Abstract
Theaflavins, the oxidation products of tea polyphenols are important biologically active components of black tea. 6-hydroxydopamine is a pro-parkinsonian neurotoxin. Theaflavins could inhibit the auto-oxidation of 6-hydroxydopamine in a dose-dependent manner from 0.5 µg/ml to 25 µg/ml. Here we investigated the protective effect of theaflavins on 6-hydroxydopamine induced SH-SY5Y cells against apoptosis (within this concentration range). It was found that pretreating SH-SY5Y cells with 0.5 µg/ml of theaflavins prevented 6-hydroxydopamine-induced loss of cell viability, condensed nuclear morphology, attenuated 6-hydroxydopamine-induced apoptosis, decrease of mitochondrial membrane potential and the increase of intracellular nitric oxide levels. Our results indicated that theaflavins had protective effect against 6-hydroxydopamine induced apoptosis at low concentrations, possibly through inhibition of reactive oxygen species and nitric oxide production.
Keywords: theaflavins (TFs), SH-SY5Y cell, apoptosis, 6-hydroxydopamine (6-OHDA), Parkinson’s disease
Introduction
Parkinson’s disease (PD) is one of the most common chronic neurodegenerative movement disorders. It is an age-related progressive disorder with a prevalence of 1–2% in people over the age of 50.(1) It is second disorders in neurodegenerative disorders only to Alzheimer’s disease.(2) The predominant motor abnormalities seen in patients with PD include bradykinesia, resting tremor, rigidity, and postural instability. The main pathological characteristic of PD is the loss of pigmented dopamine (DA)-containing neurons of the substantia nigra pars compacta (SNc) associated with the presence of intracellular protein aggregates known as Lewy bodies.(3) The prevalence of PD is likely to increase in the future as the number of elderly people increases quickly, but in present, there are no drugs which are efficient without adverse effects, the available therapies do not protect neuron against dopaminergic neurodegeneration. Therefore, it is very important to develop new drugs that slow or halt the rate of progression of PD.(4)
Although the etiologies of neurodegenerative disorders remain to be clarified, the common disease-modifying factors are confirmed including oxidative stress, apoptosis, mitochondrial dysfunction, excitotoxicity, impaired ubiquitine-proteasome system and inflammation.(5) Among the rest, data from human postmortem tissue indicate that reactive oxygen species (ROS) and decrements in mitochondrial complex I activity are important in the pathogenesis of sporadic PD.(6)
Human neuroblastoma (SH-SY5Y) cell is one of the many PD cell models used in research. SH-SY5Y cell line possesses many qualities of substantia nigra neurones and is thus suitable for use as an in vitro model to study the death of dopaminergic neurons.(7) When treated by neurotoxins such as 6-hyfroxydopamine (6-OHDA), SH-SY5Y cells mimic many aspects of the dopaminergic neuronal death observed in PD caused by the mitochondrial complex I neurotoxins.(8) 6-OHDA is a neurotoxin which selectively kills dopaminergic neurones, and is widely used to induce neuronal death in both in vitro and in vivo experimental model of PD.(7) 6-OHDA can interact with and inhibit complex I in isolated brain mitochondria,(9) and induce a ROS-related collapse in mitochondrial membrane potential. It is shown that 6-OHDA can produce oxidative stress in vivo as well as in vitro.(10)
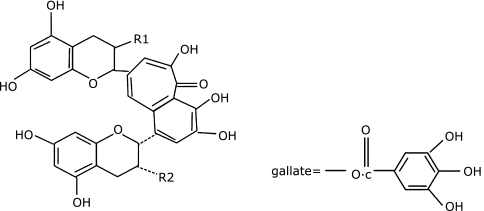
Theaflavins (TFs) are the oxidation products of green tea polyphenols which are regarded as the important biologically active components of black tea that can provide health benefits like anticancer effect.(11) TFs are a mixture that have been found to have 19 different structure related compounds, and there are four major black tea TFs: including theaflavin (TF-1), theaflavin-3-gallate (TF-2A and TF-2B), and theaflavin-3,3-digallate (TF-3) (Fig. 1).
Fig. 1.
The structures of major TFs. TF-1: R1 = R2 = OH; TF-2: R1 = OH, R2 = gallate or R1 = gallate, R2 = OH; TF-3: R1 = R2 = gallate.
