Abstract
Solid Pseudopapillary Tumor of the pancreas is a rare nonfunctioning tumor. It is considered a low-grade malignancy that is apparently curable with surgical complete excision in most instances. We present a case of solid pseudopapillary pancreatic tumor that represented a challenge to the radiologists. This case highlights its possible various appearances and the need to the radiologist to be familiar with them.
Keywords: Pancreas, pancreatic neoplasms, CT, MRI
CASE REPORT
A 35 year-old caucasian woman with no relevant medical or surgical history presented with vague abdominal discomfort for 3 months, without nausea, vomiting, diarrhea, jaundice or change in weight. On physical examination, no tenderness or abdominal mass was detected. Blood serum analyses were normal, including tumor markers.
Imaging findings
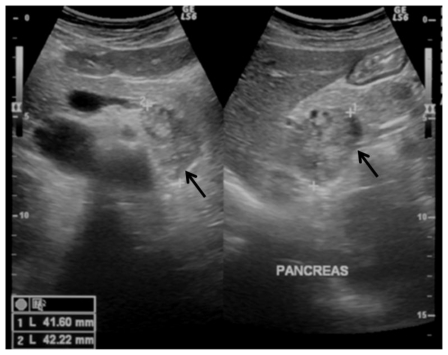
The referring physician started the work up with an ultrasound that revealed a well limited, heterogeneous nodular lesion of 42mm (the longest diameter) adjacent to the contour of the body-tail portions of the pancreas (Fig 1).
Figure 1.
35-year-old women with a solid pseudopappilary tumor. Ultrasound, transverse transabdominal image. A well circumscribed nodular retroperitoneal lesion is depicted, just posteriorly to the pancreas; it is solid, heterogeneous, slightly hypoechoic to the adjacent pancreatic parenchyma, with no signs of local invasion. (GE Logic 7 Pro, curved transducer, 3.5–5 Mhz)
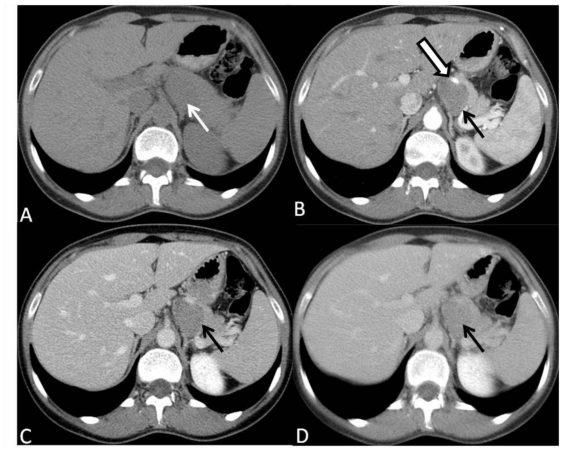
A subsequent abdominal computed tomography (CT) before and after intravenous contrast administration during parenchymal pancreatic phase and portal venous phase followed, that showed a solid, well circumscribed multilobular lesion localized on the left of the celiac trunk, posterior to the pancreatic body and tail, displacing it anteriorly, as well as splenic artery. Cleavage planes with celiac trunk and left adrenal gland seemed to be preserved, but not with pancreatic body and tail, though there were no signs of direct invasion. It was difficult to define the origin of the lesion. After intravenous contrast administration, there was slight (about 30 HU) enhancement of the lesion noticed, always lower than pancreatic parenchyma in both phases (Fig. 2). There was no other relevant alteration of the exam. The first diagnostic hypothesis was an enlarged lymph node.
Figure 2.
35-year-old women with a solid pseudopappilary tumor. Axial CT scans depict a sharply marginated retroperitoneal, solid and hypovascular lesion. (A) Precontrast scan shows homogeneous, slightly hypoattenuating lesion (arrow) posterior to the body and the tail of pancreas. (B) Scan obtained during pancreatic phase show hypoattenuating lesion, with clear border, without infiltration of perilesional fat, displacing the celiac trunk anteriorly (open arrow), and the pancreatic tail anterolaterally. (C) On scans obtained during portal venous and (D) delayed phases, the lesion shows progressive enhancement, but is still hypoattenuating compared with pancreatic parenchyma. There is neither ductal dilatation nor pancreatic atrophy. In the remaining study (not shown), there were no lymph node enlargement or nodules in abdominal solid organs, such as liver, kidney or spleen, suggesting metastasis. Positive oral contrast was given to the patient. (GE BrightSpeed S 4 slices; 3.75 mm slice thickness; 120 KV e 80 mA, 100 ml intravenous ioxitalamate meglumine 300mg/ml - injected at 3,5mL/sec)
Since the lesion was not clearly understood, a biopsy of the lesion was proposed to the patient, which refused it. One year later, the patient presented with the same symptoms. Abdominal MR was performed in the same month, including in-phase and out-of-phase axial T1-weighted images (wi), axial and coronal T2-wi and axial T1-wi after gadolinium injection. T2-wi images demonstrated a hyperintense 40mm retroperitoneal lesion, adjacent to the posterior border of body-tail of the pancreas (Fig. 3). No lipid content could be depicted on chemical-shift images, and the lesion demonstrated intermediate signal intensity on T1-wi (Fig. 4). The dynamic study showed no significant enhancement relative to pancreatic parenchyma in arterial phase, but slight enhancement during portal venous and equilibrium phases, but still lower than the adjacent parenchyma (Fig. 5).
