Abstract
Mulberry is commonly used as silkworm diet and an alternative medicine in Japan and China, has recently reported to contain many antioxidative flavanoid compounds and having the free radical scavenging effects. Antioxidants reduce cardiac oxidative stress and attenuate cardiac dysfunction in animals with pacing-induced congestive heart failure. Hence we investigated the cardioprotective effect of mulberry leaf powder in rats with experimental autoimmune myocarditis. Eight-week-old Lewis rats immunized with cardiac myosin were fed with either normal chow or a diet containing 5% mulberry leaf powder and were examined on day 21. ML significantly decreased oxidative stress, myocyte apoptosis, cellular infiltration, cardiac fibrosis, mast cell density, myocardial levels of sarco/endo-plasmic reticulum Ca2+ ATPase2, p22phox, receptor for advanced glycation end products, phospho-p38 mitogen activated protein kinase, phospho-c-Jun NH2-terminal protein kinase, glucose regulated protein78, caspase12 and osteopontin levels in EAM rats. These results may suggest that mulberry diet can preserve the cardiac function in experimental autoimmune myocarditis by modulating oxidative stress induced MAPK activation and further afford protection against endoplasmic reticulum stress mediated apoptosis.
Keywords: Apoptosis, experimental autoimmune myocarditis, mulberry leaves, oxidative stress, endoplasmic reticulum stress
Introduction
In patients with heart failure (HF), increase in plasma biochemical markers of oxidative stress has been reported. There is a definitive correlation between oxidative stress and ventricular dysfunction. Furthermore, ventricular remodeling and progressive dilation leading to end-stage HF may be mediated by oxygen-derived free radicals. Therefore, it is likely that reactive oxygen species (ROS) are involved in not only the pathogenesis but also the active progression of HF.(1) Acute myocarditis is a potentially lethal disease and frequently precedes the development of acute and chronic HF. Some patients with myocarditis show a fulminant course and die of intractable cardiogenic shock, and the treatment strategy for the disease is still unresolved.(2) A model of rat experimental autoimmune myocarditis (EAM) resembles human giant cell myocarditis, and the recurrent form of EAM leads to dilated cardiomayopathy.(3) Recent evidence suggests that oxidative stress and myocardial remodeling including myocardial apoptosis play important roles in the progression of EAM.(4,5) Oxidative stress is known to induce apoptosis in a variety of cell types by activating intracellular cell death signaling cascades including the well-characterized mitogen activated protein kinase (MAPK) pathways in the heart.(6,7) There is growing evidence that ROS may cause cell death via mediation of MAPK under various oxidative conditions. Oxidative stress in the cardiomyocyte can further produce endoplasmic reticulum (ER) stress. Both oxidative stress involving MAPK pathway and ER stress lead to myocardial apoptosis and myocardial damage.
Mulberry (Morus alba L., family Moraceae) leaves, which are commonly used a silkworm diet, contain various nutritional components. Among them, flavonoids and moracins are known to have effects as antioxidants or free radical scavengers.(8) It contains many phenolic antioxidants that can reduce cardiovascular disease.(9) Dietary mulberry has also been reported to have hypoglycemic, hypolipidemic, and antioxidant effects.(10) Mulberry showed relatively high antioxidant activity on comparison with 52 kinds of edible plant products in Japan by LDL oxidation assay.(11) Because of these interesting results we have decided to examine the effects of dietary administration of mulberry leaf powder (ML) on EAM focusing on the antioxidative effects followed by modulating influence on ER stress in cardiac dysfunction.
Materials and Methods
Animals
Eight-week old male Lewis rats were purchased from Charles River Japan Inc., Kanagawa, Japan and were maintained in our animal facilities. Throughout the studies, all the animals were treated in accordance with the guidelines for animal experiments as laid out by our institute.