We’ve known that green tea have neuroprotective effects in models of degenerative disorders.(2,12,13) Much of the research on black tea and TFs has been focused on their anticancer properties,(14) little is known for the neuroprotective effects of black tea and TFs. In this study, we tested whether TFs might protect SH-SY5Y cells against 6-OHDA-induced apoptosis through the ROS–NO pathway. Cell viability was measured by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetraz-olium bromide] and sulphorhodamine (SRB) assays. It was found that the cell viability was significantly higher in SH-SY5Y cells pretreated with 0.5 µg/ml TFs than in control cells. TFs pretreatments also prevented 6-OHDA induced nuclear morphological changes and the decrease of mitochondrial membrane potential. 6-OHDA induced increase in intracellular NO level was also inhibited by TFs. The result showed that TFs had strong protective effect against 6-OHDA induced apoptosis in SH-SY5Y cells, possibly through inhibition of ROS and NO production.
Materials and Methods
Chemicals
Dulbecco’s modified Eagle’s medium (DMEM), newborn bovine serum (FBS), 4-(2-hydroxyethyl)-piperazine-1-erhane-sulfonic acid (HEPES), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliu-mbromide (MTT) were purchased from GIBCO BRL (NY). Dimethyl sulfoxide (DMSO), 6-Hydroxydop-amine (6-OHDA), DNase I, Hoechst 33258, Propidium iodide (PI), trypsin, penicillin, and streptomycin were purchased from Sigma Chemical Co. (MO); Theaflavins were provided by Zhejiang university. All other chemicals made in China were analytical grade.
Cell Culture
Human neuroblastoma SHSY-5Y cells were maintained in a DMEM supplemented with 10% FBS, penicillin (100 IU/ml), and streptomycin (100 µg/ml) at 37°C in 5% CO2/95% air with 100% relative humidity. Cells were cultured in 6 cm2 dishes and passaged 3–5 days by trypsinization at 80–90% confluence. All experiments were performed in cells plated at a density of 2 × 105 cells/ml on 96-well plates or at a density of 5 × 105 cells/ml in 6-well plates. After 24–36 h, the cells were exposed to 100 µM 6-OHDA alone for 24 h or pretreated with different concentrations of TFs for 3 h. In order to avoid the interaction between TFs and 6-OHDA in the medium, after 3 h of TFs treatment, cells were washed three times to remove TFs and then incubated with 6-OHDA for an additional 24 h.
Measurement of cell viability by MTT assay
MTT assay was performed as described previously in the literature.(15) Cells were incubated with MTT (500 µg/ml) at 37°C for 3 h. The supernatant was removed and the colored formazan was dissolved in DMSO. The absorbance at 590 nm was measured using a Bio-Rad 3350 microplate reader. Control cells were processed in the same way, but without TFs and 6-OHDA treatments. The viability of SH-SY5Y cells was presented as the percentage of control cells.
Measurement of cell viability by SRB assay
Sulphorhodamine (SRB) assay was performed as described previously in the literature(16) with some modification. Cells were fixed with 10% ice-cold TCA for 1 h at 4°C. After fixation, cells were washed for five times with distilled water and allowed to dry in the air. Cells were then incubated with 0.4% (m/v) SRB solution at room temperature for 30 min. The unbound SRB dye was removed by washing the plates quickly with 1% (v/v) acetic acid for five times. The washed plates were dried in the air, and the bound SRB was solubilized by adding 150 µl of 10 mM unbuffered Tris Base (pH 10.5) to each well and shaking for 10 min on a shaker platform. The absorbance at 540 nm was measured using a Bio-Rad 3350 microplate reader. The viability of SH-SY5Y cells in each well was presented as the percentage of control cells.
Morphological changes
The changes in the nuclear morphology of apoptotic cells were investigated by labeling the cells with the nuclear stain Hoechst 33258 and examining them under fluorescent microscopy. Briefly, the SH-SY5Y cells pre-plated in 35 mm2 dishes were treated with 6-OHDA for 24 h. For pre-treatments, TFs were added 3 h before 6-OHDA addition. The cells were fixed with Carnoy’s fixative consisting of methanol and glacial acetic acid (3:1, v/v), and stained with Hoechst 33258 (3 mg/ml), the stained cells were then examined under fluorescence microscopy, and the apoptotic cells were distinguished from control cells by the presence of a fragmented or highly condensed nucleus.
