Abstract
Fetus-in-fetu (FIF) is a rare anomaly in which a vertebrate fetus is enclosed within the body of its twin in diamniotic monochorionic pregnancy. To the best of our knowledge, fewer than 100 cases have been reported in literature. Although a wide variety of presentations have been described in clinical reports, the characteristic features on MRI which distinguish FIF from teratoma have not been well delineated. Here we present a case of fetus-in-fetu in which characteristic MDCT and MR findings were used to diagnose FIF preoperatively and successfully differentiate it from teratoma. Although both CT and MRI can be used for definitive preoperative diagnosis of FIF, MRI is an ideal imaging modality due to inherent high tissue contrast and spatial resolution. Furthermore, MRI obviates the need for iodine contrast and eliminates the risk of ionizing radiation. We emphasize that MRI is an ideal valuable diagnostic tool for definite preoperative diagnosis of FIF and surgical planning.
Keywords: fetus-in-fetu, teratoma, axial skeleton, diamniotic monochorionic twins, parasitic twin, multidetector computed tomography, magnetic resonance imaging
CASE REPORT
A 14 year old girl presented to us with complaints of gradually increasing swelling in the upper abdomen, dull ache in the abdomen and infrequent vomiting over the past few years. Poverty, ignorance, fear from surgery and parental negligence contributed to delayed presentation. On exam the patient appeared pale and malnourished. Patient had a history of familial twinning in first cousins. There was no other significant family history. Local examination revealed a large nontender swelling with variable consistency in the epigastrium extending into the left hypochondrium and lumbar region. Laboratory tests were unremarkable.
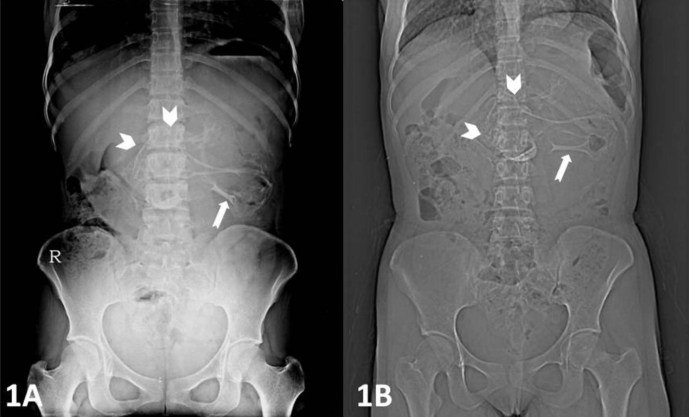
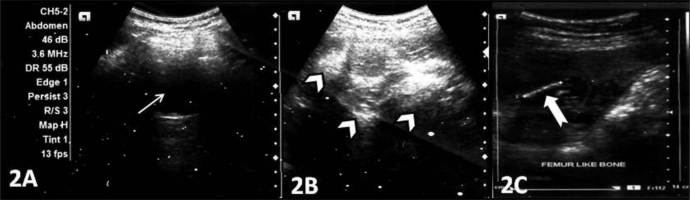
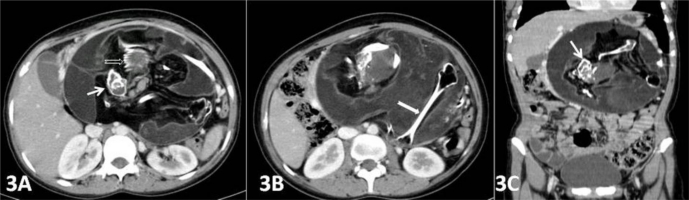
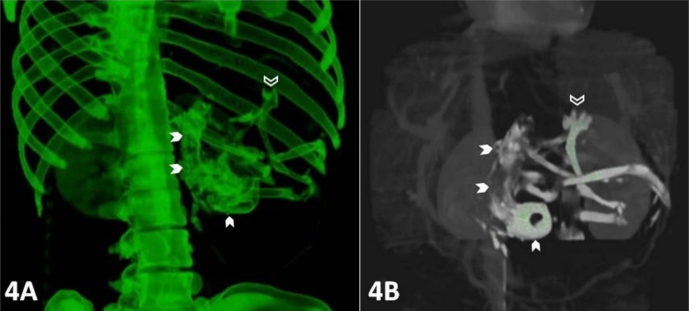
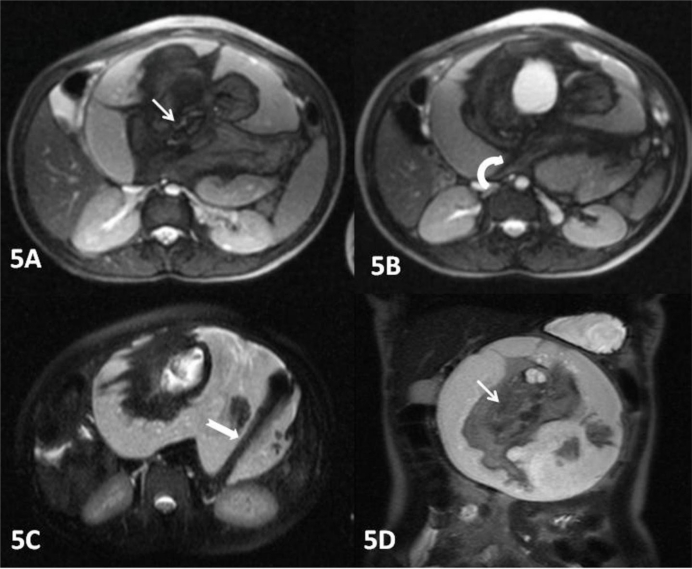