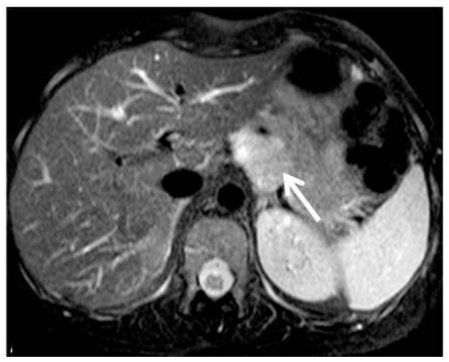
Figure 3.
35-year-old women with solid pseudopappilary tumor. Axial T2 weighted MR image demonstrates a well defined, hyperintense lesion adjacent to the posterior border of the pancreatic body-tail. Fat cleavage plane with pancreatic gland is lost, but no signs of local invasion are identified. [Philips Intera 1.5 T, 5 mm slice thickness, TE=1651, TR=70]
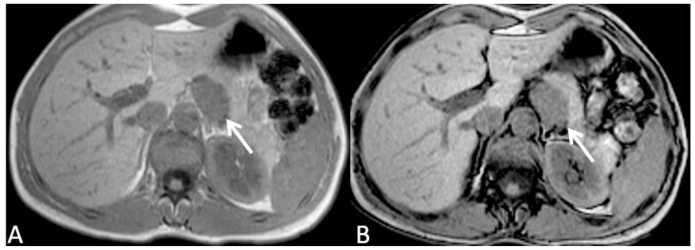
Figure 4.
35-year-old women with solid pseudopappilary tumor. Axial T1 weighted MR image in-phase (A) and out-of-phase (B) demonstrates a well defined, hypointense lesion adjacent to the posterior border of the pancreatic body-tail. No signal loss in out-of-phase images excluded the presence of microscopic fat content of the lesion. Fat cleavage plane with pancreatic gland is lost, but no signs of local invasion are identified. [Philips Intera 1.5 T, 5mm slice thickness, TE=171, TR=4,6 and TR=2,3]
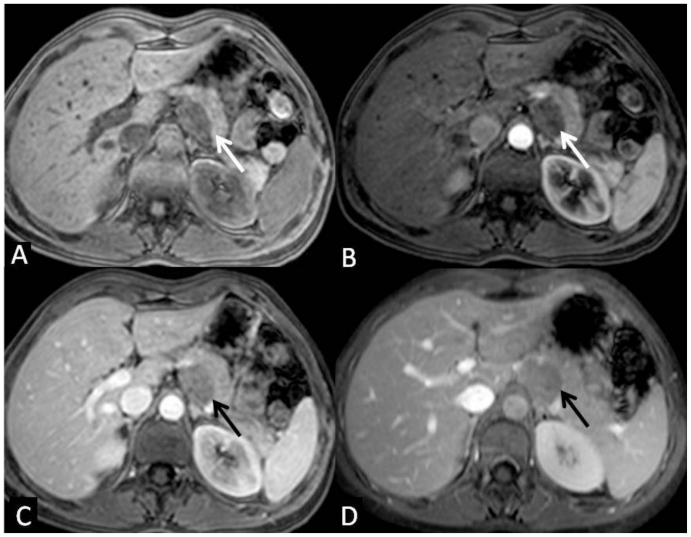
Figure 5.
35-year-old women with solid pseudopappilary tumor. Axial T1 weighted MR imagees, fat-suppressed, 3-D spoiled gradient recall echo before (A) and after contrast administration during the arterial (B), portal (C) and equilibrium phase (D) demonstrate a well defined, intermediate signal intensity lesion with progressive enhancement, lower than the adjacent normal pancreas. The lesion displaces the celiac trunk anteriorly, and the pancreatic tail anterolaterally. The boundaries with left adrenal gland and with diaphragmatic crus are preserved. [Philips Intera 1.5 T, 5 mm slice thickness, TE=3.6, TR=1.7, flip angle 15º, 90ml intravenous gadolinium at a 3,5ml/s injection rate]
Morphology and dimensions did not change when compared with previous CT, and the lesion seemed to be independent from the surrounding structures (Fig. 3).
A thoracic and pelvic CT scan was performed, as well as serum markers (beta2-microglobulin) obtained, to search for signs suggestive of lymphoproliferative disorder, but both were negative.
