Abstract
Antioxidant supplementations are commonly used as an ergogenic aid for physical exercise despite its limited evidence. The study aimed to investigate the effects of a polyphenol mixture and vitamins on exercise endurance capacity. Seventy regularly exercising male participants were randomly assigned to receive oligomerized lychee fruit extract, a mixture of vitamin C (800 mg) and E (320 IU), or a placebo for 30 consecutive days. The study results showed that oligomerized lychee fruit extract significantly elevated the submaximal running time (p = 0.01). The adjusted mean change was 3.87 min (95% CI: 1.29, 6.46) for oligomerized lychee fruit extract, 1.33 (−1.23, 3.89) for the vitamins, and 1.60 (−1.36, 4.56) for the placebo (p = 0.33 in between groups). Oligomerized lychee fruit extract significantly increased the anaerobic threshold by 7.4% (1.8, 13.0). On the other hand, vitamins significantly attenuated VO2max by −3.11 ml/kg/m (−5.35, −0.87). Their effects on plasma free radical amount, however, were similar. Our results suggest that a polyphenol-containing supplement and typical antioxidants may have different mechanisms of action and that the endurance-promoting effect of oligomerized lychee fruit extract may not directly come from the scavenging of free radicals but may be attributed to other non-antioxidant properties of polyphenols, which requires further investigation.
Keywords: polyphenol, lychee, antioxidant, vitamin, endurance capacity
Introduction
There is considerable debate about the role and clinical meaning of exercise-induced reactive oxygen species (ROS) and antioxidant supplementation for exercise performance.(1,2) Several studies have presented evidence that exercise can increase ROS production(3–5) and that exercise-induce oxidative stress can cause muscle fatigue.(6,7) Earlier studies concentrated mainly on potential harmful effects of exercise-induced ROS, and many types of antioxidant supplements were assessed as to whether they prevent muscle fatigue and enhance exercise performance.(8,9) While several animal studies have found that antioxidant supplements delay muscle fatigue(10) and improve exercise performance,(11) the clinical efficacy of antioxidants remains uncertain.(8,9,12) Why most clinical trials have failed to show a significant ergogenic effect of antioxidants remains unclear. Partial explanations may include 1) an inadequate dosage and/or inadequate duration of intake, 2) low bioavailability and the antioxidant capacity of supplements, 3) the insufficient number of subjects in those studies, or 4) the harmful effect of scavenging exercise–induced ROS, which serve as cell signaling molecules.(13–15) In spite the weak clinical evidence presented in these studies, antioxtidants are known to be very commonly used.(16)
It was recently reported that polyphenolic flavonoids have strong antioxidant effects. This may stem not only from direct scavenging ROS but also from indirect modulation of multiple cell signaling pathway.(17,18) Oligomerized lychee fruit extract (OLFE) is a supplement made from lychee fruit pericarp. It is made by technological oligomerization of polyphenol polymers in the extract and contains a relatively large proportion of catechin-type monomers and oligomers of proanthocyanidin.(19) Previous studies showed that OLFE is safe(20) and that it has strong antioxidant capacity and relatively high bioavailability.(21) These findings suggest that OLFE is an appropriate candidate supplement with specific ergogenic effects.
The objective of the study was to explore the hypothesis that an antioxidant supplementation could improve the exercise endurance performance in the form of two different types: a mixture of polyphenols with antioxidant capacity (OLFE), and a mixture of antioxidant vitamins in a placebo-controlled trial.
Materials and Methods
Eligibility and recruitment
We recruited participants between August of 2007 and January of 2008 in Samcheok City in Korea through advertisements on internet websites and local community newspapers. The participants met the eligibility criteria if they were aged between 20 and 65 years, non-smoking, male, and had been performing regular aerobic exercise at least for 30 min per day and 3 days per week for at least 3 months up to the time the study started. Subjects were excluded if they met any of the following criteria: BMI>25 kg/m2 or body fat>25%; intake of ergogenic agents or antioxidant supplements within 2 weeks prior to the study (for example, polyphenols, Vitamin E, Vitamin C, glutathione, α-lipoic acid, carotenoids, flavonoids, ubiquinone, copper, zinc, iron, selenium, manganese); having a serious disease such as severe pulmonary, gastrointestinal, hepatic, endocrinologic, neurologic, or psychologic disease; any history of arrhythmia or coronary artery disease such as angina pectoris, unstable angina, acute myocardial infarction for the last 6 months; current musculoskeletal problems such as foot and ankle pain, knee pain or pelvic, or low back pain.
Participant eligibility was determined through medical history and diet assessments; supplementation history; a physical activity questionnaire; and by measuring the height, weight and body composition in an bioelectrical impedance analysis (Zeus 9.9, Jawon Medical CO., Ltd., Seoul, Korea). Written informed consent was received from participants who had been informed of the study purpose and methods including the process, duration, possible side effects of the experimental supplements. They were also told they could drop out of the study at any time. The study protocol was approved by the Institutional Review Board of the Seoul National University Hospital. It was registered at www.clinicaltrial.gov (NCT00731926).