Plain x-ray of the abdomen (AP view) was performed which revealed an amorphous soft tissue density in the upper abdomen with numerous calcifications resembling bony structures (Figure 1). Ultrasound revealed a large heterogeneous complex solid cystic mass with multiple dense calcifications in the upper abdomen displacing stomach and small bowel loops. Fatty areas were also seen (Figure 2). Sonographic findings were nonspecific and considered to be consistent with teratoma. Further evaluation was advised with multi detector computed tomography (MDCT) and magnetic resonance imaging (MRI). A contrast enhanced (intravenous iodixanol) abdominal computed tomography (CT) examination was performed on a 16-row multidetector CT (Sensation; Siemens, Germany). MRI of abdomen was done with 1.5T scanner (Avanto; Siemens, Germany). The MRI protocol used an axial and coronal T2-weighted Half- Fourier Acquisition Single-Shot Turbo SE (HASTE) sequence, axial 2-D Fast Low Angle Shot (FLASH) plus fat suppression sequence, axial, sagittal and coronal true Fast sequence (TRUFI). CT revealed a large well defined rounded retroperitoneal mass in the epigastric region displacing stomach and adjacent bowel loops (Figure 3). The mass has cystic, fatty and bony components. 3D CT reconstruction was done with volume rendering technique (VRT) which revealed a mass with a deformed fetus like appearance in the retroperitoneum (Figure 4). On MRI CT findings were confirmed and mixed high, intermediate and low intensities were seen within the mass (Figure 5). Bony structures seen within the miniature fetus were few irregular vertebral bodies, rib like bones and a well formed femur like long bone suggesting remnants of a second fetus. Facial and calvarial bones could not be seen. Limb buds were seen with primitive phalanges and an orbital socket. Fat plane between the mass and surrounding structures were well maintained. The mass had a separate blood supply from the infrarenal abdominal aorta.
Figure 1:
A 14 year old girl with fetus in fetu. Plain x-ray abdomen AP view (1A) and CT scannogram (1B) reveals presence of an amorphous soft tissue density shadow in the upper abdomen with numerous calcifications resembling bony structures.
Figure 2:
A 14 year old girl with fetus in fetu. Ultrasonographic images (Siemens Acuson X 300, 3.5–5.0 MHz, convex probe) demonstrate a large heterogeneous complex mass in the upper abdomen with solid echogenic (white arrow heads) and cystic anechoic (thin white arrow) components with linear calcified structure, resembling a femur (thick white arrow).