In the context of a single solid retroperitoneal lesion of undetermined etiology and its difficult percutaneous assessment, patient underwent elective laparoscopic resection.
Pathologic evaluation
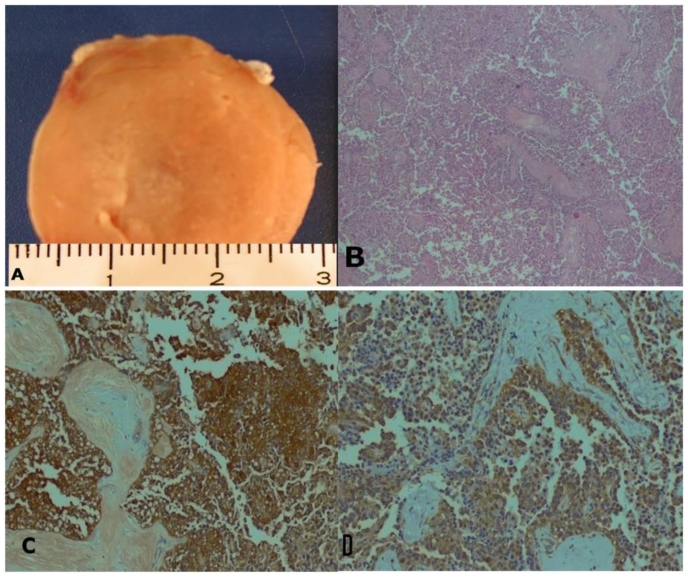
Grossly, the specimen consisted of a well circumscribed, encapsulated, yellow, solid, nodule, weighed 27 g and measured 42 × 28 × 10mm (Fig. 6). An incomplete peripheral thin rim of pancreas was seen around the nodule.
Figure 6.
35-year-old women with solid-pseudopappilary tumor. Macroscopic and microscopic pathology. A. gross appearance of the isolated pancreatic nodule. B. microscopic appearance of the tumor (haematoxylin-eosin, original magnification × 40). C - D: CD-56 and Sinaptophysin-positive tumor cells (original magnification x100).
Microscopic examination revealed a highly delicate vascular solid-papillary tumor. The solid portion disclosed an endocrine-like appearance with sheets, nests and pseudo-glandular structures of small round cells. Neoplastic cells showed excessive eosinophilic cytoplasms with round regular nuclei and inconspicuous nucleoli (Fig. 6B). There were no extensive fibrous or amyloid-like substance deposits. A careful search failed to reveal mitotic figures, cellular degeneration, obvious nuclear atipia or vascular involvement.
Immunohistochemical evaluation of the tumor revealed that the tumor cells were positive for CD 56, Sinaptophysin (Fig. 3C and D), Vimentin, CD 10 and Alpha-1-antitrypsin and negative for epithelial markers (CAM 5.2) and Chromogranin A. B-catenin mutation was not investigated.
The differential diagnosis included other tumors that share the microscopic appearance, mainly acinar cell carcinoma, mixed acinar-endocrine carcinoma, pancreatic endocrine neoplasms and pancreatoblastoma. Most of them are positive for Chromogranin A and epithelial markers and negative for vimentin and alpha-1-antitripsyn. Pancreatoblastoma is most common in childhood although it can occur in adults. This tumor shows evidence of epithelial differentiation, and the characteristic “squamoid corpuscles” were not found in our case [1,2].
The diagnosis of a solid pseudopapillary tumor of the pancreas was established. The patient was followed up periodically by abdominal MR. At the last follow-up visit (two years postoperatively) she was disease-free and had no complaints.
DISCUSSION
Solid pseudopapillary tumor (SPT) was first described by Frantz in 1959 as a “papillary tumor, benign or malignant” in a report of three cases; in 1981 was proposed as a distinct tumor entity, and in 1996, was defined by World Health Organization (WHO) as “solid pseudopapillary tumors” for the international histological classification of tumors of the exocrine pancreas. The molecular studies of solid pseudo-papillary tumor reveal that the tumor is similar to tumors originating in acinar cells and distinct from ductal cell adenocarcinoma, but previously it was misdiagnosed as nonfunctioning islet cell tumors [3]. It’s nomenclature as evolved over time and is also known as solid and papillary tumor, solid-cystic tumor, papillary-cystic tumor, papillary epithelial neoplasm, papillary and solid neoplasm, solid and pseudopapillary epithelial neoplasm, solid and cystic acinar cell neoplasm, and Frantz tumor [3, 4, 5, 6, 7].
Clinical features and behavior
SPTs occur most frequently in women in the second and third decades of life, but has also been reported in males and children [4]. There is no known genetic or hormonal factor to explain the strong female predilection [3]. It seems to have a black racial predilection [4, 7, 8].