Materials
Eligible and consenting participants were randomly allocated to receive either oligomerized lychee fruit extract (OLFE; Oligonol®, Amino-Up Chemical Co., Ltd., Japan) or vitamin mixture, or a placebo. OLFE was produced by oligomerization of polyphenols extracted from lychee fruit pericarp, which contained a relatively large amount of low-molecular-weight polyphenols (about 33%) consisting of 15.7% polyphenol monomer ((+)-catechin and (−)-epicatechin etc.) along with 13.3% polyphenol dimer (procyanidin B2 etc.). More detailed information on preparation of OLFE can be found in the previous literature.(20,22) Dried lychee fruits are extracted with 80% ethanol. The filtrate is evaporated and passed through a DIAION HP-20 column, and eluted with ethanol. The eluate is evaporated to dryness yielding a dark brown powder which contains a mixture of proanthocyanidins. The lychee extract is mixed with green tea extract, which provides an enriched source of monomeric procyanidins, and citric acid in water. The reaction mixture is heated at 60°C for 16 h, filtered through a DIAION HP-20 column, washed with water and eluted with 40% ethanol. Evaporation of the eluate finally yields a reddish brown powder containing the monomeric and oligomeric proanthocyanidin mixture. Fifty milligrams of OLFE powder was packed into one milky capsule. One capsule of the vitamin mixture contained 200 mg of vitamin C and 80 IU of vitamin E (α-tocopherol). The placebo capsule contained 50 mg of dextrin. All study supplements were contained in capsules of the same size, taste, and color. All participants were instructed to intake 2 capsules twice a day for 30 days. Therefore, the total intake dosages per day were: 200 mg of placebo, 200 mg of OLFE, or a mixture of vitamin C 800 mg and vitamin E 320 IU. Previous studies had used a variety of dosages, ranging from 750 to 1000 mg of vitamin C and 300 to 1200 IU of vitamin E but there was not a particular consensus for the optimal dose.(23) We have chosen a combination of tolerable doses from those ranges tried. A study reported that 200 mg of OLFE for 26 days of intake improved subjective feeling of sports fatigue and fatigue recovery after exercise training in amateur athletes,(24) which was considered as a reference for the choice of dose for OLFE.
Randomization and study process
A computerized block randomization sequence was prepared by the Medical Research Collaborating Center at the Seoul National University Hospital. The product manufacturer produced, packed, and labeled the study regimens in bottles of the same size, shape and color in accordance with the randomization sequence and dispatched the concealed and serially numbered packages. For the duration of the study, the product manager could be contacted at any time to open the concealment in case of an emergency.
A trained registered dietitian instructed all participants to maintain their regular exercise practice and normal diet and to eschew any other supplementation that may affect the exercise performance during the study period. All participants completed daily food records for 2 days prior to each submaximal treadmill test. The nutrition intake was calculated from recorded food items using a computer-aided nutritional analysis program (CAN pro ver. 3.0, 2005, Korea Nutrition Society, Korea).
We contacted all participants by phone every week during the study period. With every phone call, the overall functioning, difficulties with intervention adherence, and any adverse effects by the subjects were assessed. The capsule count, as the primary marker of compliance, was calculated at the end of the follow-up period as the total number of capsules dispensed minus the number returned divided by the total number of capsules dispensed.
Primary outcome
The primary outcome was the change in the submaximal running time to exhaustion at 80% of the maximal heart rate (HRmax). We conducted a treadmill test at 80% of HRmax and measured the running time to exhaustion at the baseline and after 30 days of supplementation. During the submaximal treadmill test, the subjects kept running until they felt exhausted and were incapable of continuing. The grade was controlled while fixing the speed to keep the subjects’ heart rate in the range of 80% HRmax ± 10 beats per minute under continuous monitoring. We determined exhaustion when the rating of perceived exertion (RPE) was 19, which meant ’extremely hard’ (when they were not capable of continuing for long at this pace), or when an abrupt increase in the heart rate from a plateau state or a sudden decrease of the running speed occurred. All subjects were prohibited from strenuous exercise, drinking, and additional fruits or vegetables for 3 days before the submaximal treadmill test. The submaximal test was performed between 7:00 to 10:00 AM after a minimum of 10 h overnight fasting.
Secondary outcomes
We measured the VO2max three days before the submaximal treadmill tests. To evaluate the VO2max, all subjects participated in a maximal graded exercise test using a motorized treadmill in accordance with the Bruce protocol, in which the speed and grade were elevated every 3 min(25) using a flow meter and a gas analyzer after a 5-min warm-up exercise following measurement of the resting heart rate. Prior to each test, both the flow meters and the gas analyzers were calibrated. The temperature of the laboratory was maintained between 22 and 24°C, and the humidity was controlled at 50%. Expired gas was collected and the rate of oxygen consumption (VO2) and that of carbon dioxide production (VCO2) were measured on a mixing chamber using a metabolic measurement system (TrueOne 2400, Sandy, Utah). The maximal oxygen consumption rate was defined as the highest value recorded during the test. The body fat-adjusted maximal oxygen consumption rate (VO2max/fat-free mass) was used in this study. It has been known that total body fat does not affect maximal aerobic capacity but fat-free mass is a more important factor in determining maximum aerobic capacity.(26,27) The fat-adjusted maximal oxygen consumption rate has been reported allowing a better prediction of outcomes, for example, in chronic heart failure.(28)
The anaerobic threshold is commonly used as a surrogate marker for the lactate threshold (LT), which has been suggested as the best predictor of endurance capacity.(29,30) LT refers to the workload cut-off value when the production of lactic acid overcomes its removal rate, allowing it to start accumulating in the blood and thus aggravate muscle fatigue and rapidly attenuate exercise performance.(31) During the maximal treadmill test, the anaerobic threshold was determined by the V-slope method(29) after a computerized regression analysis of the slopes of the CO2 output versus the O2 uptake.
We measured the plasma free radical amount using the free oxygen radicals test (FORT) at baseline and on the 30th day before and after the submaximal treadmill test: the radical species produced by the reaction were directly proportional to the quantity of lipid peroxides present in the sample interact with an additive (phenylenediamine derivative) that formed a radical molecule evaluable by a spectrophotometer at 505 nm (FORMox, Callegari, Parma, Italy). Results were expressed in FORT units, where 1 FORT unit corresponds to 0.26 mg/L of H2O2.