Figure 3:
A 14 year old girl with fetus in fetu. Contrast enhanced axial (3A, 3B) and coronal reconstruction (3C) abdominal computed tomography (CT) images in arterial phase showing a large well defined rounded retroperitoneal mass in epigastric region displacing stomach and adjacent bowel loops. The mass has cystic, fatty and bony components. Bony structures seen are few irregular vertebral bodies (thin white arrow), rib like bones (open white arrow) and a well formed femur like long bone (thick white arrow). (Protocol: 16-row multidetector sensation CT, Siemens, Germany, 150 mAs, 110 kVP, 2.5 mm reformation, 100 c.c. of 300 mg/ml iodine concentration intravenous iodixanol given by hand injection)
Figure 4:
A 14 year old girl with fetus in fetu. CECT images with 3D reconstruction (volume rendering technique) show a deformed fetiform structure in retro peritoneum (white arrow head) with an eye socket and few long bones. Limb buds are seen with primitive phalanges (open white arrow). (Protocol: 16-row multidetector sensation CT, Siemens, Germany, 150 mAs, 110 kVP, 2.5 mm reformation, 100 c.c. of 300 mg/ml iodine concentration intravenous iodixanol given by hand injection)
Figure 5:
A 14 year old girl with fetus in fetu. The axial true FISP (5A&5B) and axial HASTE (5C) 1.5 T MR images of the abdomen confirm the CT findings and showing a large encapsulated intraabdominal retroperitoneal mass with mixed high, intermediate and low intensities. The predominant component of the mass is liquid and the soft tissue component is suspended in the fluid by a cord-like pedicle (curved white arrow). A well formed femur like long bone is seen within the mass (thick white arrow).The coronal (5D) HASTE image shows a fetiform solid component containing areas of skeletal elements resembling a vertebral axis (thin white arrow). (Protocol: 1.5 T magnet strength, Avanto MR Siemens, Germany, FISP sequence - 4.56 TR, 1.88 TE, 6.0 mm slice thickness and HASTE sequence - 1100 TR, 117 TE, 6.0 mm slice thickness).
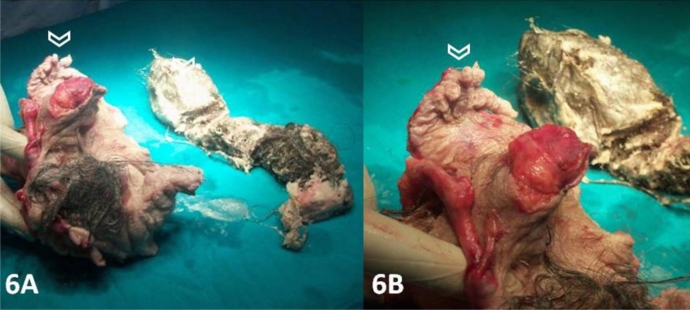
Elective laparotomy was done which confirmed the imaging findings. A large well encapsulated retroperitoneal cystic mass was seen. The FIF was surrounded by thin amniotic like fluid. FIF was seen as a large heterogeneous disorganized mass of soft tissue adhered to retroperitoneal structures by a cord like structure. A separate blood supply was seen connected to the host's abdominal aorta. After mobilization the mass was completely excised which revealed thick wrinkled skin, rudimentary limb buds, hairs, malformed vertebral column and other undifferentiated tissues. Remaining body parts could not be identified. It weighted about 1000 gm and measured 15 cm in length (Figure 6). Chromosomal analysis was not performed. The patient recovered well after surgery and was discharged. Regular follow up was advised every three months with sonography and serum tumour markers (alpha fetoprotein) to rule out any recurrence.
Figure 6:
A 14 year old girl with fetus in fetu. Post operative surgical specimen showing a large heterogeneous disorganized mass of soft tissue having thick wrinkled skin covered with hairs and rudimentary limb buds (open white arrow head) and other undifferentiated tissues.
DISCUSSION
Fetus-in-fetu (FIF) is a rare entity resulting from abnormal embryogenesis in diamniotic monochorionic twins. FIF occurs when a vertebrate fetus is enclosed within the body of a normally developing fetus. The condition is extremely rare, occurring once in 500,000 deliveries. Fetus-in-fetu (FIF), a term quoted by Lewis, was first described by Johann Friedrich Meckel (circa 1800) [1]. Hoeffel et al reviewed 88 previously reported cases and their own case after extensively reviewing the literature [2]. Characteristic imaging features on plain abdominal x-ray, sonography and computed tomography (CT) scan have all been well described previously [3–7]. The purpose of this case study is to describe the MRI appearances of this rare condition.
Rarely multiple or twin FIF are described. Multiple theories are proposed regarding the embryogenesis. One is the teratoma theory which says that FIF is a highly differentiated form of mature teratoma [8]. Another is parasitic twin theory, according to which FIF may be a parasitic twin fetus growing within its host twin. Although abdominal masses are commonly encountered in the pediatric population, the finding of FIF is rare and unexpected. FIF is a complex structure, which is composed of fetus suspended by a cord and a fluid containing thin fibrous membrane (representing chorio-amniotic complex and amniotic fluid).