It is a rare tumor, accounting for only 1%–2% of the primary non-endocrine tumors of pancreas [4, 6, 8, 10, 11]. The incidence of this neoplasm seems to be increasing, but consideration should also be given to whether it is simply recognized and diagnosed on a more consistent basis [4, 10, 12].
Its recognition is important since it’s considered a low-grade malignancy that is apparently curable with surgical complete excision in most instances, even in the presence of metastatic disease (up to 20% of cases) [8, 10, 12, 13, 14]. The correct preoperative diagnosis of the tumor makes possible to lessen or minimize the extent of surgery compared to that required for malignant pancreatic lesions [15]. The overall 5-years survival is estimated to be more than 90%, including patients with metastatic disease [14, 16]. Because of its indolent behavior and amenability of metastases to be resected, patients rarely die as a direct result of this neoplasm [10]. Tumors occurring later in life seem to be more likely to behave aggressively, especially in male gender [4, 12, 17].
The liver is the most common site for metastases and few cases of lymph node and peritoneal spread have been reported [18, 19]. Hepatic, left gastric, splenic, retropancreatic, celiac, and mesenteric lymph node group involvement have been described. Metastatic disease described by Choi et al showed CT appearance similar to that of the primary pancreatic mass: well-defined margins and mixed solid and cystic internal architecture [19].
Patients often present with vague abdominal discomfort or pain, and an enlarging upper abdominal mass that can be associated with symptoms of the upper gastrointestinal system, such as nausea, vomiting and dyspepsia. Not infrequently, patients are asymptomatic and the tumors are incidentally found by imaging studies following the workup for unrelated conditions [7, 14].
Hematologic laboratory investigations usually provide little or none additional information: normal amylase level, negative pancreatic cancer markers [7].
SPT can occur anywhere in the pancreas, and there is no consensus between the reported series in demonstrating a preferable localization (throughout the pancreas): some investigators observed a predilection for the tail [14, 20], while others, namely the largest review of literature reported almost the same frequency in head and in tail (34% and 35.9%, respectively) [21]. The tumor may be quite exophytic, as in this case, and occasionally their pancreatic origin may not be apparent, even at surgery [3]. This tumor rarely (1%) arises from an extra-pancreatic site such as retroperitoneum, mesocolon or liver [11,18, 23].
Pathological features
Grossly, a SPT is a large (described mean diameters between 58 to 100mm), sharply marginated and encapsulated pancreatic mass that usually demonstrates variable degrees of internal hemorrhage, necrosis and cystic degeneration [11, 16, 19, 22]. Nevertheless, some SPTs are almost completely solid, as in the described case [14].
The light microscopic features are quite characteristic and generally do not present diagnostic problems, making immunohistochemical analysis superfluous in most cases [9, 11]. Microscopically, the tumor is composed of cells arranged in the form of solid sheets, microcysts and pseudopapillae. The solid areas contain sheets of uniform polygonal cells admixed with a delicate vascular network that traverses this tumor. Areas of hemorrhage and necrosis are seen secondary to this easily disrupted vascular network. Pseudopapillae are formed by central thin walled blood vessel surrounded by several layers of dropped neoplastic cells. The cells have moderate amount of eosinophilic to vacuolated cytoplasm and nuclei are ovoid and folded with indistinct nucleoli and few mitoses. Areas of hemorrhage and necrosis are seen, secondary to the delicate, easily disrupted vascular network that traverses this tumor. Peripheral, capsular calcifications are rarely seen [11, 13, 14, 22].
Immunohistochemistry shows a distinctive pattern that corresponds neither to exocrine nor to endocrine pancreatic cell types, and may be useful to exclude other tumors of the pancreas in problematic cases [11]. SPT of pancreas is positive for CD56, CD10, Alpha 1-antitrypsin, Alpha 1-anti-chymotrypsin, neuron specific enolase, vimentin, synaptophysin and progesterone receptor [9, 11, 14].
Imaging features
The radiologic findings in solid and papillary epithelial neoplasm may be suggestive but not specific of SPT [8].
Abdominal x-ray of large SPT may demonstrate displacement of the stomach, colon or spleen by an extrinsic mass. A peripheral curvilinear (eggshell) calcification is seldom encountered [8, 13, 17].
On ultrasound evaluation, SPT presents as a heterogeneous and hypovascular (on Doppler evaluation) mass, as in this case. Solid component may present more hypoechoic portions due to tumor necrosis and hemorrhage. Few small echo-free areas may be seen, sometimes with fluid level, corresponding to the cystic component [7, 13].
Computed tomography is the imaging modality of choice for detection and characterization of SPTs of the pancreas and clear depicting of the relationships with adjacent structures, while the MRI can be more accurate in differentiating the cystic or solid component inside the tumor [16].