Flow cytometric analysis using PI
In order to quantitatively detect cell apoptosis induced by 6-OHDA, SH-SY5Y cells were added to each 35 mm2 dishes and treated with 6-OHDA for 24 h. For pre-treatments, TFs were added 3 h before 6-OHDA addition. Then each dish cells were centrifuged (1000 rpm, 5 min) to remove the medium, washed two times with PBS and add 70% ethanol and incubated for 12 h at 4°C, then centrifuged again (1000 rpm, 5 min) to remove the medium, washed two times with PBS, add 0.5 ml PBS with RNAase (50 µg/ml) and incubated for 1 h at 37°C. After incubation, stained with PI (Final concentration 50 µg/ml) for 30 min at 4°C, and then to analysis by flow cytometry without exposure to light prior, more than ten thousands events were analyzed per test in list mode. PI emissions were detected in the FL2-A channels of cytometry system (Becton Dickinson Immunocytometry System, San Jose, CA), using emission filters of 575 nm. Calculate the apoptosis percentage of each sample use ModifitL1 analysis software.
Measurement of mitochondrial membrane potential
Mitochondrial membrane potential was measured by the incorporation of a cationic fluorescent dye rhodamine 123 as described previously in the literature.(17) After 24 h incubation in normal medium with or without treatment, the cells were changed to serum-free medium containing 10 µM rhodamine 123 and incubated for 15 min at 37°C. The cells were then collected and the flurescence intensity was analyzed within 15 min by a spectro-photofluorimeter (Hitachi F-4500, 490 nm excitation and 515 nm emission).
Measurement of intracellular NO
The intracellular NO was detected using DAF-2DA as described previously in the literature(18) with some modifications. DAF-2DA, a nitric oxide fluorescent probe, can react with NO within viable cells to produce a fluorescent compound. The cells were collected and re-suspended in BSS (containing 130 µM NaCl, 5.4 mM KCl, 0.8 mM MgCl2, 1.8 mM CaCl2, 15 mM glucose, and 5 mM Hepes, pH 7.4) and then incubated with 2 µM DAF-2DA (in DMSO) for 45 min at 37°C. After washing out the excess probe, the fluorescence intensity was recorded with a Hitachi F-4500 at 485 nm excitation and 515 nm emission.
Determine the composition of TFs sample by HPLC
The analytical HPLC was performed as follows: the separation was completed on a Shim-pack ODS column (150 × 4.6 i.d. mm, Shimadzu, Japan), which was packed with 5 mm C18 silica. The operating temperature was maintained at 35°C. The LC mobile phase consisted of (A) 2% glacial acetic acid and (B) ethyl acetate = 21:3. The gradient was 82% A and 18% B for 28 min, 76% A and 24% B for 4 min, 82% A and 18% B for 3 min. The flow rate was kept at 0.8 ml/min. The injection volume was 10.0 µl.
Statistical analysis
All experiments were done in triplicate or more. One-way ANOVA was used to estimate overall significance followed by post hoc Tukey’s tests corrected for multiple comparisons.(19) Data are presented as means ± SD. A probability level of 1% (p<0.01) was considered to be significant.