In the mother's uterus growth of FIF initially parallels that of its twin sibling, but either due to vascular dominance of the sibling twin (host twin) or an inherent defect in FIF (parasitic twin) suddenly stops resulting in FIF. Most of the FIF are anencephalic, but in almost all cases vertebral column (91%) and limb buds (82.5%) are present. Lower limbs are more developed than upper limbs [1]. Pre natal and post natal sonography, computed tomography and MRI may be used to diagnose the condition. Early diagnosis is important as unidentified FIF can lead to a host of complications early in life.
The condition is being detected most often in infants; however age of presentation varies from ante natal period upto as old as 47 years [9]. 89% of the cases are discovered before the age of 18 months[10]. There are reports in which this anomaly remained asymptomatic until late age despite its prevalence in infants. Thakral et al [11] reported that FIF occurs equally among the male and female population but Patankar et al [4] mentioned a 2:1 male predominance in contrast to teratoma which shows female predominance. FIF may be asymptomatic. Some cases may present due to mass effect, like abdominal distension, dysphgia, emesis, feeding difficulties and jaundice [3, 10]. In 80% the case mass is retroperitoneal [12]. Uncommon sites are oral cavity, sacrococcygeal region, scrotum, cranium, GIT, vessels, lungs, adrenal glands, spleen, lymph nodes and genitourinary system of the host twin [10].
In FIF one the twin trapped within the developing sibling and starts acting as a parasite of fetal circulation of the dominant twin. Several hypotheses have been proposed on the origin of FIF such as aberration of or defective process of monochorionic diamniotic twining where a malformed parasitic fetus is found within the body of its sibling twin [13]. FIF results when unequal division of the totipotent inner cell mass of the developing blastocyst allows one twin being trapped within a maturing sibling embryo. The exact pathogenesis is unknown; however it is proposed that during the process of ventral folding of the trilaminar embryonic disc, one of the fetuses is enclosed in its host because of persistent anastomoses of vitelline circulation. As the superior mesenteric artery develops from vitelline circulation, thereby explaining the retroperitoneal location of FIF [10]. Vascular anastomoses with the host vessels are frequently identified. Absence of independent blood supply could account for fetal growth restriction in all cases. It is proposed that later due to some vascular accident it might change into an amorphous retroperitoneal structure. Twin subordinate (FIF) becomes dependent on the dominant brother, thus the fully developed twin hosts its immature twin.
Most common differential diagnosis includes a mature teratoma. The mass of FIF must demonstrate true organogenesis to differentiate it from teratoma. Some authors think that FIF is within the spectrum of anomalies that can result from the anomalous embryogenesis in a diamniotic monochorionic pregnancy. This spectrum includes conjoined symmetric twins; parasitic fetuses; embryonic vestigial fetal inclusions; and organized teratoma [14]. Thus FIF is also considered an extreme form of well differentiated highly organized teratoma with mature organs. Willis proposed that FIF is not an extremely well developed teratoma [15]. He suggested that diagnosis of FIF be restricted to those cases in which parts of axial skeleton were present with an appropriate arrangement of organs relative to skeletal axis in the body of its host, usually in the abdominal cavity. Identification of vertebral column shows that in FIF development has progressed beyond primitive streak stage (12–15 days of gestation) to notochord which is the precursor of vertebral column [4, 5, 11]. For a mass to incorporate an axial skeleton it would have to progress through the primitive streak stage. In teratoma uncontrolled growth of pluripotent stem cells occurs without the overall organization. So presence of axial skeleton within the mass represents FIF, in contrast to congenital teratoma.