The serum lactate dehydrogenase (LDH) level was measured before and after the submaximal treadmill test. Blood was sampled from the anticubital vein by venipuncture, and the serum LDH concentration was analyzed using an ultraviolet spectrometer.
Sample size calculation
To estimate the effect size to determine the proper sample size, we conducted a small pilot study involving 15 healthy male college students for 30 days. The study process and measurements were identical to those in the main trial. The sample size was calculated for an analysis of variance (ANOVA), which is a method of test to find out whether means from several groups differ, in terms of the change in the submaximal running time to exhaustion at 80% HRmax as a primary outcome. When we assumed that the maximum difference between the OLFE and placebo was 4.5 and that the common standard deviation was 4.5 min based on the results of the pilot study, the required sample size was calculated to be 23 for each group, allowing for a 15% drop-out rate at a significance level of 5% (two–sided) and power of 80%.
Statistical analysis
The homogeneity of the baseline characteristics between groups was evaluated by using an ANOVA. To test any change after supplementation within the groups, a paired t test was used for each group. The ANOVA was used to assess the comparative differences between groups. To adjust for significantly different baseline characteristics or to account for potential confounders, an analysis of covariance (ANCOVA) was also considered. As the baseline values of BMI, body fat, and fat-free mass were significantly different among groups, and as they can be regarded as the parameters that potentially affect the outcome variables, we included those variables as covariates in the ANCOVA models for adjustment. Since there was also a significant between-group difference in age, although the age itself may not substantially affect the outcomes, it was also considered as one of the adjustment covariates in the ANCOVA models. There was a significant imbalance between groups also found in body weight, but we did not include this variable in the model for adjustment as it was already counted in calculation of BMI. Statistical significance was determined when p<0.05. All statistical analyses were conducted using SPSS ver. 12.0 K.
Results
Study process
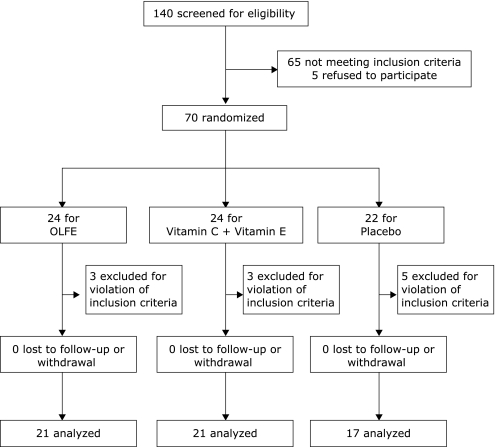
Fig. 1 shows the trial profile. After randomization of seventy participants, eleven participants were excluded as they were found to have violated the eligibility criteria (BMI>25 kg/m2), as identified during the data verification process. The other fifty nine participants completed the trial protocol and were available for data analysis. The compliance of all subjects was very good and there was no significant difference in the compliance between the groups (p = 0.06), although it was slightly lower in the placebo group: OLFE (99.0%), vitamin C + vitamin E (98.7%), placebo (97.2%). No adverse event was reported from any enrolled participants.
Fig. 1.
Flow diagram of the trial.
Anthropometric data
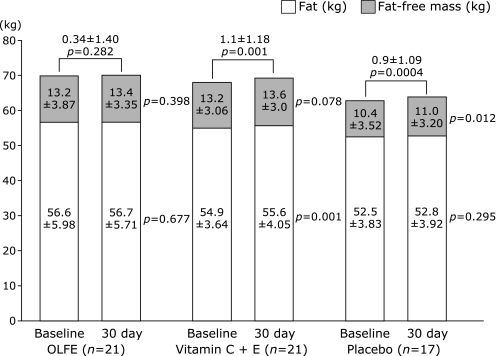
The anthropometric characteristics are presented in Table 1. Whilst the majority of the participants were in their mid-twenties in all groups, participants in the placebo group were younger on average (p = 0.02). The participants in this group were also significantly lower in body weight, BMI, body fat and fat-free mass compared to the other groups. While maintaining their normal diet and regular physical exercise routine during the 30 days of supplementation, the body weight significantly increased in the vitamin (p = 0.001) and placebo (p = 0.0004) groups by approximately 1 kg. The change patterns of the body composition, however, differed between those two groups in that the fat-free mass significantly increased (p = 0.001) in the vitamin group whereas the body fat significantly increased (p = 0.012) in the placebo group. There was little change of body weight observed in the OLFE group (Fig. 2).
Table 1.
Baseline characteristics of study subjects
| OLFE (n = 21) | Vitamin C + E (n = 21) | Placebo (n = 17) | p | |
|---|---|---|---|---|
| Age (yr) | 24.6 ± 6.60 | 23.9 ± 5.71 | 22.9 ± 3.57 | 0.02 |
| Height (cm) | 175.5 ± 6.43 | 174.0 ± 4.75 | 172.4 ± 5.34 | 0.24 |
| Weight (kg) | 69.9 ± 8.37 | 68.1 ± 6.13 | 62.9 ± 6.82 | 0.01 |
| BMI (kg/m2) | 22.7 ± 2.13 | 22.5 ± 1.50 | 21.2 ± 1.97 | 0.04 |
| Body fat (%) | 18.7 ± 4.29 | 19.2 ± 2.95 | 16.2 ± 3.80 | 0.04 |
| Fat-free mass | 56.6 ± 5.98 | 54.9 ± 3.64 | 52.5 ± 3.83 | 0.03 |
OLFE: oligomerized lychee fruit extract. Presented values are the mean ± SD. The p value was calculated for the difference among the groups by one-way ANOVA. The participants in the placebo group were significantly younger. They were also lower in body weight, BMI, body fat and fat-free mass compared to the other groups.
