Abstract
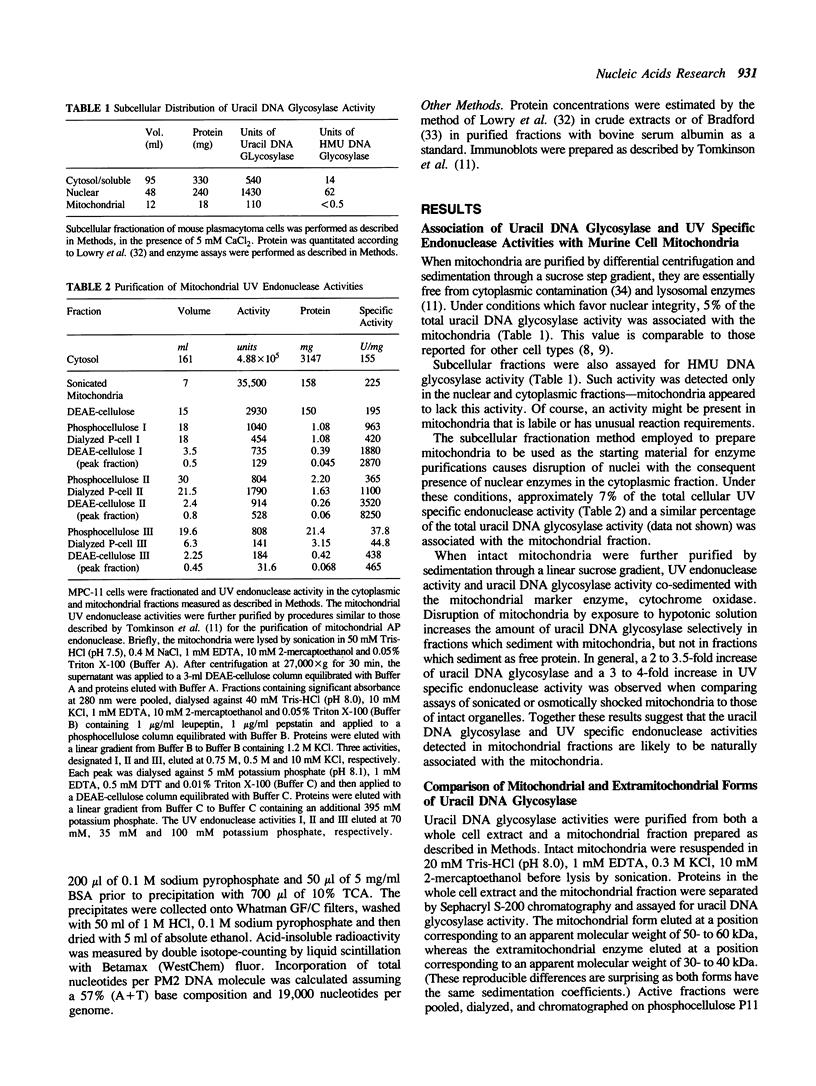
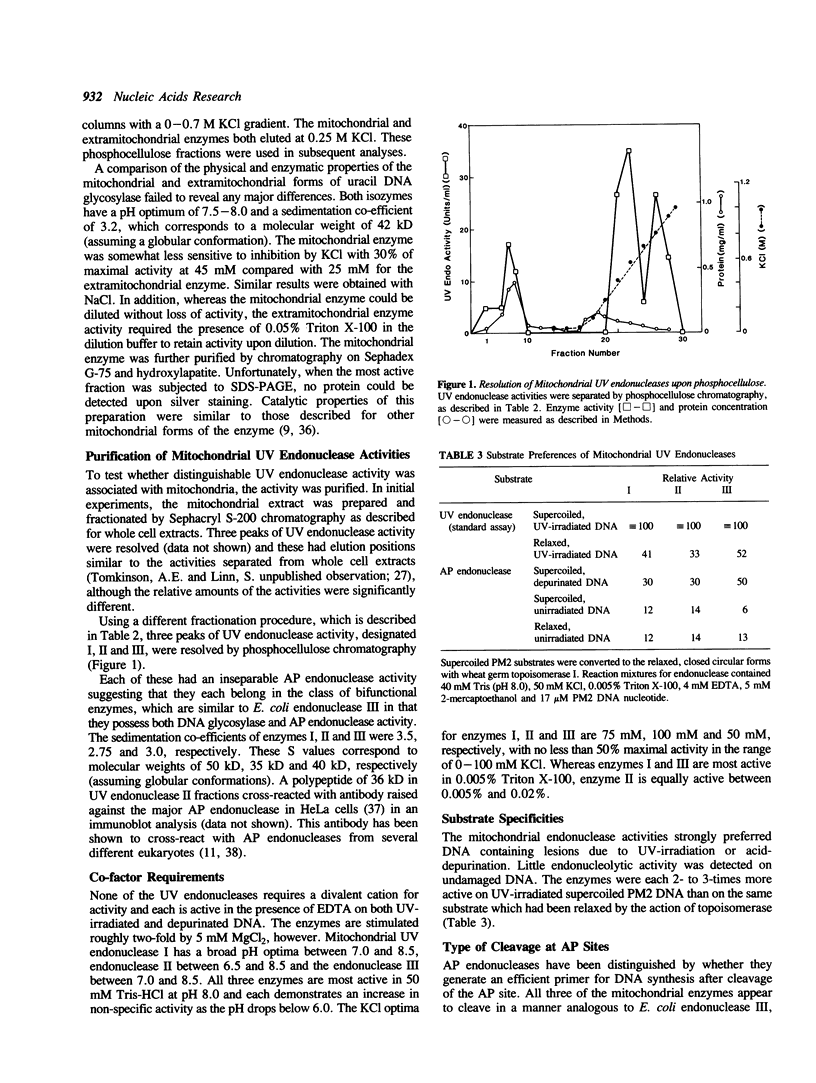
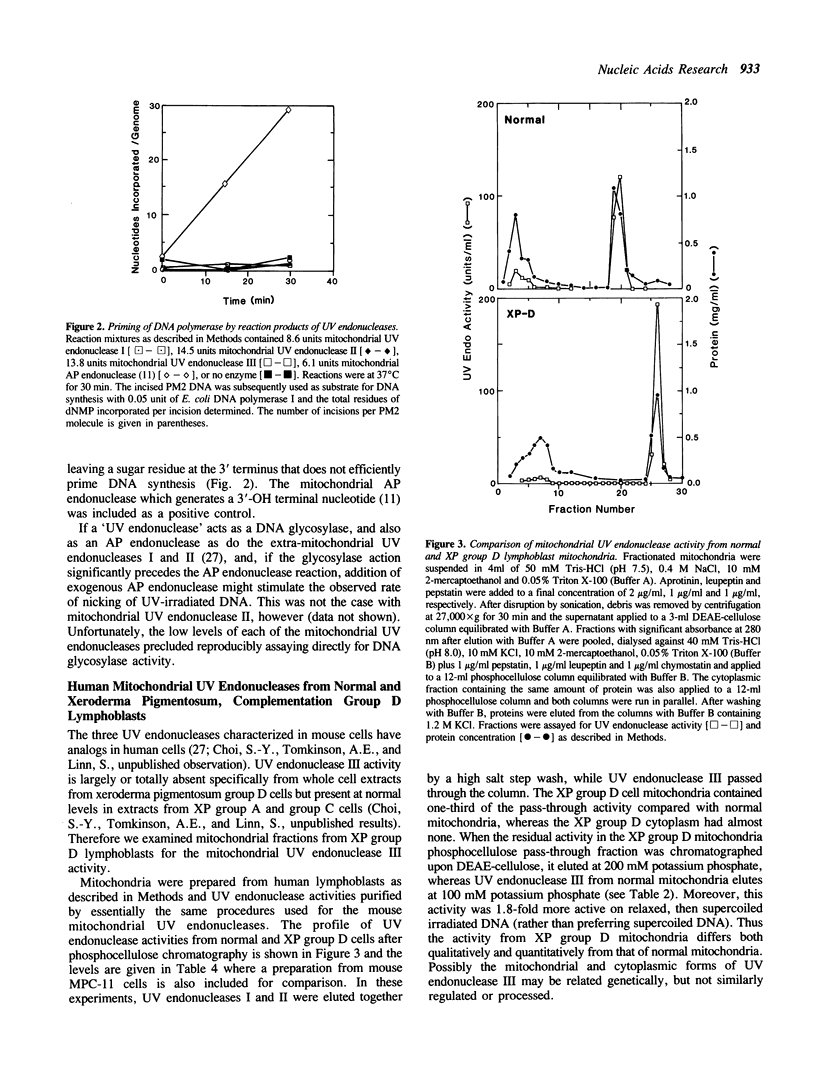
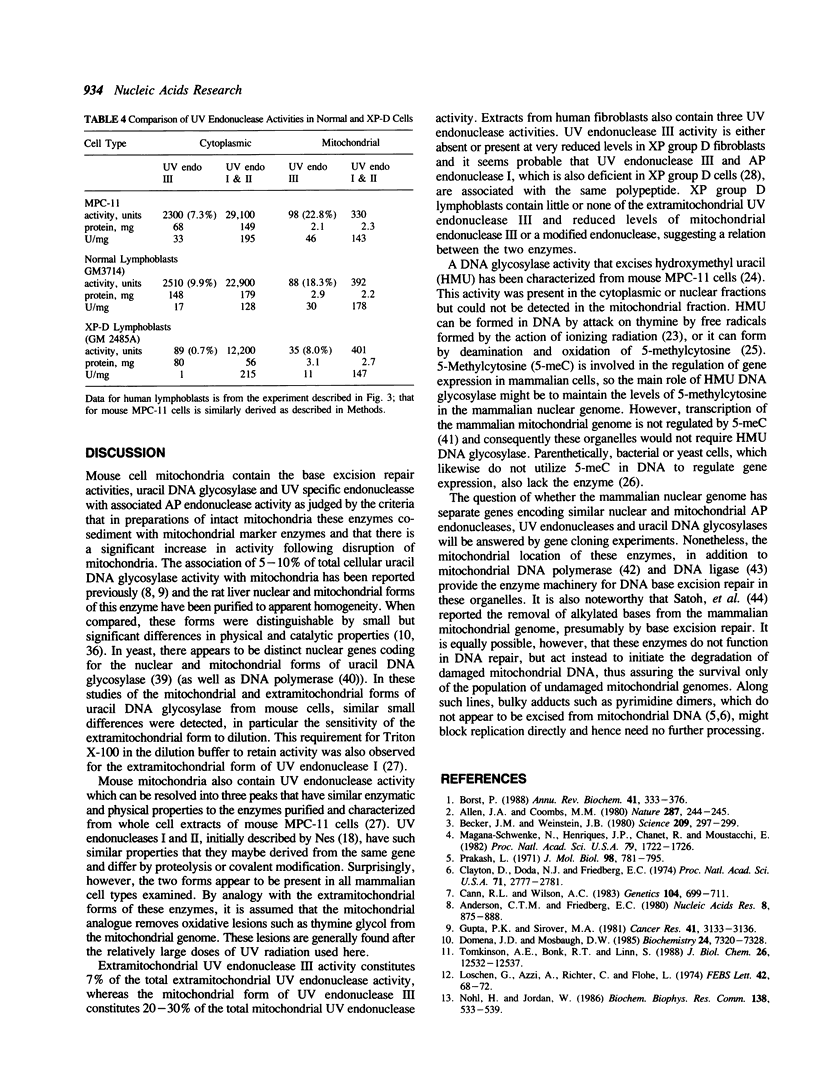
Mitochondrial forms of uracil DNA glycosylase and UV endonuclease have been purified and characterized from the mouse plasmacytoma cell line, MPC-11. As in other cell types, the mitochondrial uracil DNA glycosylase has properties very similar to those of the nuclear enzyme, although in this case the mitochondrial activity was also distinguishable by extreme sensitivity to dilution. Three mitochondrial UV endonuclease activities are also similar to nuclear enzymes; however, the relative amounts of these enzyme activities in the mitochondria is significantly different from that in the nucleus. In particular, mitochondria contain a much higher proportion of an activity analogous to UV endonuclease III. Nuclear UV endonuclease III activity is absent from XP group D fibroblasts and XP group D lymphoblasts have reduced, but detectable levels of the mitochondrial form of this enzyme. This residual activity differs in its properties from the normal mitochondrial form of UV endonuclease III, however. The presence of these enzyme activities which function in base excision repair suggests that such DNA repair occurs in mitochondria. Alternatively, these enzymes might act to mark damaged mitochondrial genomes for subsequent degradation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. A., Coombs M. M. Covalent binding of polycyclic aromatic compounds to mitochondrial and nuclear DNA. Nature. 1980 Sep 18;287(5779):244–245. doi: 10.1038/287244a0. [DOI] [PubMed] [Google Scholar]
- Anderson C. T., Friedberg E. C. The presence of nuclear and mitochondrial uracil-DNA glycosylase in extracts of human KB cells. Nucleic Acids Res. 1980 Feb 25;8(4):875–888. [PMC free article] [PubMed] [Google Scholar]