Diagnosis of FIF is mainly by imaging. FIF can be diagnosed with pre natal sonography. X-ray abdomen of the involved part may detect amorphous soft tissue density shadow in the upper abdomen with numerous calcifications, axial skeleton (vertebral column) and limb buds in half of the cases, similar to our case. Now with increasingly use of sonography, CT scan and MRI more cases of FIF are diagnosed [13]. On sonography a complex amorphous mixed echogenic mass is seen with ill defined solid and cystic components. Long bones are seen as linear hyperechoic areas with distal acoustic shadowing. CT scan of involved part identifies the site, vascular supply and mass effect by the FIF. The mass is better seen in CT scan as compared to ultrasound, which can very well demonstrate a large well defined rounded retroperitoneal mass in the epigastric region having solid, cystic and fatty components, limb buds with long bones and spinal column. 3D reconstruction with VRT helps in exact anatomical localization of FIF. Hoeffel et al [2] emphasized on this fact that non visualization of vertebral column on CT and radiograph does not exclude the diagnosis of FIF. It may be found on pathological examination of the specimen. This may occur due to markedly dysplastic and underdeveloped spinal axis. Despite requirement of presence of spinal axis for diagnosis of FIF, there are case reports of FIF without vertebral column [16]. This is probably due to markedly dysplastic and an underdeveloped spinal column preventing the identification of spinal axis at imaging. Although MRI is a powerful diagnostic modality, there are few case reports of use of MRI in diagnosis of FIF [17–19]. MRI produces high quality images of the human anatomy with great inherent contrast. MRI can play a crucial role in evaluation and detection of FIF. Many advantages of MRI such as high contrast resolution, multiplanar capability and choice of various pulse sequences make the diagnosis of FIF accurately. MRI has some distinct advantage over other diagnostic modalities particularly CT in evaluation of FIF: (1) lack of ionizing radiation, (2) it does not depend on detection of tissue calcification, this helps in identification of insufficiently calcified vertebral column [17], (3) absence of bony artifacts, (4) no need of iodine based contrast agents that can cause considerable risk of kidney damage and allergic reaction. MR images may reveal a large fetiform retroperitoneal mass with mixed high, intermediate and low intensities and bony structures in the form of crumpled vertebral bodies, rib like bones and well formed long bones suggesting remnants of a second fetus. Limb buds can be seen with primitive phalanges and an orbital socket. Fat plane between the mass and surrounding structures is well maintained. Both MRI and USG lack ionizing radiation and are ideal imaging modalities. Due to its wide availability, low cost and real time imaging, USG has become the investigation of choice for diagnosis of fetal structures and various growth anomalies. However there are few intrinsic limitations of USG with regard to tissue contrast, especially in maternal obesity and reduced amniotic fluid. MRI has emerged as an excellent alternate safe technique in evaluation of FIF. It gives a large field of view with better spatial resolution. There is no operator and interpreter dependence. Recently popularity of fetal MRI has increased due to development of ultrafast MRI pulse sequences allowing minimal image degradation due to fetal motion. MRI is considered safe in pregnancy as there is no known adverse biological effect on the fetus in utero.
Treatment of FIF is complete resection with the surrounding sac as it may cause symptoms because of mass effect over intra abdominal organs. Other risks associated with FIF are chances of infection, bleeding and pleura-peritoneal inflammation due to leak of sac contents. Although it is a benign condition, there is one case report of malignant degeneration best of our knowledge in which recurrence occurred after 4month as a yolk sac tumour [20]. Recurrence has been attributed to the presence of immature tissues in the small areas and remnants of the surrounding capsule of the mass [4].
Gross specimen is covered with thin fibrous sac containing straw colored fluid along with an anencephalic fetus (100%) with limb buds (83%) and vertebral column. Pathological diagnosis of FIF is confirmed by the presence of vertebral column and core distinct bodies of members. Another criterion for diagnosis includes presence of a membrane covering fetal vasculature connections [5]. Rounded or tubular collection of very low density fat surrounding amorphous soft tissue is characteristic of FIF. Other structures which may be seen in FIF are skin, skin extensions, bone, bone marrow, venous structures, skeletal muscles and peripheral nerves. Uncommonly teeth, tongue, salivary glands, lymphatics, trachea, thyroid, parathyroid, lower respiratory tract, pancreas, spleen, kidney, gonads, testis, ovaries, urinary bladder, digits and nails are present in FIF[3,8,9]. Karyotyping, serological markers and restriction site mapping demonstrates that FIF actually represents a diamniotic monochorionic twin of the host, thus confirming a separate etiology of FIF as compared to teratoma [5]. No placenta or chorionic villi are found at involved site.
Prognosis is more favorable in FIF as compared with cystic teratoma; however presence of immature elements emphasizes the need of close follow up with sonography and serology (alpha fetoprotein) and a possible chance of recurrence must be kept in mind.