Fig. 2.
Change in body weight. Presented values are the mean ± SD. p value was calculated for the difference between baseline value and that after 30 days of supplementation within each group by a paired t test. After supplementation, body weight of vitamin and placebo group significantly changed. Fat-free mass increased in vitamin group and body fat did in placebo group.
Dietary assessment
The average intake of daily calories, protein, carbohydrate, fat, and antioxidant vitamins for the two consecutive days preceding the submaximal treadmill test were assessed. The participants’ diets were adequate in terms of calories, macronutrients, and antioxidant vitamins when compared to the daily recommended intake (DRI) for their age group. No statistically or nutritionally significant differences between sessions/groups were noted (Table 2).
Table 2.
Dietary assessment for 2 consecutive days before the submaximal treadmill test
| Session | Total calories (kcal) | Protein (g) | Fat (g) | Carbohydrate (g) | Vitamin C (mg) | Vitamin E (mg) | Retinol (µg) | β-carotene (µg) | Zinc (mg) | |
|---|---|---|---|---|---|---|---|---|---|---|
| OLFE (n = 21) | Baseline | 1963.6 ± 391.1 | 82.9 ± 19.2 | 59.9 ± 20.6 | 279.0 ± 55.2 | 90.2 ± 48.9 | 17.6 ± 9.6 | 128.3 ± 86.6 | 4219.9 ± 2602.4 | 9.5 ± 2.2 |
| Final | 1953.3 ± 295.5 | 87.8 ± 18.3 | 57.4 ± 11.6 | 273.1 ± 58.6 | 84.2 ± 44.2 | 15.3 ± 6.2 | 130.8 ± 106.1 | 3874.5 ± 2578.1 | 9.6 ± 2.3 | |
| Vitamin C + E (n = 21) | Baseline | 1997.3 ± 282.4 | 89.0 ± 19.3 | 68.0 ± 25.6 | 274.7 ± 35.6 | 96.8 ± 49.0 | 14.6 ± 6.5 | 114.0 ± 103.2 | 2982.2 ± 906.7 | 9.6 ± 2.1 |
| Final | 1916.8 ± 303.1 | 89.5 ± 27.7 | 71.3 ± 50.7 | 254.2 ± 45.7 | 95.1 ± 46.6 | 14.9 ± 6.4 | 109.2 ± 80.7 | 3803.0 ± 2679.9 | 9.4 ± 2.1 | |
| Placebo (n = 17) | Baseline | 1917.1 ± 375.0 | 81.3 ± 30.9 | 54.6 ± 16.6 | 273.8 ± 64.3 | 102.2 ± 49.8 | 16.1 ± 5.3 | 129.8 ± 172.8 | 3487.0 ± 1963.0 | 8.4 ± 2.0 |
| Final | 1952.9 ± 371.8 | 90.0 ± 27.6 | 57.7 ± 19.7 | 272.0 ± 64.1 | 103.4 ± 47.5 | 13.4 ± 6.1 | 115.2 ± 66.6 | 5090.5 ± 2892.5 | 9.3 ± 2.6 |
OLFE: oligomerized lychee fruit extract. Presented values are the mean ± SD. The p value was calculated for the difference among the groups by one-way ANOVA. The participants in the placebo group were significantly younger. They were also lower in body weight, BMI, body fat and fat-free mass compared to the other groups.
Submaximal endurance time
Running times to exhaustion at 80% HRmax are presented in Table 3. There was no significant difference observed between the groups in the mean submaximal endurance time upon the baseline test. The change from baseline after supplementation increased only in the OLFE group by 3.3 min with statistically borderline significance (p = 0.05). Neither of the other groups showed any significant change from the baseline, with a reading of 1.5 (p = 0.18) for the vitamin C + E group and 2.1 (p = 0.27) for the placebo group.
Table 3.
Submaximal running time to exhaustion at 80% of the maximal heart rate
| Submaximal running time | Baseline | 30 day | Change (p) | |
|---|---|---|---|---|
| OLFE (n = 21) | Up to RER1 | 9.7 ± 1.84 | 9.2 ± 1.92 | −0.5 ± 2.46 (0.39) |
| Beyond RER1 | 16.8 ± 13.14 | 20.5 ± 14.40 | 3.7 ± 4.71 (0.01) | |
| Total | 26.5 ± 13.14 | 29.7 ± 15.85 | 3.3 ± 4.78 (0.05) | |
| Adjusted total (95% CI) | 3.87 (1.29–6.46) | |||
| Vitamin C + E (n = 21) | Up to RER1 | 9.2 ± 1.68 | 8.7 ± 1.52 | −0.5 ± 1.62 (0.17) |
| Beyond RER1 | 13.0 ± 9.50 | 15.1 ± 8.71 | 2.0 ± 5.36 (0.10) | |
| Total | 22.2 ± 10.36 | 23.8 ± 9.21 | 1.5 ± 5.07 (0.18) | |
| Adjusted total (95% CI) | 1.33 (−1.23–3.89) | |||
| Placebo (n = 17) | Up to RER1 | 9.2 ± 1.45 | 9.7 ± 1.88 | 0.5 ± 2.16 (0.35) |
| Beyond RER1 | 18.8 ± 9.77 | 20.4 ± 9.45 | 1.6 ± 6.53 (0.33) | |
| Total | 28.1 ± 9.77 | 30.2 ± 9.58 | 2.1 ± 7.57 (0.27) | |
| Adjusted total (95% CI) | 1.60 (−1.36–4.56) | |||
OLFE: oligomerized lychee fruit extract. RER1: respiratory exchange ratio of 1. CI: confidence interval. Presented values are the mean ± SD, unless otherwise noted, in minutes. The p value was calculated for the difference between the baseline value and that after 30 days of supplementation within each group by a paired t test. RER1 denotes a respiratory exchange ratio of 1. OLFE significantly increased both the total submaximal running time and the running time beyond RER1. The mean change in the submaximal running time together with 95% CI was estimated after adjusting for the baseline values of BMI, body fat, and fat-free mass, and age. The 95% confidence interval for the change excludes zero in the OLFE group, suggesting a significant within-group improvement. The p value for difference in change among groups did not reach a statistical significance at the 5% level by ANCOVA (p = 0.33) as well as by ANOVA without the adjustment (p = 0.62).