Induction of EAM
Cardiac myosin was prepared from the ventricular muscle of porcine hearts as previously described.(12) It was dissolved in phosphate buffered saline (PBS) at 5 mg/ml and emulsified with and equal volume of complete Freund’s adjuvant supplemented with 11 mg/ml Mycobacterium tuberculosis H37 RA (Difco, Detriot, Michigan). On day 0, the rats received a single immunization at two subcutaneous sites (both footpads) with a total of 0.2 ml of emulsion for each rat.
Test dose
The immunized rats were randomly divided into two groups, control and 5% ML, and were started on the test diets. The control group was fed a normal powder chow diet, whereas the 5% ML received a normal powder diet supplemented with 5% w/w of ML (Taimatsu Co., Gosen, Japan) on 0 day of immunization. Age-matched Lewis rats without immunization used as normal group and fed with normal powder diet.
Hemodynamic and echocardiography
On day 21, cardiac function of each rat was measured by hemodynamic study and cardiac pressure changes were recorded as described previously.(12) Similarly heart dimensions were studied using echocardiography followed by the calculation of ejection fraction (EF) and fractional shortening (FS).(12)
Histopathological analysis
After the analysis of myocardial function, rats were sacrificed and the hearts were excised and weighed. The heart weight (HW) to body weight (BW) ratios was calculated. The excised hearts were cut into about 2-mm transverse slices and fixed in 10% formalin. After being embedded in paraffin, several transverse sections were prepared from the ventricle, and stained with hematoxylin-eosin (H-E) and Azan-Mallory. Infiltration of inflammatory cells was examined in the H-E stained slides viewed under a high-power light microscope.(13,14) The area of myocardial fibrosis was measured quantitatively using a color image analyzer (CIA-102; Olympus, Tokyo, Japan), making use of the differences in the Azan-Mallory stained color (blue fibrotic area opposed to red myocardium). The results were presented as the ratio of the fibrotic area to the whole area of myocardium.
Mast cell staining and quantification
Histochemical staining with toluidine blue was performed to identify mast cells.(15) For toluidine blue staining, slides of paraffinized sections of the mid ventricle were dewaxed, dehydrated and incubated with 0.05% w/v toluidine blue for 30 min followed by counterstaining with 0.01% w/v eosin for 1 min. Metachromatic staining of mast cell granules was handy to identify these cells. Mast cell density was quantified by counting the number of toluidine blue-positive mast cells per field (100X). At least 15 fields were included from each slide for counting.
Western blotting analyses
Protein lysate was prepared from heart tissue as described previously.(16) The total protein concentration in samples was measured by the bicinchoninic acid method. For Western blotting experiments, 30 µg of total protein was loaded in each lane and proteins were separated by SDS-PAGE (200 V for 40 min) and electrophoretically transferred to nitrocellulose filters (semi-dry transfer at 10 V for 30 min). Filters were blocked with 5% non-fat dry milk in Tris buffered saline (20 mM Tris, pH 7.6, 137 mM NaCl) with 0.1% Tween 20, washed, and then incubated with primary antibody. Primary antibodies employed include: glyceraldehyde-3-phosphate dehydrogenase (GAPDH), p22phox, receptor for advanced glycation end products (RAGE), sarcoendoplasmic reticulum Ca2+ ATPase2 (SERCA2), phospho-p38 MAPK, c-Jun NH2-terminal protein kinase (JNK), phospho-JNK, Glucose regulated protein 78 (GRP78), caspase12 and osteopontin (OPN). All the antibodies were purchased either from Cell Signaling Technology Inc., MA, USA or Santa Cruz Biotechnology Inc., CA. After incubation with the primary antibody, the bound antibody was visualized with horseradish peroxidase-coupled secondary antibodies (Santa Cruz Biotechnology) and chemiluminescence developing agents (Amersham Biosciences, Buckinghamshire, UK). For western blotting analysis, all primary antibodies were used at a dilution of 1:1000 and secondary antibodies were used at a dilution of 1:5000. Films were scanned and band densities were quantified by densitometric analysis using Scion image software (Epson GT-X700; Tokyo, Japan).