Results
TFs prevent 6-OHDA-induced decrease of SH-SY5Y cell viability
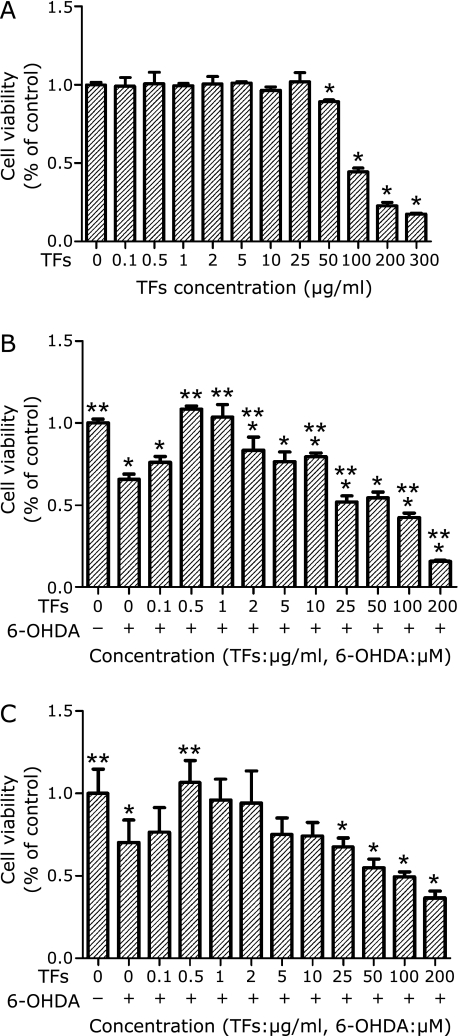
MTT and SRB assay are two major techniques used to assess the cell growth. In this study, we investigated the effect of TFs on 6-OHDA-induced neuronal cell toxicity using both MTT and SRB assays. SH-SY5Y cells were incubated with different concentrations (0.1–300 µg/ml) of TFs and 6-OHDA, and the cell viability was determined. It was found that the survival rate of cells treated with low concentrations (0.1–25 µg/ml) of TFs alone for 24 h didn’t have significant difference when compared with control cells. However, TFs showed a significant toxicity to SH-SY5Y cells at higher concentrations (50–300 µg/ml) (Fig. 2A). The result also showed that 6-OHDA induced cell death in a dose-dependent manner (data not shown), and the survival rate of SH-SY5Y was about 60% when the cells were treated with 100 µM 6-OHDA for 24 h. The effects of the pre-treatment of TFs on the cytotoxicity induced by 6-OHDA were shown to have a dose-dependent manner as shown in Fig. 2 B and C. At 0.1 µg/ml concentration, TFs has no significant effects on 6-OHDA-induced cytotoxicity. At concentrations from 0.5 µg/ml to 25 µg/ml, TFs pre-treatments showed protective effects against cytotoxicity. When cells were pretreated with 0.5–2 µg/ml TFs, the cell viability was significantly higher than that of the control cells (Fig. 2 B and C). However, at high concentrations (50–300 µg/ml), TF pretreatments caused more toxicity than 6-OHDA treatment alone, which is not surprising since TFs themselves induced cell damage at those concentrations (data not shown).
Fig. 2.
Effects of 6-OHDA and TFs on SH-SY5Y cell viability. (A) Cells were incubated in medium containing different concentrations of TFs for 24 h. The cell viability was determined by MTT assay. (B) Cells were pre-incubated with different concentrations of TFs for 3 h, then treated with 100 µM 6-OHDA for an additional 24 h and the cell viability was measured by MTT assay. (C) Cells were pre-incubated with different concentrations of TFs for 3 h, then treated with 100 µM 6-OHDA for an additional 24 h and the cell viability was measured by SRB assay. Data are expressed as percentage of the untreated control means ± SD, n = 7. *p<0.01 significantly different from untreated control cells and **p<0.01 significantly different from 6-OHDA group.
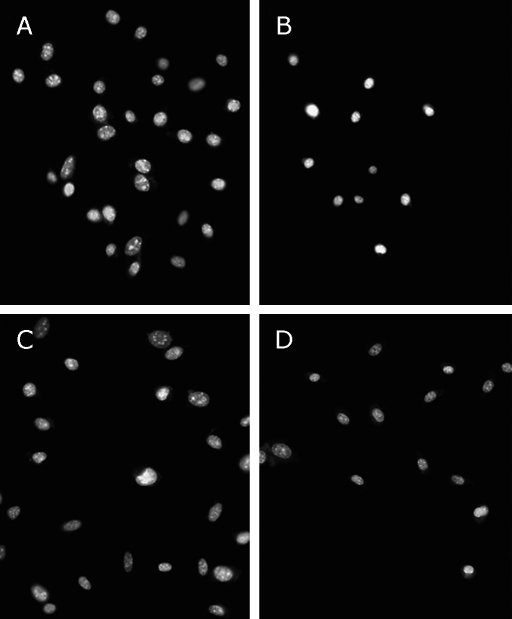
TFs attenuate 6-OHDA-induced changes in nuclear morphology
Normal SH-SY5Y cell nuclei are suborbicular, much bigger and darker than treatment groups cells (Fig. 2A). Nuclei apoptosis indicated by condensed nuclei and nuclear fragmentation were apparent after exposure to 100 µM 6-OHDA for 24 h (Fig. 3B). These changes in nuclear characteristics of apoptosis were attenuated significantly in the cells pretreated with 0.5 µg/ml and 1 µg/ml TFs (Fig. 3 C and D).
Fig. 3.