TEACHING POINT
Fetus-in-fetu (FIF) is a rarely diagnosed condition. FIF could be considered as a rare differential of teratoma in an infant or young child presenting with progressively increasing abdominal swelling and vomiting. Presence of axial skeleton distinguishes FIF from teratoma; however it can be seen only in 91% of cases on imaging. Both MDCT and MRI findings are useful however MRI provides excellent definition of the salient anatomy without ionizing radiation and should be considered the imaging modality of choice. We emphasize that MRI is a valuable adjunctive tool to sonography for definitive preoperative diagnosis of FIF and surgical planning.
Table 1:
Differential diagnoses for fetus-in-fetu
| Differential diagnosis | X-Ray | US | CT | MRI |
|---|---|---|---|---|
| Fetus in fetu |
|
|
|
|
| Mature teratoma |
|
|
|
|
| Pseudocyst |
|
|
|
|
Table 2:
Summary table of fetus-in-fetu
| Etiology | Several hypotheses have been proposed on the origin of FIF such as aberration of or defective process of monochorionic diamniotic twining where a malformed parasitic fetus is found within body of its sibling twin. FIF results when unequal division of the totipotent inner cell mass of the developing blastocyst allows one twin being trapped within a maturing sibling embryo |
| Incidence | The condition is extremely rare occurring once in 500,000 deliveries. |
| Gender ratio | 2: 1 male predominance in fetus in fetu, In contrast to teratoma which shows female predominance. |
| Age predilection | The condition is being detected most often in early infancy; however age of presentation varies from ante natal period upto as old as 47 years. 89% of the cases are discovered before the age of 18 months. |
| Risk factors | Not known |
| Treatment | Treatment of FIF is complete resection with the surrounding sac |
| Prognosis | favorable |
| Findings on imaging (can be adopted from the differential table) |
Plain x ray abdomen (AP view) -presence of an amorphous soft tissue density shadow in the upper abdomen with numerous calcifications. Ultrasound - a large heterogeneous complex solid cystic mass with multiple dense calcified parts and fatty areas in upper abdomen displacing stomach and small bowel loops. A contrast enhanced abdominal computed tomography (CT) - a large well defined rounded retroperitoneal mass in epigastric region displacing stomach and adjacent bowel loops The mass has cystic, fatty and bony components. 3D CT reconstruction revealed a deformed fetus like appearance in retro peritoneum. MRI abdomen – a large fetiform retroperitoneal mass with mixed high, intermediate and low intensities. Bony structures seen in miniature fetus were few irregular vertebral bodies, rib like bones and a well formed femur like long bone suggesting remnants of second fetus. Limb buds can be seen with primitive phalanges and an orbital socket. Fat plane between the mass and surrounding structures is well maintained. |
Acknowledgments
We want to acknowledge Dr Shobha Khanduri, Dr S.K. Srivastava, Dr Pramod Dixit, Dr Kailash Singh and Dr Anit Parihar for their contribution.
ABBREVIATIONS
- 3D =
Three dimension
- AP =
view antero-posterior view
- FIF =
fetus-in-fetu
- FLASH =
Fast Low Angle Shot
- GIT =
gastrointestinal tract
- HASTE =
Half- Fourier Acquisition Single-Shot Turbo spin echo
- MDCT =
multi detector computed tomography
- MRI =
magnetic resonance imaging
- SE =
spin echo
- TRUFI =
True Fast sequence
- UB =
Urinary bladder
- USG =
ultrasonography
- VRT =
Volume Rendering Technique
- WIs =
weighted images
Footnotes
Online access
This publication is online available at: www.radiologycases.com/index.php/radiologycases/article/view/833
REFERENCES