Detection of apoptosis
Frozen left ventricular (LV) tissues embedded in OCT compound were cut into 4 µM thick sections and fixed in 4% paraformaldehyde (pH 7.4) at room temperature. Terminal transferase-mediated DNA nick end labeling (TUNEL) assay was performed as specified in the in situ apoptosis detection kit (Takara Bio Inc. Shiga, Japan). Sections were mounted and examined using light microscopy. For each animal, five sections were scored for apoptotic nuclei. Only nuclei that were clearly located in cardiac myocytes were considered.
Statistical analysis
All values are expressed as mean ± SEM. Statistical analysis of differences between the groups was performed by one-way ANOVA, followed by Tukey’s multiple comparison method. A value of p<0.05 was considered statistically significant.
Results
ML treatment improved cardiac structure and function
The present study identified a significant suppression of the cardiac function in the EAM rats after three weeks of cardiac myosin injection. There was a significant impairment of the systolic and diastolic components of cardiac contraction as per the hemodynamic and echocardiographic analyses. The control rats demonstrated LV remodeling with increased LV end diastolic pressure (LVEDP), LV dimension in systole (LVDs), LV dimension in diastole (LVDd) and reduced FS in vehicle-treated EAM rats in comparison to control, indicating the impairment of myocardial function. ML treatment has reversed these changes significantly (Table 1). There was a marked inflammatory cellular infiltration and increased myocardial cell size in the control group as identified by histochemical analysis using hematoxylin-eosin staining whereas the ML treated rats showed normal cardiac architecture without much inflammatory cellular infiltration (Fig. 1A).
Table 1.
Changes in histopathological, hemodynamic and echocardiographic parameters after 3 weeks of treatment with mulberry leaf powder in rats with EAM
| Normal | Control | Mulberry 5% | |
|---|---|---|---|
| Histopathology | |||
| BW (g) | 328.4 ± 1.94 | 228 ± 4.55## | 236 ± 4.76 |
| HW (g) | 0.94 ± 0.01 | 1.27 ± 0.09## | 1.21 ± 0.06 |
| HW/BW (g/kg) | 2.86 ± 0.04 | 5.57 ± 0.34## | 5.13 ± 0.34 |
| Area of fibrosis (%) | 3.4 ± 0.5 | 78.57 ± 4.04## | 44 ± 2.45** |
| Hemodynamic data | |||
| CVP | 0.73 ± 0.37 | 10.58323 ± 0.66## | 6.48 ± 4.90** |
| AP | 113.88 ± 2.08 | 38.96667 ± 0.63## | 66.63 ± 3.46 |
| LVP | 130.5 ± 6.60 | 54.79 ± 1.61## | 91 ± 1.25** |
| LVEDP | 1.98 ± 0.81 | 14.28 ± 0.14## | 10.72 ± 0.57* |
| dP/dt max | 9013 ± 100.80 | 2891.66 ± 22.92## | 4389.5 ± 568.5* |
| dP/dt min | 8422 ± 615.19 | 2167.33 ± 50.22## | 3252 ± 226 |
| Echocardiographic data | |||
| HR (beats/min) | 437.58 ± 6.56 | 237.33 ± 14.43## | 342.5 ± 30.5* |
| LVDd (mm) | 6.2 ± 0.37 | 6.61 ± 0.53 | 7.7 ± 0.53 |
| LVDs (mm) | 3.8 ± 0.32 | 6.1 ± 0.5## | 5 ± 0.32 |
| FS (%) | 42.7 ± 2 | 7.1 ± 0.59## | 14.4 ± 1.87* |
| EF (%) | 79 ± 2.1 | 18.6 ± 1.49## | 34.8 ± 3.76** |
BW, body weight; HW, heart weight; HW/BW, ratio of heart weight to body weight; CVP, central venous pressure; AP, arterial pressure; LVP, left ventricular pressure; LVEDP, left ventricular end-diastolic pressure; dP/dt, rate of intraventricular pressure change; HR, heart rate; LVDd, left ventricular dimension in diastole; LVDs, left ventricular dimension in systole; FS, fractional shortening; EF, ejection fraction; Normal, age-matched untreated rats; Control, rats with EAM treated with vehicle; Mulberry 5%, rats with EAM treated with ML (5%) Results are expressed as mean ± SEM. ##p<0.01 vs normal; *p<0.05 and **p<0.01 vs control.