- Backer J. M., Weinstein I. B. Mitochondrial DNA is a major cellular target for a dihydrodiol-epoxide derivative of benzo[a]pyrene. Science. 1980 Jul 11;209(4453):297–299. doi: 10.1126/science.6770466. [DOI] [PubMed] [Google Scholar]
- Bolden A., Noy G. P., Weissbach A. DNA polymerase of mitochondria is a gamma-polymerase. J Biol Chem. 1977 May 25;252(10):3351–3356. [PubMed] [Google Scholar]
- Boorstein R. J., Levy D. D., Teebor G. W. 5-Hydroxymethyluracil-DNA glycosylase activity may be a differentiated mammalian function. Mutat Res. 1987 May;183(3):257–263. doi: 10.1016/0167-8817(87)90008-3. [DOI] [PubMed] [Google Scholar]
- Borst P. Mitochondrial nucleic acids. Annu Rev Biochem. 1972;41:333–376. doi: 10.1146/annurev.bi.41.070172.002001. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brent T. P. Properties of a human lymphoblast AP-endonuclease associated with activity for DNA damaged by ultraviolet light, gamma-rays, or osmium tetroxide. Biochemistry. 1983 Sep 13;22(19):4507–4512. doi: 10.1021/bi00288a024. [DOI] [PubMed] [Google Scholar]
- Burgers P. M., Klein M. B. Selection by genetic transformation of a Saccharomyces cerevisiae mutant defective for the nuclear uracil-DNA-glycosylase. J Bacteriol. 1986 Jun;166(3):905–913. doi: 10.1128/jb.166.3.905-913.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cann R. L., Wilson A. C. Length mutations in human mitochondrial DNA. Genetics. 1983 Aug;104(4):699–711. doi: 10.1093/genetics/104.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon-Carlson S. V., Gokhale H., Teebor G. W. Purification and characterization of 5-hydroxymethyluracil-DNA glycosylase from calf thymus. Its possible role in the maintenance of methylated cytosine residues. J Biol Chem. 1989 Aug 5;264(22):13306–13312. [PubMed] [Google Scholar]