- 1.Lewis RH. Fetus-in-fetu and the retroperitoneal teratoma. Arch Dis Child. 1961;36:220–226. doi: 10.1136/adc.36.186.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoeffel CC, Nguyen KQ, Phan HT, Truong NH, Nguyen TS, Tran TT, Fornes P. Fetus-in-fetu: a case report and literature review. Pediatrics. 2000;105:1335–1344. doi: 10.1542/peds.105.6.1335. [DOI] [PubMed] [Google Scholar]
- 3.Knox JS, Webb AJ. The clinical features and treatment of fetus-in-fetu: two case reports and review of literature. J Pediatr Surg. 1975;10:483–489. doi: 10.1016/0022-3468(75)90188-8. [DOI] [PubMed] [Google Scholar]
- 4.Patankar T, Fatterpekar GM, Prasad S, et al. Fetus-in-fetu: CT appearance--report of two cases. Radiology. 2000;214:735–737. doi: 10.1148/radiology.214.3.r00mr32735. [DOI] [PubMed] [Google Scholar]
- 5.Magnus KG, Millar AJ, Sinclair-Smith CC, et al. Intrahepatic fetus-in-fetu: a case report and review of the literature. J Pediatr Surg. 1999;34:1861–1864. doi: 10.1016/s0022-3468(99)90333-0. [DOI] [PubMed] [Google Scholar]
- 6.Gangopadhyay AN, Srivastava A, Srivastava P, Gupta DK, Sharma SP, Kumar V. Twin fetus-in-fetu in a child: a case report and review of the literature. Journal of Medical Case Reports. 2010;4:96. doi: 10.1186/1752-1947-4-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varanelli MJ, Jamal Bokhari SA, Katai FM, Scoutt L. Case 55: Fetus in Fetu. Radiology. 2003;226(2):517–520. doi: 10.1148/radiol.2262010624. [DOI] [PubMed] [Google Scholar]
- 8.Basu A, Jagdish S, Iyengar KR, Basu D. Fetus-in-fetu or differentiated teratomas. Indian J Pathol Microbiol. 2006 Oct;49:563–5. [PubMed] [Google Scholar]
- 9.Taori KB, Khurana SD, Dhomne SP, Rathi V. Fetus-in-fetu - a rare case. Indian J Radiol Imaging. 2003;13:85–7. [Google Scholar]
- 10.Escobar MA, Rossman JE, Caty MG. Fetus-in-fetu: report of a case and a review of the literature. Journal of Pediatric Surgery. 2008;43:943–6. doi: 10.1016/j.jpedsurg.2008.01.061. [DOI] [PubMed] [Google Scholar]
- 11.Thakral CL, Maji DC, Sajwani MJ. Fetus-in-fetu: a case report and review of literature. J Pediatr Surg. 1998;33:1432–1434. doi: 10.1016/s0022-3468(98)90029-x. [DOI] [PubMed] [Google Scholar]
- 12.Willis RA. The borderland of embryology and pathology. Washington, DC: Butterworths; 1962. pp. 442–462. 2. [Google Scholar]
- 13.ARahman LO, AKadir AY, Rahman AG. Fetus -in -fetu in a 6-month-old. Afr J Paediatr Surg. 2008;5:96–8. doi: 10.4103/0189-6725.44187. [DOI] [PubMed] [Google Scholar]
- 14.Potter EL. Pathology of the fetus and the newborn. In: Potter EL, editor. Pathology of the fetus and the newborn. 2nd ed. Chicago, Ill: Year Book; 1961. pp. 183–187. [Google Scholar]
- 15.Willis RA. Structure of teratomatous. J Pathol Bacteriol. 1935;40(January):1–36. [Google Scholar]
- 16.Karaman I, Erdogan D, Ozalevli S, Karaman A, Cavusoglu YH, Aslan MK, Cakmak O. Fetus-in-fetu: A report of two cases. J Indian Assoc Pediatr Surg. 2008;13:30–2. doi: 10.4103/0971-9261.42572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sinha A, Sarin YK, Sengar M. Magnetic Resonance Imaging (MRI) in the diagnosis of fetus-in-fetu. Indian Pediatr. 2003;40:63–64. [PubMed] [Google Scholar]
- 18.Aoki K, Matsumoto Y, Hamazaki M, et al. MRI reveals fetus-in-fetu in the mediastinum. Pediatr Radiol. 2004;34:1017–1019. doi: 10.1007/s00247-004-1295-4. [DOI] [PubMed] [Google Scholar]
- 19.Chang Chia-Chien, Peng Hui-Ling, Wu Chin-Chu, Lu Ta-Nien, Chen Liang-Kuang, Su Cheng-Tau. Fetus-in-fetu: MR Appearance and a case report. Chin J Radiol. 2006;31:323–327. [Google Scholar]
- 20.Hopkin KL, Dickson PK, Ball TI, Ricketts RR, O'Shea PA, Abramovosky CR. Fetus-in-fetu with malignant recurrence. J Pediatr Surg. 1997;32:1476–1479. doi: 10.1016/s0022-3468(97)90567-4. [DOI] [PubMed] [Google Scholar]