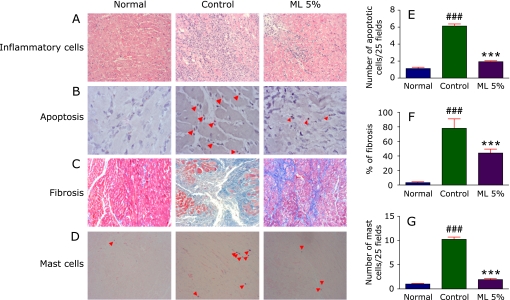
Fig. 1.
A–D, Hematoxylin and eosin staining depicting interstitial edema, vacuolization and degeneration of cardiac fibers, TUNEL staining depicting myocardial apoptosis, Azan-Mallory staining for fibrosis (blue area) and cross-sectional cardiac tissue slices with Toluidine blue staining depicting the mast cells respectively (×200). E–G, Bar graph showing the TUNEL positive apoptotic cells (25 fields), % fibrosis and number of mast cells (25 fields). Each bar represents mean ± SEM. Normal, age-matched normal rats; Control, Immunized rats fed with normal diet; ML 5%, Immunized rats administered with normal diet with 5% Mulberry leaf powder. ***p<0.001 vs normal; ###p<0.001 vs control.
ML afforded protection from oxidative stress
The untreated rats showed a marked elevation in the myocardial expression of p22phox, which is a membrane-bound NADPH oxidase subunit indicating the oxidative stress mediated by cardiac myosin. This elevation was prevented by the ML supplementation in the treated rats confirming the possible protection of the EAM rats from oxidative stress. Similarly the expression of RAGE in the myocardium of the ML treated rats were significantly less when compared with the control rats.
ML treatment modulates MAPK pathway
There was a significant increase in the expression of p38MAPK and p-JNK in the control rats. This result suggests that oxidative stress mediates the stimulation of MAPK pathway. ML supplementation through diet modified this change in the EAM rats. Protein expression of both these markers was attenuated significantly in the ML treated rats.
ML afforded protection from ER stress
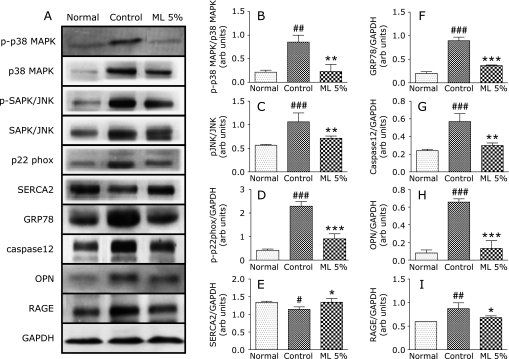
GRP78 is a marker of ER stress whose expression is reported to be increased in the EAM rats. ER stress leads to the activation of caspase 12 and other markers involved in the myocardial changes associated with several autoimmune reactions. In our study with EAM rats also revealed the involvement of ER stress as evidenced by significant increase in the myocardial GRP78 and caspase12 expression. Prevention of ER stress is one of the strategies involved in the reduction of myocardial complications of EAM. In the present study ML treated animals had shown significant attenuation of these ER stress markers when compared with the control group (Fig. 2).
Fig. 2.