- Clayton D. A., Doda J. N., Friedberg E. C. The absence of a pyrimidine dimer repair mechanism in mammalian mitochondria. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2777–2781. doi: 10.1073/pnas.71.7.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. A. Transcription of the mammalian mitochondrial genome. Annu Rev Biochem. 1984;53:573–594. doi: 10.1146/annurev.bi.53.070184.003041. [DOI] [PubMed] [Google Scholar]
- Costas M. J., Cameselle J. C., Sillero A. Mitochondrial location of rat liver dinucleoside triphosphatase. J Biol Chem. 1986 Feb 15;261(5):2064–2067. [PubMed] [Google Scholar]
- Demple B., Linn S. 5,6-Saturated thymine lesions in DNA: production by ultraviolet light or hydrogen peroxide. Nucleic Acids Res. 1982 Jun 25;10(12):3781–3789. doi: 10.1093/nar/10.12.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demple B., Linn S. DNA N-glycosylases and UV repair. Nature. 1980 Sep 18;287(5779):203–208. doi: 10.1038/287203a0. [DOI] [PubMed] [Google Scholar]
- Doetsch P. W., Helland D. E., Haseltine W. A. Mechanism of action of a mammalian DNA repair endonuclease. Biochemistry. 1986 Apr 22;25(8):2212–2220. doi: 10.1021/bi00356a054. [DOI] [PubMed] [Google Scholar]
- Doetsch P. W., Henner W. D., Cunningham R. P., Toney J. H., Helland D. E. A highly conserved endonuclease activity present in Escherichia coli, bovine, and human cells recognizes oxidative DNA damage at sites of pyrimidines. Mol Cell Biol. 1987 Jan;7(1):26–32. doi: 10.1128/mcb.7.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domena J. D., Mosbaugh D. W. Purification of nuclear and mitochondrial uracil-DNA glycosylase from rat liver. Identification of two distinct subcellular forms. Biochemistry. 1985 Dec 3;24(25):7320–7328. doi: 10.1021/bi00346a045. [DOI] [PubMed] [Google Scholar]