Multidetector computed tomography demonstrates a well-circumscribed, round or lobulated lesion with a clear-depicted peritumoral capsule. Its internal architecture ranges from solid muscle density, through mixed solid and cystic, to thick-walled cyst, depending on the degree of hemorrhagic necrosis. Attenuation values (Hounsfield units) in the cystic areas are higher than those of water, corresponding to hemorrhagic degeneration [7, 19]. In the majority of cases, intravenous contrast administration produces a peripheral, heterogeneous enhancement in pancreatic phase due to intratumoral cystic component/hemorrhage [15, 20].
In a study by Baek et al, the authors compared the imaging features of large (>3 cm) and small (< 3cm) SPTs, and found that small SPTs frequently appear as purely solid tumors, with a sharp margin and gradual enhancement, weak during pancreatic phase and progressive during the hepatic venous phase [15].
Our case presented a well-circumscribed exophytic lesion from the pancreatic gland, and although it is larger than 3 cm, it shares the imaging features of small SPTs.
SPTs complications usually reflect local compression of the surrounding structures rather than invasion [12]. As described above, the tumor tends to displace the adjacent organs and structures (vessels, pancreatic duct), showing the slow-growing and noninvasive tendencies of this tumor [17, 19], as it can be seen in this case.
Atypical SPT manifestations described by Choi et al, Buetow et al, Petrakis et al, and assessed by Wang included, in decreasing order: extracapsular invasion of the surrounding parenchyma and vessels (portal vein, superior mesenteric vein, splenic vein), peripancreatic fat invasion, calcifications, invasion of surrounding organs (spleen, duodenum and stomach), lymph node metastases, ductal obstruction and pseudocyst formation, liver metastases and tumor rupture [13, 16, 17]. Wang et al found that when tumor size was > 6 cm, the probability of vascular involvement and capsular invasion increased significantly, and it also was more likely to present an aggressive behavior such as metastasis and recurrence [16].
Thus, imaging findings are predictive of the malignant potential of theses lesions and are of high value to the patient’s surgical planning [6, 16].
Magnetic resonance (MR) imaging offers more specificity by directly demonstrating a surrounding fibrous capsule (of low signal intensity on T1 and T2-wi) and internal hemorrhage [3]; the presence of blood products in either the solid or cystic component of the mass result in areas of high signal intensity on T1-wi and of low or inhomogeneous signal intensity on T2-wi; hemorrhagic cystic degeneration can also present as a fluid-fluid or fluid-debris level (hematocrit effect) [23]. Solid components with little or no hemorrhagic necrosis present low signal intensity on T1-wi and slightly high signal intensity on T2-wi [13, 22]. In our case, MR did not add value to the diagnosis once there was no evidence of blood degradation products that could suggest the final diagnosis.
On gadolinium-enhanced dynamic MR, the enhancement pattern is similar to that seen at CT, the most common consisting of peripheral and heterogeneous enhancement during the arterial phase with progressive but also heterogeneous fill-in of the lesion during portal venous and equilibrium phases [3, 13].
Differential diagnosis
The complex anatomy of the upper abdomen (both peritoneal and retroperitoneal) may give rise to diagnostic challenges in the presence of an exophytic pancreatic lesion. Depending on the lesion location, a wide range of pancreatic and peri-pancreatic diseases might be considered, such as normal anatomic variants, inflammatory and infectious diseases, peripancreatic nodal enlargement and lymphoma, vascular lesions, disease in surrounding structures, metastases to the pancreas, and miscellaneous pancreatic diseases [24]. Our case had an exophytic posterior growth from the pancreatic gland, which raised the suspicion of a retroperitoneal adenomegaly.
In mixed type (solid-cystic) SPT, differential diagnosis includes serous cystadenoma, mucinous cystic adenoma, pancreatoblastoma, and calcified/hemorrhagic pseudocyst [4]. A misdiagnosed SPT as a hydatid cyst has also been reported [12]. The first three are unlikely in patients below the age of 30, and patients with pseudocyst are usually older than those with SPTs and have a history of pancreatitis [13].
Serous cystadenoma is common located in the pancreatic head and is composed of innumerable cysts smaller than 2 cm in diameter, producing a honeycomb pattern, with central calcified scar (not a feature of SPT) [22].
Mucinous cystic neoplasms, including cystadenoma (macrocystic adenoma) and cystadenocarcinoma, are large unilocular or multilocular cysts filled with mucin with or without solid components [22]. On CT, a clue to the correct diagnosis is the HU values in the cystic region: water density of mucinous material, as opposed to ones compatible with blood in SPT [8]. On MR, mucin is typically hyperintense on T1 and hyperintense on T2-wi, but may show different signal intensities depending on the proteinaceous concentration of the fluid. Additionally, fluid-debris levels that demonstrate a hematocrit effect or signal intensity on MR images, suggesting hemorrhagic components, may be also seen, difficulting the differential [13]. On the other hand, it doesn’t have the peripheral enhancement of SPTs, showing enhancement of the internal septa and cyst wall [4, 19].