Myocardial expressions of phospho-p38 MAPK, p38 MAPK, phospho-SAPK/JNK, SAPK/JNK, p22phox, SERCA2, GRP78, caspase12, OPN, RAGE and GAPDH. A, Representative western blots showing specific bands for phospho-p38 MAPK, p38 MAPK, phospho-SAPK/JNK, SAPK/JNK, p22phox, SERCA2, GRP78, caspase12, OPN, RAGE and GAPDH as an internal control. Equal amounts of protein sample (30 µg) obtained from whole ventricular homogenate were applied in each lane. These bands are representative of five separate experiments. B–H, Densitometric data of protein analysis. B and C, The mean density values of phospho-p38 MAPK, phospho-SAPK/JNK, expressed as ratios relative to their respective unphosphorylated protein mean density values. D–I, The mean density values of p22phox, SERCA2, GRP78, caspase12, OPN and RAGE expressed as ratios relative to that of GAPDH. Each bar represents mean ± SEM. Normal, age-matched normal rats; Control, Immunized rats fed with normal diet; ML 5%, Immunized rats administered with normal diet with 5% Mulberry leaf powder. *p<0.05, **p<0.01, ***p<0.001 vs normal; #p<0.05, ##p<0.01, ###p<0.001 vs control.
ML afforded protection from myocardial apoptosis
Further ER stress mediated apoptosis was confirmed by the TUNEL staining of the myocardial tissue slices where the control animals presented numerous TUNEL positive apoptotic nuclei and ML treated animals showed less apoptosis on comparison with the control group (Fig. 1 B and E).
ML treatment decreased myocardial fibrosis
Several areas of the control group rat myocardial tissue were replaced by the fibrotic tissue as stained by the Azan-Mallory technique which was prevented by the ML supplementation (Fig. 1 C and F). Extensive myocardial fibrosis was also confirmed by the expression of OPN. Control animals showed significantly increased expression of OPN in the myocardial protein lysate when compared to the normal rats. This increased expression was attenuated by the ML supplementation. The stained tissue sections of ML treated rats showed near normal cardiac architecture with least fibrotic tissue replacement.
ML treatment normalized the myocardial calcium handling
Rats with EAM showed impaired calcium handling as evidenced by the significant reduction of the myocardial SERCA2 level, which is essential for the maintenance of calcium movement across the myocardial cells. ML supplemented rats showed significant increase in the expression of SERCA2 in the cardiomyocytes indicating the protection offered by ML against the cardiac myosin induced defect in myocardial calcium handling.
ML treatment decreased the mast cell count
The number of mast cells present in the myocardial tissue slices of all the groups of rats was identified using toluidine blue staining. Toluidine blue positive mast cell count was significantly higher in the EAM control rats when compared with the normal whereas ML treated showed possible protection from this increase confirmed by the significant decrease in the mast cell count when compared with the control animals (Fig. 1 D and G).
Discussion
Oxidative stress plays a major role in the pathogenesis of EAM(17) and several antioxidants were screened for their protective role in the treatment of EAM. We have carried out the study with dietary supplementation of ML in cardiac myosin induced EAM rats. The hemodynamic and echocardiographic analyses demonstrated LV remodeling with LVEDP, LVDs, LVDd and reduced FS in vehicle-treated EAM rats in comparison to control, indicating impaired systolic and diastolic function of the myocardium. ML treatment produced significant beneficial effects on hemodynamic properties by reversing the ventricular pressure changes, fibrosis, EF and FS significantly.
Mast cells participate in the acute inflammatory reaction and the onset of ventricular remodeling associated with acute myocarditis and that the inhibition of their function may be therapeutic in this disease.(18) Several flavonoids including quercetin and myricetin were reported to inhibit mast cell activation.(19) Similarly the number of mast cells in the myocardium of the ML treated rats was reduced significantly than the control rats evidenced by histopathological analysis (Fig. 1). In line with several studies with antioxidants against mast cells in EAM, our study also confirmed that ML a rich source of antioxidants has a protective role in EAM.