- Domena J. D., Timmer R. T., Dicharry S. A., Mosbaugh D. W. Purification and properties of mitochondrial uracil-DNA glycosylase from rat liver. Biochemistry. 1988 Sep 6;27(18):6742–6751. doi: 10.1021/bi00418a015. [DOI] [PubMed] [Google Scholar]
- Gates F. T., 3rd, Linn S. Endonuclease V of Escherichia coli. J Biol Chem. 1977 Mar 10;252(5):1647–1653. [PubMed] [Google Scholar]
- Genga A., Bianchi L., Foury F. A nuclear mutant of Saccharomyces cerevisiae deficient in mitochondrial DNA replication and polymerase activity. J Biol Chem. 1986 Jul 15;261(20):9328–9332. [PubMed] [Google Scholar]
- Gossett J., Lee K., Cunningham R. P., Doetsch P. W. Yeast redoxyendonuclease, a DNA repair enzyme similar to Escherichia coli endonuclease III. Biochemistry. 1988 Apr 5;27(7):2629–2634. doi: 10.1021/bi00407a054. [DOI] [PubMed] [Google Scholar]
- Gupta P. K., Sirover M. A. Stimulation of the nuclear uracil DNA glycosylase in proliferating human fibroblasts. Cancer Res. 1981 Aug;41(8):3133–3136. [PubMed] [Google Scholar]
- Hollstein M. C., Brooks P., Linn S., Ames B. N. Hydroxymethyluracil DNA glycosylase in mammalian cells. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4003–4007. doi: 10.1073/pnas.81.13.4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen T. J., Kow Y. W., Wallace S. S., Henner W. D. Mechanism of action of Micrococcus luteus gamma-endonuclease. Biochemistry. 1987 Oct 6;26(20):6436–6443. doi: 10.1021/bi00394a021. [DOI] [PubMed] [Google Scholar]
- Kane C. M., Linn S. Purification and characterization of an apurinic/apyrimidinic endonuclease from HeLa cells. J Biol Chem. 1981 Apr 10;256(7):3405–3414. [PubMed] [Google Scholar]
