 7 insertion. J Bacteriol. 1978 Jan;133(1):43–52. doi: 10.1128/jb.133.1.43-52.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth P. T., Grinter N. J. Map of plasmid RP4 derived by insertion of transposon C. J Mol Biol. 1977 Jul 5;113(3):455–474. doi: 10.1016/0022-2836(77)90233-9. [DOI] [PubMed] [Google Scholar]
- Been M. D., Champoux J. J. DNA breakage and closure by rat liver type 1 topoisomerase: separation of the half-reactions by using a single-stranded DNA substrate. Proc Natl Acad Sci U S A. 1981 May;78(5):2883–2887. doi: 10.1073/pnas.78.5.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brevet J., Faure F., Borowski D. Tn7-encoded proteins. Mol Gen Genet. 1985;201(2):258–264. doi: 10.1007/BF00425668. [DOI] [PubMed] [Google Scholar]
- Cole S. T., Raibaud O. The nucleotide sequence of the malT gene encoding the positive regulator of the Escherichia coli maltose regulon. Gene. 1986;42(2):201–208. doi: 10.1016/0378-1119(86)90297-0. [DOI] [PubMed] [Google Scholar]
- Craig N. L., Nash H. A. The mechanism of phage lambda site-specific recombination: site-specific breakage of DNA by Int topoisomerase. Cell. 1983 Dec;35(3 Pt 2):795–803. doi: 10.1016/0092-8674(83)90112-5. [DOI] [PubMed] [Google Scholar]
- Craigie R., Mizuuchi K. Transposition of Mu DNA: joining of Mu to target DNA can be uncoupled from cleavage at the ends of Mu. Cell. 1987 Nov 6;51(3):493–501. doi: 10.1016/0092-8674(87)90645-3. [DOI] [PubMed] [Google Scholar]
- Derbyshire K. M., Hwang L., Grindley N. D. Genetic analysis of the interaction of the insertion sequence IS903 transposase with its terminal inverted repeats. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8049–8053. doi: 10.1073/pnas.84.22.8049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd I. B., Egan J. B. Systematic method for the detection of potential lambda Cro-like DNA-binding regions in proteins. J Mol Biol. 1987 Apr 5;194(3):557–564. doi: 10.1016/0022-2836(87)90681-4. [DOI] [PubMed] [Google Scholar]
- Echols H. Multiple DNA-protein interactions governing high-precision DNA transactions. Science. 1986 Sep 5;233(4768):1050–1056. doi: 10.1126/science.2943018. [DOI] [PubMed] [Google Scholar]
- Gay N. J., Tybulewicz V. L., Walker J. E. Insertion of transposon Tn7 into the Escherichia coli glmS transcriptional terminator. Biochem J. 1986 Feb 15;234(1):111–117. doi: 10.1042/bj2340111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosti-Testu F., Brevet J. Détermination des séquences terminales du transposon Tn7. C R Seances Acad Sci III. 1982 Jan 25;294(4):193–196. [PubMed] [Google Scholar]
- Harley C. B., Reynolds R. P. Analysis of E. coli promoter sequences. Nucleic Acids Res. 1987 Mar 11;15(5):2343–2361. doi: 10.1093/nar/15.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. J., Heffron F., Twu J. S., Schloemer R. H., Lee C. H. Analysis of Tn3 sequences required for transposition and immunity. Gene. 1986;41(1):23–31. doi: 10.1016/0378-1119(86)90263-5. [DOI] [PubMed] [Google Scholar]
- Iida S., Huber H., Hiestand-Nauer R., Meyer J., Bickle T. A., Arber W. The bacteriophage P1 site-specific recombinase cin: recombination events and DNA recognition sequences. Cold Spring Harb Symp Quant Biol. 1984;49:769–777. doi: 10.1101/sqb.1984.049.01.087. [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Bruist M. B., Glaccum M. B., Simon M. I. In vitro analysis of Hin-mediated site-specific recombination. Cold Spring Harb Symp Quant Biol. 1984;49:751–760. doi: 10.1101/sqb.1984.049.01.085. [DOI] [PubMed] [Google Scholar]
- Krishnapillai V., Nash J., Lanka E. Insertion mutations in the promiscuous IncP-1 plasmid R18 which affect its host range between Pseudomonas species. Plasmid. 1984 Nov;12(3):170–180. doi: 10.1016/0147-619x(84)90041-6. [DOI] [PubMed] [Google Scholar]