NADPH oxidases are the major sources of superoxide in vascular cells and myocytes.(20) Several membrane-bound and cytosolic subunits of them are implicated in oxidative stress. Pathogenic inflammatory reaction stimulates these subunits and produces ROS mediated tissue damage. Antioxidants lower the NADPH oxidase activity and reduce the levels of its preexisting subunits. Dietary supplementation of several plant products rich in anthocyanins were reported to suppress the oxidative stress.(21) It was reported that ML, a good source of antioxidants including anthocyanins, can ameliorate oxidative stress by inhibiting NADPH oxidase subunits(22) and thus we have screened the effect of ML treatment on a membrane bound NADPH oxidase subunit, p22phox expression in the myocardium. There was a significant increase in the myocardial p22phox level of control rats indicating the increased level of oxidative stress and ML treatment attenuated this increase significantly (Fig. 2) ensuring that ML can modulate the oxidative stress mediated by NADPH oxidase. Further, the involvement of oxidative stress was confirmed by assessing the myocardial expression of RAGE. Oxidation of cellular proteins and lipids produce advanced glycation end products,(23) which act as ligand for RAGE and lead to tissue damage.(24) RAGE expression was significantly suppressed in the ML treated rats (Fig. 2) indicating the protection offered by its supplementation. These results suggest that ML treatment can protect the animals from cardiac damage induced by oxidative stress following EAM.
Oxidative stress and MAPKs are involved in myocardial remodeling and play a major role in the development of cardiotoxicity.(25) It was reported that mulberry extracts can modulate the MAPK pathway and induce antioxidant defense system.(21) In order to investigate the possible relationship between NADPH oxidase and the activation of redox-sensitive kinases, we measured the phosphorylation of p38 MAPK, and SAPK/JNK. The p38 MAPK is a subfamily of the MAPK superfamily and is stress responsive. Activation of p38 MAPK is associated with accumulation of reactive oxygen species generated under stress conditions.(26) Various antioxidants were reported to inhibit JNK and p38 MAPK activation.(27) The expression of phospho-p38 MAPK and phospho-JNK were significantly increased in the diseased animals whereas ML supplementation has provided protection from the cardiac damage evidenced by decreased levels of both proteins in this pathway (Fig. 2). These results confirm the correlation between oxidative stress and MAPK pathway. ML treatment can protect the animals from cardiac damage induced by oxidative stress following MAPK activation.
ER stress has been attracting considerable attention since it triggers several inflammatory disorders. There are reports suggesting that oxidative stress can lead to ER stress and show increased expression of ER stress markers.(6) In response to ER stress, there is a marked upregulation of ER chaperone like GRP78. Cell apoptosis can proceed through caspase12, which is localized in the ER and activated by ER stress.(28) This stress includes disruption of ER calcium homeostasis and accumulation of excessive proteins in ER.(29) It was reported that quercetin which is the major component of ML suppressed the ER stress caused by calcium dynamics dysregulation.(30) In the present study there was a significant increase in the myocardial levels of GRP78 and caspase12 in the control rats, pointing the ER stress mediated by EAM. In line with the reports of quercetin, ML supplemented rats also showed reduced levels of both GRP78 and caspase12 indicating the prevention of ER stress.
Dysregulated apoptosis has been implicated in many diseases including cancer, autoimmune and degenerative disorders, and is now emerging as a likely suspect in cardiomyocyte death.(31) Identification of TUNEL positive apoptotic cells in the myocardium provides a useful tool for determining the intensity of the myocardial damage.(32) There are several reports indicating the increase in number of apoptotic cells in the myocardium of the EAM rats and our results with histopathological study also confirmed the increase in apoptotic cells present in the control rats. Our study also revealed the antiapoptotic role of ML by decreasing the number of TUNEL positive cells. From these data we can establish that ML can prevent apoptosis in EAM rats and protect them from myocardial damage.