When there is little or no hemorrhage or necrosis of the SPT, pancreatic ductal adenocarcinoma, islet cell tumor, fibrous tumor, pancreatoblastoma and pancreatic metastases are difficult to exclude [8, 25].
Pancreatic ductal adenocarcinoma normally presents as an ill-defined, heterogeneous mass with associated upstream pancreatic duct dilatation and parenchymal atrophy, secondary changes not encountered in SPT. In opposition to SPT, pancreatic ductal adenocarcinoma typically shows infiltrative changes to the surrounding pancreatic parenchymal or fat tissue, locally invasive, with vascular encasement and mesenteric, duodenal and possibly gastric extension. Distant metastases are common finding, especially into regional lymph nodes, liver and peritoneum [8, 15, 25, 26].
Functional islet cell tumors can be excluded by a lack of clinical manifestations of hormonal activity, but the nonfunctional variant should not be forgotten [8]. Nonfunctioning islet cell tumor often shows findings similar to those of SPTs: appear as well-defined solid masses without pancreatic ductal dilatation and/or parenchymal atrophy, may appear cystic, contain calcification and demonstrate areas of internal hemorrhage [13,15]. Nonfunctioning islet cell tumors appear as areas of low intensity or isointensity on T1-wi; however, the enhancement pattern of the tumor is somewhat different: this tumor is characteristically hypervascular in arterial phase and has prominent early draining veins, in contrast with the generally hypovascular behavior of SPT, specially the solid ones [8, 15, 19].
Solitary fibrous tumor of the pancreas are extremely rare, most of the reported patients are women, and the average age at presentation and the size of the tumor vary widely; at imaging they appear as well-defined masses with variable arterial phase enhancement as well as progressive enhancement on venous and delayed phase images; areas of cystic change or necrosis may be seen, so it should be included at differential diagnosis; in contrast to SPTs, most of the reported solitary fibrous tumor occur in women above 40 years of age [27].
The evidence of a pancreatic tumor with distant metastasis may aid to narrow the differential diagnosis, that includes: pancreatic adenocarcinoma, nonfunctioning islet cell tumor and mucinous cystadenocarcinoma [19].
Pancreatic metastases are usually clinically silent and mostly affect older population. Most of times (78%) are solitary lesions, and there is no evidence of pancreatic or biliary ductal obstruction. Common primary tumors include renal, breast, lung, melanoma and ovarian cancer, and might be suggested by the clinical context [25].
TEACHING POINT
Solid pseudopapillary tumor (SPT) is a rare low grade malignancy more frequent in young women. The typical imaging features include a well-circumscribed encapsulated lesion with peripheral and heterogeneous enhancement in pancreatic phase, due to its variable degree of hemorrhagic necrosis. However, small SPTs frequently present as purely solid lesions with sharp margins and gradual enhancement, weak during pancreatic phase and progressive during the hepatic venous phase.
Table 1.
Differential diagnosis table for solid pseudopapillary tumors.
| Local Morphology | X-Ray | US | CT | MR | Pattern of contrast enhancement | |
|---|---|---|---|---|---|---|
| Solid Pseudopapillary tumor | Tail (43%) | Curvilinear Ca2+ | Well-circumscribed, heterogeneous (hypoechoic solid and anechoic cystic components) and hypovascular | Well-circumscribed, encapsulated, round or lobulated lesion. Variable internal architecture (solid, mixed solid and cystic, thick-walled cyst) depending on the degree of hemorrhagic necrosis. |
Degradation blood products: high SI onT1-wi, low or inhomogeneou s SI on T2-wi Solid component without hemorrhagic foci: low SI on T1-wi, high SI on T2-wi Fibrous capsule: low SI on T1 and T2-wi |
Iodinate contrast: Peripheral, heterogenous enhancement in pancreatic phase Gadolinium: Early, peripheral enhancement, progressive heterogeneous fill-in in portal and equilibrium phases |
| Mucinous neoplasm | Tail Uni/multilocular cyst, from 2 to 12cm |
Sunburst Ca2+ | Large unilocular or multilocular cysts | Water HU of the cyst Distant metastasis (mucinous adenocarcinoma) |
High SI on T1 and T2-wi (but variable with concentration) | Enhancement of the internal septa and cyst wall |
| Serous cystadenoma | Head Innumerable small cysts |
Central calcified scar | Multiple milimetric hypoechoic or anechoic cysts | Honeycomb pattern of multiple milimetric cysts | Hypointense on T1 and hyperintense on T2-wi clustered cysts | Parietal enhancement |
| Nonhyper-functioning islet cell tumors | Small or large in size | May contain Ca2+ | Homogeneously hypoechoic lesion | Isoattenuating to the parenchyma Distant metastasis | Low SI or isointensity on T1-wi High to isointense on T2-wi |
Enhancement in arterial phase |
| Pancreatic adeno-carcinoma | Head Ill defined lesion with contour deformity of the gland |
Ca2+ very rare | Hypoechoic lesion Dilated pancreatic duct Atrophic gland | Isodense to the parenchyma Dilated pancreatic duct and atrophic gland Obliteration of peripancreatic fat Contiguous organ invasion, vascular invasion and distant metastases |
Low SI on T1- wi Variable SI on T2-wi Contiguous organ invasion and distant metastases |
Poor or non-enhancing lesion Vascular encasement/invasion |
Table 2.