Effect of TFs on 6-OHDA-induced apoptotic nuclear morphology in SH-SY5Y cells. Fluorescence micrographs of SH-SY5Y cell nuclei from untreated cells (A); cells exposed to 100 µM 6-OHDA for 24 h (B); Cells pre-incubated with 0.5 µg/ml TFs (C) or 1 µg/ml TFs (D) for 3 h, and treated with 100 µM 6-OHDA for 24 h. The cells were stained with the DNA-binding fluorochrome Hoechst 33258. Scale bar = 50 µm.
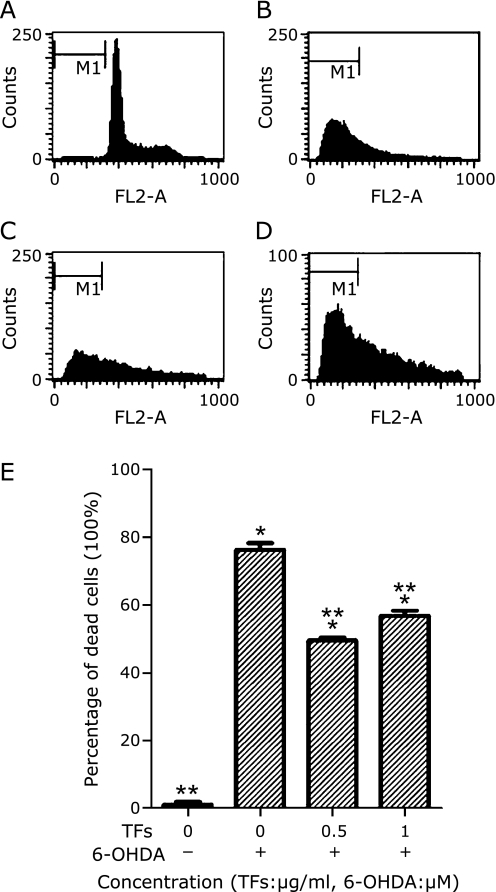
TFs attenuate 6-OHDA-induced apoptosis through flow cytometric analysis
Using PI single-stain system to measure SH-SY5Y cell apoptosis. Typical examples are shown Fig. 4 A–D. After 24 h of incubation with 6-OHDA, results demonstrated that the percentage of apoptosis increased significantly (p<0.01) (Fig. 4B) compared to control cells (Fig. 4A), and TFs showed a positive effect at 0.5 µg/ml and 1 µg/ml concentration (Fig. 4 C–D). The cell apoptosis rate showed at Fig. 4E.
Fig. 4.
Cell apoptosis detected by flow cytometry. SH-SY5Y cells were incubated in drug-free medium (A) or medium containing with 100 µM 6-OHDA for 24 h (B); or cells were pre-incubated with 0.5 µg/ml TFs (C) or 1 µg/ml TFs (D) for 3 h. The results shown in (E) are the cell apoptosis rate of the means and SE for two independent experiments. The cells were stained with PI. Data are expressed as percentage of the dead cells means ± SD, n = 2. *p<0.01 significantly different from untreated control cells and **p<0.01 significantly different from 6-OHDA group.
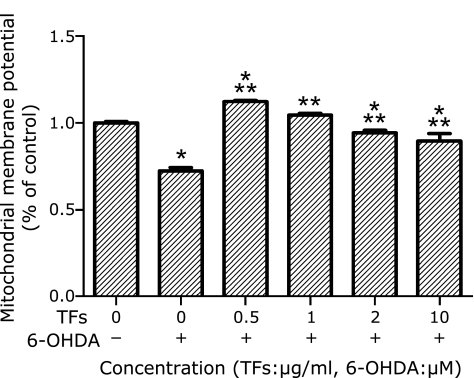
TFs attenuate 6-OHDA-induced decrease in mitochondrial membrane potential
Mitochondria are important organelles in the cell. It has been shown that mitochondrial dysfunction plays an important role in the induction of apoptosis and the mitochondrial membrane potential is an affected by apoptotic factors, the earliest recorded change is the reduction of mitochondrial membrane potential, and then cells enter the irreversible apoptotic process.(20)
After SH-SY5Y cells were exposed to 6-OHDA for 24 h, the fluorescent intensity of rhodamine 123 staining of the cells was decreased, representing a fall in the mitochondrial membrane potential. Since low concentrations of TFs (0.5–25 µg/ml) protected cells against 6-OHDA toxicity, we tested whether pre-treatments of TFs (0.5 µg/ml, 1 µg/ml, 2 µg/ml) before 100 µM 6-OHDA treatment affected mitochondrial membrane potential. The results showed that TFs at this concentrations have significantly inhibited the fall of mitochondrial membrane potential caused by 6-OHDA at 100 µM (Fig. 5).