Calcium dynamics dysregulation was identified in the control rats evidenced by the decreased myocardial levels of SERCA2. The sequence of events following oxidative stress leads to an inotropic increase in intracellular calcium concentrations.(33) The coordinating role of calcium in cardiac hypertrophic response has been reported.(26) SERCA2 is chiefly responsible for the regulation of intracellular calcium ion concentration in cardiac myocytes during the excitation-contraction cycle.(33) Dysregulation of the intracellular calcium content and the myocardial contractility should lead to the development of myocarditis.(34) Conversely OPN is abundantly expressed by infiltrating macrophages during wound healing and has been implicated in cardiac tissue repair.(35) High expression levels of OPN in acute myocarditis are associated with the development of extensive fibrosis.(36) It was reported that quercetin, an antioxidant flavonoid compound found in ML, normalized the calcium movement in transgenic mouse model.(37) In our study, the myocardial level of SERCA2 was significantly decreased and OPN level was increased in the control rats which was found to be normalized in the ML treated group (Fig. 2). Thus ML treated rats can handle calcium movement in the cardiac cells properly to maintain normal cardiac function.
Conclusion
In EAM, the pathologic involvement of oxidative stress, ER stress, improper calcium handling and apoptosis were confirmed by our study. An interesting view is that all of these changes are inter-related as both oxidative stress and reduced SERCA2 expression lead to ER stress and subsequently to apoptosis and cardiovascular damage. It was reported that agents with effect on subcellular remodeling, oxidative stress, apoptosis and defect in calcium handling are important in preventing the alterations in the failing heart.(38) ML treatment showed possible protection from the oxidative stress in the EAM rats, which may be mainly due to its antioxidant property and further avoided the ER stress and myocardial apoptosis. Along with this, EAM rats treated with ML can maintain the normal cardiac function by preserving the normal calcium ion movement in the cardiomyocytes. The protection afforded by the ML treatment is by blockade of ER stress either via modulation of oxidative stress and/or SERCA2 expression in the hearts of EAM rats.
Our study with ML provided the evidences for the protective effect of ML on EAM rats with 21 days treatment as confirmed by the maintenance of normal cardiac anatomy and physiology. Mulberry leaves are known for their good antioxidant activity and supplementation of ML with the normal diet has reversed the changes induced by cardiac myosin, indicating the role of this antioxidant in treating the cardiac dysfunction in EAM.
Acknowledgments
This research was supported by Yujin Memorial Grant, Ministry of Education, Culture, Sports, Science and Technology, Japan, and by a grant from the promotion and Mutual Aid Corporation for Private Schools, Japan.
References
- 1.Keith M, Geranmayegan A, Sole MJ, et al. Increased oxidative stress in patients with congestive heart failure. J Am Coll Cardiol. 1998;31:1352–1356. doi: 10.1016/s0735-1097(98)00101-6. [DOI] [PubMed] [Google Scholar]
- 2.Fuse K, Kodama M, Okura Y, et al. Predictors of disease course in patients with acute myocarditis. Circulation. 2000;102:2829–2835. doi: 10.1161/01.cir.102.23.2829. [DOI] [PubMed] [Google Scholar]
- 3.Mitsuma W, Ito M, Kodama M, et al. Cardioprotective effects of recombinant human erythropoietin in rats with experimental autoimmune myocarditis. Biochem Biophys Res Commun. 2006;344:987–994. doi: 10.1016/j.bbrc.2006.03.230. [DOI] [PubMed] [Google Scholar]