- Lichtenstein C., Brenner S. Site-specific properties of Tn7 transposition into the E. coli chromosome. Mol Gen Genet. 1981;183(2):380–387. doi: 10.1007/BF00270644. [DOI] [PubMed] [Google Scholar]
- Lichtenstein C., Brenner S. Unique insertion site of Tn7 in the E. coli chromosome. Nature. 1982 Jun 17;297(5867):601–603. doi: 10.1038/297601a0. [DOI] [PubMed] [Google Scholar]
- Lindahl L., Archer R. H., McCormick J. R., Freedman L. P., Zengel J. M. Translational coupling of the two proximal genes in the S10 ribosomal protein operon of Escherichia coli. J Bacteriol. 1989 May;171(5):2639–2645. doi: 10.1128/jb.171.5.2639-2645.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl L., Zengel J. M. Ribosomal genes in Escherichia coli. Annu Rev Genet. 1986;20:297–326. doi: 10.1146/annurev.ge.20.120186.001501. [DOI] [PubMed] [Google Scholar]
- Liu-Johnson H. N., Gartenberg M. R., Crothers D. M. The DNA binding domain and bending angle of E. coli CAP protein. Cell. 1986 Dec 26;47(6):995–1005. doi: 10.1016/0092-8674(86)90814-7. [DOI] [PubMed] [Google Scholar]
- McKown R. L., Orle K. A., Chen T., Craig N. L. Sequence requirements of Escherichia coli attTn7, a specific site of transposon Tn7 insertion. J Bacteriol. 1988 Jan;170(1):352–358. doi: 10.1128/jb.170.1.352-358.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKown R. L., Waddell C. S., Arciszewska L. K., Craig N. L. Identification of a transposon Tn7-dependent DNA-binding activity that recognizes the ends of Tn7. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7807–7811. doi: 10.1073/pnas.84.22.7807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens G., Hoffmann A., Blöcker H., Frank R., Kahmann R. Gin-mediated site-specific recombination in bacteriophage Mu DNA: overproduction of the protein and inversion in vitro. EMBO J. 1984 Oct;3(10):2415–2421. doi: 10.1002/j.1460-2075.1984.tb02148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Mizuuchi K., Gellert M., Nash H. A. Involement of supertwisted DNA in integrative recombination of bacteriophage lambda. J Mol Biol. 1978 May 25;121(3):375–392. doi: 10.1016/0022-2836(78)90370-4. [DOI] [PubMed] [Google Scholar]
- Moore R. J., Krishnapillai V. Tn7 and Tn501 Insertions into Pseudomonas aeruginosa plasmid R91-5: mapping of two transfer regions. J Bacteriol. 1982 Jan;149(1):276–283. doi: 10.1128/jb.149.1.276-283.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisato D., Kleckner N. Tn10 transposition and circle formation in vitro. Cell. 1987 Oct 9;51(1):101–111. doi: 10.1016/0092-8674(87)90014-6. [DOI] [PubMed] [Google Scholar]
- Ogawa H. I., Tolle C. L., Summers A. O. Physical and genetic map of the organomercury resistance (Omr) and inorganic mercury resistance (Hgr) loci of the IncM plasmid R831b. Gene. 1984 Dec;32(3):311–320. doi: 10.1016/0378-1119(84)90006-4. [DOI] [PubMed] [Google Scholar]
- Ohlendorf D. H., Anderson W. F., Matthews B. W. Many gene-regulatory proteins appear to have a similar alpha-helical fold that binds DNA and evolved from a common precursor. J Mol Evol. 1983;19(2):109–114. doi: 10.1007/BF02300748. [DOI] [PubMed] [Google Scholar]
- Plasterk R. H., Kanaar R., van de Putte P. A genetic switch in vitro: DNA inversion by Gin protein of phage Mu. Proc Natl Acad Sci U S A. 1984 May;81(9):2689–2692. doi: 10.1073/pnas.81.9.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadri M. I., Flores C. C., Davis A. J., Lichtenstein C. P. Genetic analysis of attTn7, the transposon Tn7 attachment site in Escherichia coli, using a novel M13-based transduction assay. J Mol Biol. 1989 May 5;207(1):85–98. doi: 10.1016/0022-2836(89)90442-7. [DOI] [PubMed] [Google Scholar]