Fig. 5.
TFs attenuate 6-OHDA-induced decrease of mitochondrial membrane potential. Cells exposed to 6-OHDA were incubated with rhodamine 123, and the fluorescence intensity was measured. MMP of control is defined as 100% and the data are expressed as percentage of the untreated control means ± SD, n = 5. *p<0.01 significantly different from untreated control cells and **p<0.01 significantly different from 6-OHDA group.
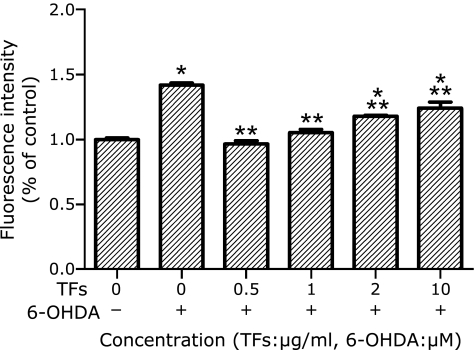
TFs attenuate 6-OHDA-induced increase of intracellular NO in SH-SY5Y cells
High concentration of NO shows cytotoxic effects, causing nerve damage. Excessive NO is produced from the reaction of peroxynitrite (ONOO−) with O2, and causes damage to cell NDA, enhancing the expression of P53, and leading to cell apoptosis.(21)
SH-SY5Y cells treated with 6-OHDA increased intracellular NO level by 45%. TFs pretreatments inhibited the increase of intracellular NO levels. The increase in NO levels was reduced in a concentration-dependent manner by pretreatment of TFs in 0.5–2 µg/ml range (Fig. 6).
Fig. 6.
TFs attenuated 6-OHDA-induced increase of intracellular NO in SH-SY5Y cells. Cells were incubated with DAF-2DA, and the fluorescence intensity was recorded. Data are expressed as percentage of the untreated control means ± SD, n = 3, *p<0.01 significantly different from untreated control cells and **p<0.01 significantly different from 6-OHDA group.
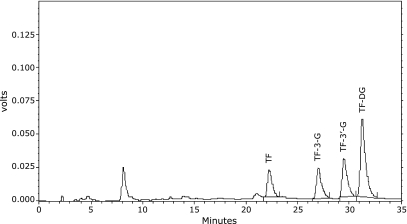
The composition of TFs sample
The result (Fig. 7) showed that the purity of our TFs is 81.89%, containing TF, TF-3-G, TF-3'-G and TFDG (the peak of retention time at 8 min in Fig. 7 is EGCG, one monomer of green tea polyphenols). Like EGCG that is considered as the main active component of green tea polyphenols, there may be a monomer in TFs which is the main active component against neuronal cell death. We are in process to separate the monomers from TFs, and may find out which monomer is the most efficient for PD prevention in the future.
Fig. 7.
The constituents of TFs detected by HPLC.
Discussion
The direct cause of neuro-degeneration in PD is the selective loss of dopaminergic neurons in the substantia nigra, and apoptosis has also been suggested to be involved in this process.(22) 6-OHDA is a mitochondrial complex I inhibitor which can reproduce PD-like cell damage both in vivo and in vitro.(23) 6-OHDA can induce apoptosis in many kinds of cells that secrete dopamine and possess dopamine transporters. SH-SY5H and PC12 are both frequently used as cell models for PD. Previous literature showed that pre-treatment of black tea extracts (BT) at the concentrations of 0.6–6 µM had protective effects against neurotoxin-induced NB SH-SY5Y and PC12 cell death by MTT test,(24) whereas the full composition of the BT was not known (20% similar polyphenols concentrations as green tea extracts). Meanwhile, researches about neuroprotective effects of TFs are not as much as catechins. According to this, our research focused on the research of neuroprotective effects of TFs, and the results showed that low concentrations of TFs prevented 6-OHDA-induced loss of SH-SY5Y cell viability, rescued 6-OHDA-induced nuclear morphological changes and the decrease of mitochondrial membrane potential, and attenuated 6-OHDA-induced apoptosis through flow cytometric analysis. In addition, the increase of intracellular NO levels induced by 6-OHDA was also inhibited by TFs. These results indicated that TFs had strong protective effect against 6-OHDA-induced apoptosis in SH-SY5Y cells, possibly through inhibition of NO production. This suggests that TFs may have the effect for PD prevention.