To investigate an enhanced carbohydrate metabolizing capacity further in relation to a respiratory exchange ratio of 1 (RER1), we divided the total submaximal running time into: 1) the running time up to RER1, and 2) the running time beyond RER1. While there were no significant changes in ”running time up to RER1” in the three groups, only the OLFE group showed a significant increase in ”running time beyond RER1”, of 3.7 min (p = 0.01). The amount of change in the submaximal running time coincides well with the amount of increase in the running time beyond RER1 in all groups with the correlation coefficients between 0.86 and 0.96.
An estimated mean change, adjusted for imbalanced baseline characteristics, in the submaximal running time in the OLFE group became greater, at 3.87 min (95% CI; 1.29, 6.46). On the other hand, the small increases shown in the other groups were reduced after the adjustment: 1.33 (−1.23, 3.89) for the vitamin group and 1.60 (−1.36, 4.56) for the placebo group. However, the differences in the change were not statistically significant between groups (p = 0.33) as also in the results by ANOVA without the adjustment (p = 0.62).
Maximal oxygen capacity
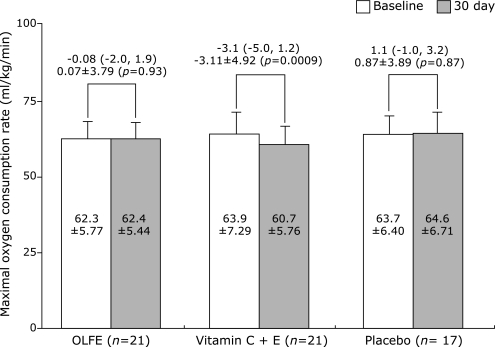
Fig. 3 presents the maximal oxygen consumption rate and its change after supplementation. When we compared the VO2max/fat-free mass, there was no significant difference (p = 0.84) observed in the values at baseline between groups: OLFE group, 62.3 ± 5.77; vitamin group, 63.9 ± 7.29; placebo, 63.7 ± 6.40. After 30 days, we observed that the maximal oxygen consumption rate significantly decreased in the vitamin group by −3.11 ml/min/kg (95% CI; −5.35, −0.87). On the other hand, the OLFE and placebo groups showed non-significant or little change in their maximal oxygen consumption rate: OLFE group, 0.07 (−1.70, 1.85); placebo, 0.87 (−1.13, 2.87). The difference in observed changes after supplementation was statistically significant between groups (p = 0.012). The results were similar after adjustment for the covariates which did not include the baseline fat free mass as it is already a part of the outcome.
Fig. 3.
Change in maximal oxygen consumption rate (VO2max/fat free mass). For each group, the mean ± SD are presented in ml/kg/min. There was no significant difference in baseline values between groups (p = 0.837). The maximal oxygen consumption rate of the vitamin group decreased significantly after 30 days of supplementation (Paired t test, p = 0.009). The mean change after supplementation was also adjusted for age as well as baseline values of BMI and body fat, and it was presented together with the 95% CI in bracket for each group. The overall p value for the difference in change among groups was 0.012 by ANOVA and was 0.015 by ANCOVA.
Anaerobic threshold
The endurance capacities measured by the anaerobic threshold (AT) are shown in Table 4. At the baseline, the endurance capacities of the three groups were similar at approximately 60% VO2max, which suggests that the participants in this study were intermediately exercised and that their endurance capacity was not significantly different between groups. After supplementation, the OLFE group showed a significant increase in their anaerobic threshold, by 7.4% (p = 0.01), whereas the vitamin and placebo groups showed no significant changes. The change in the AT by supplementation was also significantly different between the groups (p = 0.03). The results after adjustment for the imbalanced baseline characteristics were also very similar as presented in the same table.
Table 4.