Summary table for solid pseudopapillary tumors.
| Etiology | Controversial (probably from a pluripotent stem cell) |
| Incidence | 1%–2% of all exocrine pancreatic tumors |
| Gender ratio | Strong female predilection |
| Age predilection | Second and third decades of life |
| Risk factors | Unknown, may be black racial predilection |
| Treatment | Surgical |
| Prognosis | 5y survival 90% Tumors occurring later in life behave more aggressively, especially in male gender. |
| Findings on imaging | |
| US | Well circumscribed, heterogeneous (hypoechoic solid and anechoic cystic components) and hypovascular |
| CT | Encapsulated round or lobulated lesion Variable internal architecture (solid, mixed solid and cystic, thick-walled cyst) depending on the degree of hemorrhagic necrosis. Peripheral, heterogeneous enhancement in pancreatic phase |
| MRI | Demonstration of degradation blood products (high SI on T1-wi, low or inhomogeneous SI on T2-wi) Solid components: low SI on T1-wi, slightly high SI on T2-wi Hypointense onT1 and T2-wi fibrous capsule Early, peripheral and heterogeneous enhancement, progressive heterogeneous fill-in |
ACKNOWLEDGMENTS
We thank A. Freitas for technical support and L. Leite for translation review.
ABBREVIATIONS
- CT
Computed Tomography
- MR
Magnetic Resonance
- SPT
Solid Pseudopapillary Tumor
- US
Ultrasound
- wi
weighted images
REFERENCES
- 1.Klöppel G, Klimstra DS. Diagnostic Histopathology of Tumors. 3th ed. Vol. 1. Elsevier; Philadelphia: 2007. Tumors of the exocrine Pancreas Fletcher CDR. [Google Scholar]
- 2.Klimstra DS, Adsay WV. Benign and malignant tumors of the Pancreas. In: Odze RD, Goldblum JR, editors. Surgical Pathology of the GI Tract, Liver, Biliary Tract, and Pancreas. Philadelphia (PA): Elsevier; 2004. pp. 699–736. [Google Scholar]
- 3.Chung EM, Travis MD, Conran RM. Pancreatic tumors in children: radiologic-pathologic correlation. Radiographics. 2006 Jul-Aug;26(4):1211–38. doi: 10.1148/rg.264065012. [DOI] [PubMed] [Google Scholar]
- 4.Cantisani V, Mortele K, Levy A, et al. MR Imaging Features of Solid Pseudopapillary Tumor of the Pancreas in Adult and Pediatric Patients. AJR. 2003;181(2):395–401. doi: 10.2214/ajr.181.2.1810395. [DOI] [PubMed] [Google Scholar]
- 5.Kato T, Egawa N, Kamisawa T, et al. A case of solid pseudopapillary neoplasm of the pancreas and tumor doubling time. Pancreatology. 2002;2:495–498. doi: 10.1159/000064711. [DOI] [PubMed] [Google Scholar]
- 6.Sperti C, Berselli M, Pasquali C, Pastorelli D, Pedrazzoli S. Aggressive behaviour of solid-pseudopapillary tumor of the pancreas in adults: A case report and review of the literature. World J Gastroenterol. 2008 Feb;14(6):960–965. doi: 10.3748/wjg.14.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman KM, Doherty MC, Bigler SA. Solidpseudopapillary tumor of the pancreas. RadioGraphics. 2003;23:1644–1648. doi: 10.1148/rg.236035006. [DOI] [PubMed] [Google Scholar]
- 8.Friedman AC, Lichtenstein JE, Fishman EK, Oertel JE, Dachman AH, Siegelman SS. Solid and papillary epithelial neoplasm of the pancreas. Radiology. 1985;154(2):333–337. doi: 10.1148/radiology.154.2.3880903. [DOI] [PubMed] [Google Scholar]
- 9.Darius T, Brouwers J, Van Dijck H, Bernard P. Solid and cystic papillary neoplasm of the pancreas: A rare tumour in young women. Acta Chir Belg. 2006;106(6):726–729. doi: 10.1080/00015458.2006.11679994. [DOI] [PubMed] [Google Scholar]
- 10.Washington K. Solid-Pseudopapillary Tumor of the Pancreas: Challenges Presented by an Unusual Pancreatic Neoplasm. Ann Surgl Oncol. 2022 Jan-Feb;9(1):3–4. doi: 10.1245/aso.2002.9.1.3. [DOI] [PubMed] [Google Scholar]
- 11.Srilatha P, Manna V, Kanthilatha P. Solid pseudopapillary tumour of the pancreas: Report of five cases. The Internet Journal of Pathology. 2009;8(2) [Google Scholar]