In this study, we used two ways to testify the cell viability changes, MTT and SRB essay. MTT assay is much simpler than SRB and easy to manipulate so may cause less subjective errors. But the SRB assay is sensitive, reproducible and more rapid than the formazan-based assays and gives better linearity, a good signal-to-noise ratio and has a stable end-point that does not require a time-sensitive measurement, as do the MTT assay.
In previous researches, TFs have been mostly used for their anticancer properties, little is known for their cell protection effect. We noticed that the concentrations of TFs used in the anticancer researches were much higher than the concentration used in our study. So TFs may have different effects when used at different concentrations. They may protect neuronal cells at low concentrations and cause apoptosis of cancer cells at high concentrations.
In our study, the HPLC result (Fig. 7) showed that our TFs mainly containing TF, TF-3-G, TF-3'-G and TFDG. Like EGCG that is considered as the main active component of green tea polyphenols, there may be a monomer in TFs which is the main active component against neuronal cell death. We are in process to separate the monomers from TFs, and may find out which monomer is the most efficient for PD prevention in the future.
Acknowledgments
This research was partly supported by a grant from the National Natural Science Foundation of China (30870587) and (30930036).
Abbreviations
- COX
Cyclooxygenase
- DA
Dopamine
- DAF-2DA
4,5-diaminofluorescein diacetate
- DMSO
Dimethyl sulfoxide
- DMPO
5,5-Dimethyl Pyrroline-N-Oxide
- DMEM
Dulbecco’s modified Eagle’s medium
- HEPES
4-(2-hydroxyethyl)-piperazine-1-erhane-sulfonic acid
- HPLC
High performance liquid chromatography
- 6-OHDA
6-hyfroxydopamine
- PD
Parkinson’s disease
- SH-SY5Y
Human neuroblastoma cell
- SRB
Sulphorhodamine
- TFs
Theaflavins
References
- 1.Xie HR, Hu LS, Li GY. SH-SY5Y human neuroblastoma cell line: in vitro cell model of dopaminergic neurons in Parkinson’s disease. Chin Med J. 2010;123:1086–1092. [PubMed] [Google Scholar]
- 2.Zeng G, Tang T, Wu HJ, et al. Salvianolic acid B protects SH-SY5Y neuroblastoma cells from 1-methyl-4-phenylpyridinium-induced apoptosis. Biol Pharm Bull. 2010;33:1337–1342. doi: 10.1248/bpb.33.1337. [DOI] [PubMed] [Google Scholar]
- 3.Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 4.Wang T, Liu YY, Wang X, Yang N, Zhu HB, Zuo PP. Protective effects of octacosanol on 6-hydroxydopamine-induced Parkinsonism in rats via regulation of ProNGF and NGF signaling. Acta Pharmacol Sin. 2010;31:765–774. doi: 10.1038/aps.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naoi M, Maruyama W. Monoamine oxidase inhibitors as neuroprotective agents in age-dependent neurodegenerative disorders. Curr Pharm Des. 2010;16:2799–2817. doi: 10.2174/138161210793176527. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Dawson VL, Dawson TM. Oxidative stress and genetics in the pathogenesis of Parkinson’s disease. Neurobiol Dis. 2000;7:240–250. doi: 10.1006/nbdi.2000.0319. [DOI] [PubMed] [Google Scholar]
- 7.Tiong CX, Lu M, Bian JS. Protective effect of hydrogen sulphide against 6-OHDA-induced cell injury in SH-SY5Y cells involves PKC/PI3K/Akt pathway. Br J Pharmacol. 2010;161:467–480. doi: 10.1111/j.1476-5381.2010.00887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beal MF. Experimental models of Parkinson’s diasese. Nat Rev Neurosci. 2001;2:325–334. doi: 10.1038/35072550. [DOI] [PubMed] [Google Scholar]