Change of anaerobic threshold, resting free radical amount and exercise induced LDH increase
| OLFE (n = 21) | Vitamin C + E (n = 21) | Placebo (n = 17) | p2 | ||
|---|---|---|---|---|---|
| Anaerobic threshold (%) | Baseline | 59.1 ± 7.63 | 62.9 ± 16.16 | 62.8 ± 13.87 | 0.56 |
| 30 day | 67.3 ± 9.29 | 57.6 ± 15.02 | 60.1 ± 7.73 | 0.02 | |
| Change (p1) | 7.4 ± 11.96 (0.01) | −5.3 ± 20.30 (0.25) | −3.6 ± 12.70 (0.28) | 0.03 | |
| Adjusted change (95% CI) | 7.6 (0.3, 15.0) | −5.5 (−12.6, 1.7) | −3.6 (−12.0, 4.8) | 0.03* | |
| Resting free radical amount (FORT unit) | Baseline | 232.5 ± 58.4 | 225.0 ± 42.6 | 223.2 ± 39.0 | 0.81 |
| 30 day | 222.2 ± 45.2 | 213.5 ± 39.3 | 218.8 ± 37.7 | 0.79 | |
| Change (p) | −10.3 ± 44.9 (0.30) | −11.5 ± 31.5 (0.11) | –4.4 ± 27.4 (0.52) | 0.82 | |
| Adjusted change (95% CI) | −10.8 (−26.8, 5.2) | −13.2 (−29.0, 2.7) | –1.8 (–20.1, 16.6) | 0.64* | |
| Exercise-induced LDH increase (IU/l) | Baseline | 46.6 ± 72.0 | 7.1 ± 72.2 | 25.8 ± 90.8 | 0.27 |
| 30 day | 10.5 ± 43.4 | 33.2 ± 43.1 | 5.2 ± 50.1 | 0.13 | |
| Change (p) | −36.1 ± 89.5 (0.08) | 26.1 ± 87.1 (0.18) | −20.6 ± 98.1 (0.40) | 0.08 | |
| Adjusted change (95% CI) | −26.1 (−64.5, 12.2) | 22.7 (−15.3, 60.6) | −28.6 (−72.5, 15.3) | 0.08* | |
OLFE: oligomerized lychee lruit extract. FORT: free oxygen radicals test. LDH: lactate dehydrogenase. CI: conficdence interval. Presented values are the mean ± SD in each specified unit. 1p value for change from the baseline after 30 days of supplementation within group calculated by a paired t test 2p value for difference in change among groups by ANOVA, or *ANCOVA after adjusting for the baseline values of BMI, body fat, and fat-free mass, and age.
Free radical amount
We compared the resting free radical amount between the baseline value and the result after 30 days of supplementation. Although it was not statistically significant, the resting state free radical amount tended to be reduced by roughly the same amount in the two intervention groups, by 10.3 (SD; 44.9) and 11.5 (31.5) FORT units for the OLFE and the vitamin group, respectively. However, it changed by only 4.4 (27.4) in the placebo group (Table 4). The change values were also similar after adjusting for the imbalanced baseline characteristics.
Lactate dehydrogenase (LDH)
We observed opposite patterns in the OLFE and vitamin groups for an exercise-induced increase in their LDH levels after the treadmill test (Table 4). The initially observed increase in the LDH after the treadmill test for the OLFE group at baseline was reduced after 30 days of supplementation to over one fourth of the initial observation. On the other hand, the vitamin group showed an increasing pattern in exercise-induced LDH after supplementation. Although there was a relatively small change in the LDH level after running as observed at baseline in this group, the amount of LDH increase after running was inflated by more than fourfold on average after 30 days of supplementation. The differently observed patterns were not statistically significant at the 5% level (p = 0.08) between groups.
Discussion
The aim of this double-blind, randomized, placebo-controlled trial was to investigate the efficacy of antioxidant supplementations regarding an improvement in submaximal endurance performance. OLFE significantly increased the total submaximal running time, although a differential effect in that group was not statistically significanct compared to the other groups. In addition, the running time beyond RER1 and the anaerobic threshold were also increased significantly only in the OLFE group. These consistent results imply that OLFE enhances the endurance capacity. On the other hand, the result of maximal oxygen consumption rate indicates that supplementation of a large amount of antioxidant vitamins with exercise training possibly inhibits an improvement of the endurance capacity.
The effect size was estimated to be approximately 4.4 min for the submaximal running time in the pilot study conducted earlier, based on which the sample size for this study was determined. In the main trial, however, the observed maximum difference between groups was found to be smaller than what was expected from the pilot study, and individual variances were larger than those in the pilot study. In consequence, the primary test for the treatment difference between groups was characterized by a lack of statistical power. The mean change in the submaximal running time was close to 4 min, which was a 15% increase from the baseline; this can be considered a clinically meaningful improvement. Moreover, the running time beyond RER1, not the time up to RER1, increased significantly and contributed to an increase in the total submaximal running time. RER can be used as an indicator of which fuel, carbohydrate or fat, is being metabolized to supply energy. A RER value of 1.0 or higher indicates that carbohydrate is the predominant source of fuel being used and that exhaustion is imminent. In addition, only OLFE significantly elevated the anaerobic threshold. AT can be used as a surrogate indicator of the lactate threshold, which is the best predictor of endurance capacity.(29,30) The exercise-induced increase in the LDH also decreased after supplementation with OLFE. On the other hand, the maximal oxygen consumption rate did not change in the OLFE group, whereas the vitamin mixture group showed a significant decrease.
The increase in the submaximal running time (especially the time beyond RER1) and the AT, without a change in the VO2max value, and the decrease in the exercise-induced amplification of the LDH suggest OLFE as a possible enhancer of the endurance capacity. According to previous studies,(17,18,32) the antioxidant effect of polyphenols does not appear to come from the direct scavenging effect on ROS; however, polyphenols may instead increase the redox buffer capacity by modulating multiple redox signaling pathways while not inhibiting ROS–induced muscle adaptation to exercise stress.
As we were not able to restrict or entirely control all of the physical activities of the participants, possible differences in the duration or frequency of the physical exercise done by the participants during the study period might have had an influence on the results. Nevertheless, the VO2max values in the OLFE and placebo groups changed little over the study period. Hence, it is reasonable to conclude that additional exercise training did not lead to an improvement in the endurance capacity in the OLFE group.