- Raibaud O., Vidal-Ingigliardi D., Richet E. A complex nucleoprotein structure involved in activation of transcription of two divergent Escherichia coli promoters. J Mol Biol. 1989 Feb 5;205(3):471–485. doi: 10.1016/0022-2836(89)90218-0. [DOI] [PubMed] [Google Scholar]
- Reidl J., Römisch K., Ehrmann M., Boos W. MalI, a novel protein involved in regulation of the maltose system of Escherichia coli, is highly homologous to the repressor proteins GalR, CytR, and LacI. J Bacteriol. 1989 Sep;171(9):4888–4899. doi: 10.1128/jb.171.9.4888-4899.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richet E., Raibaud O. MalT, the regulatory protein of the Escherichia coli maltose system, is an ATP-dependent transcriptional activator. EMBO J. 1989 Mar;8(3):981–987. doi: 10.1002/j.1460-2075.1989.tb03461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richet E., Raibaud O. Purification and properties of the MalT protein, the transcription activator of the Escherichia coli maltose regulon. J Biol Chem. 1987 Sep 15;262(26):12647–12653. [PubMed] [Google Scholar]
- Robinson M. K., Bennett P. M., Richmond M. H. Inhibition of TnA translocation by TnA. J Bacteriol. 1977 Jan;129(1):407–414. doi: 10.1128/jb.129.1.407-414.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers M., Ekaterinaki N., Nimmo E., Sherratt D. Analysis of Tn7 transposition. Mol Gen Genet. 1986 Dec;205(3):550–556. doi: 10.1007/BF00338097. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer R. T., Yocum R. R., Doolittle R. F., Lewis M., Pabo C. O. Homology among DNA-binding proteins suggests use of a conserved super-secondary structure. Nature. 1982 Jul 29;298(5873):447–451. doi: 10.1038/298447a0. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. M., Jones P. Effects of deletions in transposon Tn7 on its frequency of transposition. J Bacteriol. 1984 Mar;157(3):962–964. doi: 10.1128/jb.157.3.962-964.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. M., Jones P. Tn7 transposition: a multigene process. Identification of a regulatory gene product. Nucleic Acids Res. 1986 Oct 24;14(20):7915–7927. doi: 10.1093/nar/14.20.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sor F., Bolotin-Fukuhara M., Nomura M. Mutational alterations of translational coupling in the L11 ribosomal protein operon of Escherichia coli. J Bacteriol. 1987 Aug;169(8):3495–3507. doi: 10.1128/jb.169.8.3495-3507.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell C. S., Craig N. L. Tn7 transposition: recognition of the attTn7 target sequence. Proc Natl Acad Sci U S A. 1989 Jun;86(11):3958–3962. doi: 10.1073/pnas.86.11.3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell C. S., Craig N. L. Tn7 transposition: two transposition pathways directed by five Tn7-encoded genes. Genes Dev. 1988 Feb;2(2):137–149. doi: 10.1101/gad.2.2.137. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B., Jacquemin-Sablon A., Live T. R., Fareed G. C., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. VI. Further purification and properties of polynucleotide ligase from Escherichia coli infected with bacteriophage T4. J Biol Chem. 1968 Sep 10;243(17):4543–4555. [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- de Crombrugghe B., Busby S., Buc H. Cyclic AMP receptor protein: role in transcription activation. Science. 1984 May 25;224(4651):831–838. doi: 10.1126/science.6372090. [DOI] [PubMed] [Google Scholar]
- von Hippel P. H., Bear D. G., Morgan W. D., McSwiggen J. A. Protein-nucleic acid interactions in transcription: a molecular analysis. Annu Rev Biochem. 1984;53:389–446. doi: 10.1146/annurev.bi.53.070184.002133. [DOI] [PubMed] [Google Scholar]