- 9.Lotharius J, Dugan LL, O’Malley KL. Distinct mechanisms underlie neurotoxin-mediated cell death in cultured dopaminergic neurons. J Neurosci. 1999;19:1284–1293. doi: 10.1523/JNEUROSCI.19-04-01284.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi WS, Yoon SY, Oh TH, Choi EJ, O’Malley KL, Oh YJ. Two distinct mechanisms are involved in 6-hydroxydopamine- and MPP+-induced dopaminergic neuronal cell death: role of caspases, ROS, and JNK. J Neurosci Res. 1999;57:86–94. doi: 10.1002/(SICI)1097-4547(19990701)57:1<86::AID-JNR9>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 11.Babich H, Gottesman RT, Liebling EJ, Schuck AG. Theaflacin-3-gallate and Theaflavin-3'-gallate, polyphenols in black tea with prooxidant properties. Basic Clin Pharmacol Toxicol. 2008;103:66–74. doi: 10.1111/j.1742-7843.2008.00232.x. [DOI] [PubMed] [Google Scholar]
- 12.Lung HL, Ip WK, Wong CK, Mak NK, Chen ZY, Leung KN. Anti-proliferative and differentiation-inducing activities of the green tea catechin epigallocatechin-3-gallate (EGCG) on the human eosinophilic leukemia EoL-1 cell line. Life Sci. 2002;72:257–268. doi: 10.1016/s0024-3205(02)02236-1. [DOI] [PubMed] [Google Scholar]
- 13.Guo S, Bezard E, Zhao B. Protective effect of green tea polyphenols on the SH-SY5Y cells against 6-OHDA induced apoptosis through ROS-NO pathway. Free Radic Biol Med. 2005;39:682–695. doi: 10.1016/j.freeradbiomed.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 14.Beltz LA, Bayer DK, Moss AL, Simet IM. Mechanisms of cancer prevention by green and black tea polyphenols. Anticancer Agents Med Chem. 2006;6:389–406. doi: 10.2174/187152006778226468. [DOI] [PubMed] [Google Scholar]
- 15.Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival: modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 16.Houghton P, Fang R, Techatanawat I, Steventon G, Hylands PJ, Lee CC. The sulphorhodamine (SRB) assay and other approaches to testing plant extracts and derived compounds for activities related to reputed anticancer activity. Methods. 2007;42:377–387. doi: 10.1016/j.ymeth.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Zhao B. Green tea polyphenols enhance sodium nitroprusside-induced neurotoxicity in human neuroblastoma SH-SY5Y cells. J Neurochem. 2003;86:1189–1200. doi: 10.1046/j.1471-4159.2003.01928.x. [DOI] [PubMed] [Google Scholar]
- 18.Nakatsubo N, Kojima H, Kikuchi K, et al. Direct evidence of nitric oxide production from bovine aortic endothelial cells using new fluorescence indicators: diaminofluoresceins. FEBS Lett. 1998;427:263–266. doi: 10.1016/s0014-5793(98)00440-2. [DOI] [PubMed] [Google Scholar]
- 19.Stoline MR. The status of multiple comparisons: simultaneous estimation of all pairwise comparisons in one-way ANOVA designs. Am Stat. 1981;35:134–141. [Google Scholar]
- 20.Wadia JS, Chalmers-Redman RM, Ju WJ, et al. Mitochondrial membrane potential and nuclear changes in apoptosis caused by serum and nerve growth factor withdrawal: time course and modification by (–)-deprenyl. J Neurosci. 1998;18:932–947. doi: 10.1523/JNEUROSCI.18-03-00932.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malysheva EV, Kruglov SV, Nazarov VA, Manukhina EB, Malyshev IY. Intracellular and extracellular NO differentially modulates the stress response and apoptosis in macrophages exposed to the influence of biological or physical factors. Bull Exp Biol Med. 2007;143:673–677. doi: 10.1007/s10517-007-0211-y. [DOI] [PubMed] [Google Scholar]
- 22.Anglade P, Vyas S, Javoy-Agid F, et al. Apoptosis and autophagy in nigral neurons of patients with Parkinson’s disease. Histol Histopathol. 1997;12:25–31. [PubMed] [Google Scholar]
- 23.Blum D, Torch S, Lambeng N, et al. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson’s disease. Prog Neurobiol. 2001;65:135–172. doi: 10.1016/s0301-0082(01)00003-x. [DOI] [PubMed] [Google Scholar]
- 24.Levites Y, Youdim MB, Maor G, Mandel S. Attenuation of 6-hydroxydopamine (6-OHDA)-induced nuclear factor-KappaB (NF-κB) activation and cell death by tea extracts in neuronal cultures. Biochem Pharmacol. 2002;63:21–29. doi: 10.1016/s0006-2952(01)00813-9. [DOI] [PubMed] [Google Scholar]