The vitamin C + E mixture was found to attenuate VO2max and the results are in accordance with findings from several previous studies in which the adverse effects of antioxidant vitamins were reported both in animals and humans. One study(13) presented similar results, in that high doses of vitamin E attenuated the muscle contractile force. Another study(14) reported that daily supplementation of vitamin C 1 g slowed racing greyhounds. Another study found that large amounts of vitamin C for 6 weeks with exercise inhibited the exercise-induced biogenesis of muscle mitochondria and the induction of antioxidant enzymes.(15) Another report suggested that high doses of a vitamin C and vitamin E mixture prohibited exercise from inducing antioxidant enzyme, thus prevented an improvement in insulin sensitivity.(33) As exercise-induced ROS acts as signaling molecules to adapt muscle cells to exercise stress,(34) it was suggested that an intake of strong antioxidant scavengers during exercise eliminates exercise-induced free radicals in the early phase, therefore possibly preventing muscle adaptation to oxidative stress and improvements in endurance capacity.
There has been much debate over the use of antioxidant supplements to promote endurance capacity. The findings of this study suggest that, even though similar reductions of plasma free radicals were observed after ingestion of polyphenol and a vitamin mixture, those supplements can have differential effects on endurance capacity. However, our study was of an exploratory nature for the generation of hypotheses due to an insufficient power to detect statistically significant difference between supplements and the limitations of the analyses based on secondary outcomes. Further studies are therefore recommended to confirm the clinical efficacy of OLFE on endurance exercise and to verify more explicitly how OLFE affects redox signaling. It would also be useful and interesting to conduct a similar study that involved individuals engaged in an endurance training program or trained endurance athletes. Although we recruited people who exercised regularly at a moderate level, the effect of antioxidant supplements may differ depending upon the oxidative burden generated by the quantity of exercise and on the interaction with the induction of antioxidant enzymes as a result of training.
Acknowledgments
We are grateful to Dr. Jose Vina, Dr. Mari-Carmen Gomez-Cabrera, Professor Sehyun Kim, and Professor Yoo Kyoung Park for their helpful advice on the study.
An independent research fund from Amino Up Chemical Co., Ltd. was granted and the company has not played any role in the design, conduct, analysis and interpretation of the research, apart from supplying the supplementations according to the study protocol.
We thank Dr. Kentaro Kitadate and Dr. Hajime Fujii for providing consultation on the potency of supplementations.
The authors’ responsibilities were as follows: Seung Wan Kang and Seokyung Hahn contributed to the planning, design, coordination of the study, the conducting of statistical analysis, interpretation of the results, and the writing of the manuscript; Jung Kyu Kim and Seung Min Yang contributed to the study design and collection of data; Byung-Joo Park contributed to the protocol development and comments on the final draft; Sang Chul Lee contributed to the planning of the study and the preparation of the manuscript.
Abbreviations
- ANOVA
analysis of variance
- BMI
body mass index
- CI
confidence interval
- DRI
daily recommended intake
- FORT
free oxygen radicals test
- HRmax
maximal heart rate
- LDH
lactate dehydrogenase
- OLFE
oligomerized lychee fruit Extract
- ROS
reactive oxygen species
- RPE
rating of perceived exertion
- VCO2
rate of carbon dioxide production
- VO2
rate of oxygen consumption
Clinical trial registration: registered at www.clinicaltrial.gov (NCT00731926).
References
- 1.Nieß AM, Striegel H, Hipp A, Hansel J, Simon P. Zusätzliche antioxidanziengabe im sport – Sinnvoll oder unsinnig? 2008;59:55–61. [Google Scholar]
- 2.Packer L, Cadenas E, Davies KJ. Free radicals and exercise: an introduction. Free Radic Biol Med. 2008;44:123–125. doi: 10.1016/j.freeradbiomed.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 3.Ashton T, Rowlands CC, Jones E, et al. Electron spin resonance spectroscopic detection of oxygen-centred radicals in human serum following exhaustive exercise. Eur J Appl Physiol Occup Physiol. 1998;77:498–502. doi: 10.1007/s004210050366. [DOI] [PubMed] [Google Scholar]
- 4.Alessio HM. Exercise-induced oxidative stress. Med Sci Sports Exerc. 1993;25:218–224. [PubMed] [Google Scholar]
- 5.Mastaloudis A, Leonard SW, Traber MG. Oxidative stress in athletes during extreme endurance exercise. Free Radic Biol Med. 2001;31:911–922. doi: 10.1016/s0891-5849(01)00667-0. [DOI] [PubMed] [Google Scholar]
- 6.Roussos CS, Macklem PT. Diaphragmatic fatigue in man. J Appl Physiol. 1977;43:189–197. doi: 10.1152/jappl.1977.43.2.189. [DOI] [PubMed] [Google Scholar]
- 7.Shindoh C, DiMarco A, Thomas A, Manubay P, Supinski G. Effect of N-acetylcysteine on diaphragm fatigue. J Appl Physiol. 1990;68:2107–2113. doi: 10.1152/jappl.1990.68.5.2107. [DOI] [PubMed] [Google Scholar]
- 8.Clarkson PM, Thompson HS. Antioxidants: what role do they play in physical activity and health? Am J Clin Nutr. 2000;72:637S–646S. doi: 10.1093/ajcn/72.2.637S. [DOI] [PubMed] [Google Scholar]