- 12.Petrakis I, Vrachassotakis N, Kogerakis N, et al. Solid pseudopapillary neoplasm of the pancreas: Report of a case after a 10-year follow-up and review of the literature. Pancreatology. 2001;1(2):123–28. doi: 10.1159/000055804. [DOI] [PubMed] [Google Scholar]
- 13.Buetow PC, Buck JL, Pantongrag-Brown L, Beck KG, Ros PR, Adair CF. Solid and papillary epithelial neoplasm of the pancreas: imaging-pathologic correlation in 56 cases. Radiology. 1996;199(3):707–11. doi: 10.1148/radiology.199.3.8637992. [DOI] [PubMed] [Google Scholar]
- 14.Matos J, Grützman R, Agaram NP, et al. Solid Pseudopapillary Neoplasms of the Pancreas: A Multi-institutional Study of 21 Patients. J Surg Res. 2009 Nov;57(1):e137–42. doi: 10.1016/j.jss.2009.03.091. [DOI] [PubMed] [Google Scholar]
- 15.Baek JH, Lee JM, Kim S, et al. Small (? 3 cm) solid pseudopapillary tumors of the pancreas at multiphasic multidetector CT. Radiology. 2010 Oct;257(1):97–106. doi: 10.1148/radiol.10092089. [DOI] [PubMed] [Google Scholar]
- 16.Wang DB, Wang QB, Chai WM, Chen KM, Deng XX. Imaging features of solid pseudopapillary tumor of the pancreas on multi-detector row computed tomography. World J Gastroenterol. 2009 Feb 21;15(7):829–835. doi: 10.3748/wjg.15.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi JY, Kim MJ, Kim JH, et al. Solid pseudopapillary tumor of the pancreas: typical and atypical manifestations. AJR Am J Roentgenol. 2006;187:W178–W186. doi: 10.2214/AJR.05.0569. [DOI] [PubMed] [Google Scholar]
- 18.Hibi T, Ojima H, Sakamoto Y, et al. A solid pseudopapillary tumor arising from the greater omentum followed by multiple metastases with increasing malignant potential. J Gastroenterol. 2006;41:276–81. doi: 10.1007/s00535-005-1753-2. [DOI] [PubMed] [Google Scholar]
- 19.Choi BI, Kim KW, Han MC, et al. Solid and papillary epithelial neoplasm of the pancreas: CT findings. Radiology. 1988;166(2):413–16. doi: 10.1148/radiology.166.2.3336716. [DOI] [PubMed] [Google Scholar]
- 20.Balthazar EJ, Subramanyam BR, Lefleur RS, Barone CM. Solid and papillary epithelial neoplasm of the pancreas: radiographic, CT, sonographic, and angiographic features. Radiology. 1984;150:39–40. doi: 10.1148/radiology.150.1.6689785. [DOI] [PubMed] [Google Scholar]
- 21.Papavramidis T, Papavramidis S. Solid pseudopapillary tumors of the pâncreas: review of the 718 patients reported in English literature. J Am Coll Surg. 2005 Jun;200(6):965–972. doi: 10.1016/j.jamcollsurg.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 22.Ohtomo K, Furui S, Onoue M, et al. Solid and papillary epithelial neoplasms of the pancreas: MR imaging and pathologic correlation. Radiology. 1992;184:567–70. doi: 10.1148/radiology.184.2.1620866. [DOI] [PubMed] [Google Scholar]
- 23.Siegelman E. Body MRI. Elsevier Saunders; Philadelphia, Pennsylvania: 2005. [Google Scholar]
- 24.To’o K, Raman S, Yu Nam, et al. Pancreatic and Peripancreatic Diseases Mimicking Primary Pancreatic Neoplasia. Radiographics. 2005 Jul-Aug;25(4):949–65. doi: 10.1148/rg.254045167. [DOI] [PubMed] [Google Scholar]
- 25.Federle MP. Diagnostic Imaging: Abdomen. Amirsys; Salt Lake City: 2004. [Google Scholar]
- 26.Nakatani K, Waanabe Y, Okumura A, et al. MR Imaging features of Solid-pseudopapillary tumor of the pancreas. Magn Reson Med Sci. 2007;6(2):121–6. doi: 10.2463/mrms.6.121. [DOI] [PubMed] [Google Scholar]
- 27.Shanbhogue AK, Prasad SR, Takahashi N, Vikram R, Zaheer A, Sandrasegaran K. Somatic and Visceral Solitary Fibrous Tumors in the Abdomen and Pelvis: Cross-sectional Imaging Spectrum. RadioGraphics. 2011 Mar-Apr;31:393–408. doi: 10.1148/rg.312105080. [DOI] [PubMed] [Google Scholar]