- Kelley M. R., Venugopal S., Harless J., Deutsch W. A. Antibody to a human DNA repair protein allows for cloning of a Drosophila cDNA that encodes an apurinic endonuclease. Mol Cell Biol. 1989 Mar;9(3):965–973. doi: 10.1128/mcb.9.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Linn S. Purification and characterization of UV endonucleases I and II from murine plasmacytoma cells. J Biol Chem. 1989 Feb 15;264(5):2739–2745. [PubMed] [Google Scholar]
- Kim J., Linn S. The mechanisms of action of E. coli endonuclease III and T4 UV endonuclease (endonuclease V) at AP sites. Nucleic Acids Res. 1988 Feb 11;16(3):1135–1141. doi: 10.1093/nar/16.3.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnlein U., Penhoet E. E., Linn S. An altered apurinic DNA endonuclease activity in group A and group D xeroderma pigmentosum fibroblasts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1169–1173. doi: 10.1073/pnas.73.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levin C. J., Zimmerman S. B. A DNA ligase from mitochondria of rat liver. Biochem Biophys Res Commun. 1976 Mar 22;69(2):514–520. doi: 10.1016/0006-291x(76)90551-9. [DOI] [PubMed] [Google Scholar]
- Linn S., Lehman I. R. An endonuclease from mitochondria of Neurospora crassa. J Biol Chem. 1966 Jun 10;241(11):2694–2699. [PubMed] [Google Scholar]
- Loschen G., Azzi A., Richter C., Flohé L. Superoxide radicals as precursors of mitochondrial hydrogen peroxide. FEBS Lett. 1974 May 15;42(1):68–72. doi: 10.1016/0014-5793(74)80281-4. [DOI] [PubMed] [Google Scholar]
- Magaña-Schwencke N., Henriques J. A., Chanet R., Moustacchi E. The fate of 8-methoxypsoralen photoinduced crosslinks in nuclear and mitochondrial yeast DNA: comparison of wild-type and repair-deficient strains. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1722–1726. doi: 10.1073/pnas.79.6.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nes I. F. Purification and properties of a mouse-cell DNA-repair endonuclease, which recognizes lesions in DNA induced by ultraviolet light, depurination, gamma-rays, and OsO4 treatment. Eur J Biochem. 1980 Nov;112(1):161–168. doi: 10.1111/j.1432-1033.1980.tb04997.x. [DOI] [PubMed] [Google Scholar]