- 9.Urso ML, Clarkson PM. Oxidative stress, exercise, and antioxidant supplementation. Toxicology. 2003;189:41–54. doi: 10.1016/s0300-483x(03)00151-3. [DOI] [PubMed] [Google Scholar]
- 10.Supinski G, Nethery D, Stofan D, DiMarco A. Effect of free radical scavengers on diaphragmatic fatigue. Am J Respir Crit Care Med. 1997;155:622–629. doi: 10.1164/ajrccm.155.2.9032204. [DOI] [PubMed] [Google Scholar]
- 11.Asha Devi S, Prathima S, Subramanyam MV. Dietary vitamin E and physical exercise: I. Altered endurance capacity and plasma lipid profile in ageing rats. Exp Gerontol. 2003;38:285–290. doi: 10.1016/s0531-5565(02)00206-1. [DOI] [PubMed] [Google Scholar]
- 12.Finaud J, Lac G, Filaire E. Oxidative stress: relationship with exercise and training. Sports Med. 2006;36:327–358. doi: 10.2165/00007256-200636040-00004. [DOI] [PubMed] [Google Scholar]
- 13.Coombes JS, Powers SK, Rowell B, et al. Effects of vitamin E and alpha-lipoic acid on skeletal muscle contractile properties. J Appl Physiol. 2001;90:1424–1430. doi: 10.1152/jappl.2001.90.4.1424. [DOI] [PubMed] [Google Scholar]
- 14.Marshall RJ, Scott KC, Hill RC, et al. Supplemental vitamin C appears to slow racing greyhounds. J Nutr. 2002;132:1616S–1621S. doi: 10.1093/jn/132.6.1616S. [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Cabrera MC, Domenech E, Romagnoli M, et al. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr. 2008;87:142–149. doi: 10.1093/ajcn/87.1.142. [DOI] [PubMed] [Google Scholar]
- 16.Hathcock JN, Azzi A, Blumberg J, et al. Vitamins E and C are safe across a broad range of intakes. Am J Clin Nutr. 2005;81:736–745. doi: 10.1093/ajcn/81.4.736. [DOI] [PubMed] [Google Scholar]
- 17.Williams RJ, Spencer JP, Rice-Evans C. Flavonoids: antioxidants or signalling molecules? Free Radic Biol Med. 2004;36:838–849. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Virgili F, Marino M. Regulation of cellular signals from nutritional molecules: a specific role for phytochemicals, beyond antioxidant activity. Free Radic Biol Med. 2008;45:1205–1216. doi: 10.1016/j.freeradbiomed.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka T, Yoshitake N, Zhao P, Matsuo Y, Kouno I, Nonaka G. Production of oligomeric proanthocyanidins by fragmentation of polymers. Jpn J Food Chem. 2007;14:134–139. [Google Scholar]
- 20.Tomita K, Oishi S, Ohno H, Fujii N. Structure-activity relationship study and NMR analysis of fluorobenzoyl pentapeptide GPR54 agonists. Biopolymers. 2008;90:503–511. doi: 10.1002/bip.20968. [DOI] [PubMed] [Google Scholar]
- 21.Nishioka H, Fujii H, Sun B, Aruoma OI. Comparative efficacy of oligonol, catechin and (–)-epigallocatechin 3-O-gallate in modulating the potassium bromate-induced renal toxicity in rats. Toxicology. 2006;226:181–187. doi: 10.1016/j.tox.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 22.Miura T, Kitadate K, Fujii H. Biotechnology in Functional Foods and Nutraceuticals. Boca Raton, FL: CRC Press; 2010. The Function of the Next GEneration Polyphenol, ”Oligonol”; pp. 91–102. [Google Scholar]
- 23.Dawson B, Henry GJ, Goodman C, et al. Effect of vitamin C and E supplementation on biochemical and ultrastructural indices of muscle damage after a 21 km run. Int J Sports Med. 2002;23:10–15. doi: 10.1055/s-2002-19273. [DOI] [PubMed] [Google Scholar]
- 24.Ohno H, Sakurai T, Hisajima T, et al. The supplementation of Oligonol, the new lychee fruit-derived polyphenol converting into a low-molecular form, has a positive effect on fatigue during regular trackand-field training in young athletes. Adv Exerc Sports Physiol. 2008;13:93–99. [Google Scholar]
- 25.Bruce RA, Kusumi F, Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J. 1973;85:546–562. doi: 10.1016/0002-8703(73)90502-4. [DOI] [PubMed] [Google Scholar]
- 26.Goran M, Fields DA, Hunter GR, Herd SL, Weinsier RL. Total body fat does not influence maximal aerobic capacity. Int J Obes Relat Metab Disord. 2000;24:841–848. doi: 10.1038/sj.ijo.0801241. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi A, Yoneda T, Yoshikawa M, et al. The relation of fat-free mass to maximum exercise performance in patients with chronic obstructive pulmonary disease. Lung. 2000;178:119–127. doi: 10.1007/s004080000014. [DOI] [PubMed] [Google Scholar]
- 28.Osman AF, Mehra MR, Lavie CJ, Nunez E, Milani RV. The incremental prognostic importance of body fat adjusted peak oxygen consumption in chronic heart failure. J Am Coll Cardiol. 2000;36:2126–2131. doi: 10.1016/s0735-1097(00)00985-2. [DOI] [PubMed] [Google Scholar]
- 29.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol. 1986;60:2020–2027. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- 30.Wasserman K, Beaver WL, Whipp BJ. Gas exchange theory and the lactic acidosis (anaerobic) threshold. Circulation. 1990;81:II14–II30. [PubMed] [Google Scholar]
- 31.Brooks GA. Anaerobic threshold: review of the concept and directions for future research. Med Sci Sports Exerc. 1985;17:22–34. [PubMed] [Google Scholar]
- 32.Lotito SB, Frei B. Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: cause, consequence, or epiphenomenon? Free Radic Biol Med. 2006;41:1727–1746. doi: 10.1016/j.freeradbiomed.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 33.Ristow M, Zarse K, Oberbach A, et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci USA. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Powers SK, Duarte J, Kavazis AN, Talbert EE. Reactive oxygen species are signalling molecules for skeletal muscle adaptation. Exp Physiol. 2010;95:1–9. doi: 10.1113/expphysiol.2009.050526. [DOI] [PMC free article] [PubMed] [Google Scholar]