- Nohl H., Jordan W. The mitochondrial site of superoxide formation. Biochem Biophys Res Commun. 1986 Jul 31;138(2):533–539. doi: 10.1016/s0006-291x(86)80529-0. [DOI] [PubMed] [Google Scholar]
- Prakash L. Repair of pyrimidine dimers in nuclear and mitochondrial DNA of yeast irradiated with low doses of ultraviolet light. J Mol Biol. 1975 Nov 15;98(4):781–795. doi: 10.1016/s0022-2836(75)80010-6. [DOI] [PubMed] [Google Scholar]
- Richter C., Park J. W., Ames B. N. Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6465–6467. doi: 10.1073/pnas.85.17.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh M. S., Huh N., Rajewsky M. F., Kuroki T. Enzymatic removal of O6-ethylguanine from mitochondrial DNA in rat tissues exposed to N-ethyl-N-nitrosourea in vivo. J Biol Chem. 1988 May 15;263(14):6854–6856. [PubMed] [Google Scholar]
- Teebor G. W., Frenkel K., Goldstein M. S. Ionizing radiation and tritium transmutation both cause formation of 5-hydroxymethyl-2'-deoxyuridine in cellular DNA. Proc Natl Acad Sci U S A. 1984 Jan;81(2):318–321. doi: 10.1073/pnas.81.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkinson A. E., Bonk R. T., Linn S. Mitochondrial endonuclease activities specific for apurinic/apyrimidinic sites in DNA from mouse cells. J Biol Chem. 1988 Sep 5;263(25):12532–12537. [PubMed] [Google Scholar]
- Tomkinson A. E., Linn S. Purification and properties of a single strand-specific endonuclease from mouse cell mitochondria. Nucleic Acids Res. 1986 Dec 22;14(24):9579–9593. doi: 10.1093/nar/14.24.9579. [DOI] [PMC free article] [PubMed] [Google Scholar]