- 4.Lowenstein CJ. Exogenous thioredoxin reduces inflammation in autoimmune myocarditis. Circulation. 2004;110:1178–1179. doi: 10.1161/01.CIR.0000143048.05940.0D. [DOI] [PubMed] [Google Scholar]
- 5.Alter P, Jobmann M, Meyer E, Pankuweit S, Maisch B. Apoptosis in myocarditis and dilated cardiomyopathy: does enterovirus genome persistence protect from apoptosis? An endomyocardial biopsy study. Cardiovasc Pathol. 2001;10:229–234. doi: 10.1016/s1054-8807(01)00077-1. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Y, Wang Q, Evers BM, Chung DH. Signal transduction pathways involved in oxidative stress-induced intestinal epithelial cell apoptosis. Pediatr Res. 2005;58:1192–1197. doi: 10.1203/01.pdr.0000185133.65966.4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Y, Wang Q, Evers BM, Chung DH. Oxidative stress-induced intestinal epithelial cell apoptosis is mediated by p38 MAPK. Biochem Biophys Res Commun. 2006;350:860–865. doi: 10.1016/j.bbrc.2006.09.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shibata Y, Kume N, Arai H, et al. Mulberry leaf aqueous fractions inhibit TNF-alpha-induced nuclear factor kappaB (NF-kappaB) activation and lectin-like oxidized LDL receptor-1 (LOX-1) expression in vascular endothelial cells. Atherosclerosis. 2007;193:20–27. doi: 10.1016/j.atherosclerosis.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Chan KC, Ho HH, Huang CN, Lin MC, Chen HM, Wang CJ. Mulberry leaf extract inhibits vascular smooth muscle cell migration involving a block of small GTPase and Akt/NF-kappaB signals. J Agric Food Chem. 2009;57:9147–9153. doi: 10.1021/jf902507k. [DOI] [PubMed] [Google Scholar]
- 10.El-Beshbishy HA, Singab AN, Sinkkonen J, Pihlaja K. Hypolipidemic and antioxidant effects of Morus alba L. (Egyptian mulberry) root bark fractions supplementation in cholesterol-fed rats. Life Sci. 2006;78:2724–2733. doi: 10.1016/j.lfs.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Enkhmaa B, Shiwaku K, Katsube T, et al. Mulberry (Morus alba L.) leaves and their major flavonol quercetin 3-(6-malonylglucoside) attenuate atherosclerotic lesion development in LDL receptor-deficient mice. J Nutr. 2005;135:729–734. doi: 10.1093/jn/135.4.729. [DOI] [PubMed] [Google Scholar]
- 12.Kodama M, Matsumoto Y, Fujiwara M, Masani F, Izumi T, Shibata A. A novel experimental model of giant cell myocarditis induced in rats by immunization with cardiac myosin fraction. Clin Immunol Immunopathol. 1990;57:250–262. doi: 10.1016/0090-1229(90)90039-s. [DOI] [PubMed] [Google Scholar]
- 13.Thandavarayan RA, Watanabe K, Ma M, et al. Dominant-negative p38alpha mitogen-activated protein kinase prevents cardiac apoptosis and remodeling after streptozotocin-induced diabetes mellitus. Am J Physiol Heart Circ Physiol. 2009;297:H911–919. doi: 10.1152/ajpheart.00124.2009. [DOI] [PubMed] [Google Scholar]
- 14.Thandavarayan RA, Watanabe K, Ma M, et al. 14-3-3 protein regulates Ask1 signaling and protects against diabetic cardiomyopathy. Biochem Pharmacol. 2008;75:1797–1806. doi: 10.1016/j.bcp.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Palaniyandi SS, Watanabe K, Ma M, Tachikawa H, Kodama M, Aizawa Y. Inhibition of mast cells by interleukin-10 gene transfer contributes to protection against acute myocarditis in rats. Eur J Immunol. 2004;34:3508–3515. doi: 10.1002/eji.200425147. [DOI] [PubMed] [Google Scholar]
- 16.Thandavarayan RA, Watanabe K, Sari FR, et al. Modulation of doxorubicin-induced cardiac dysfunction in dominant-negative p38α mitogen-activated protein kinase mice. Free Radic Biol Med. 2010;49:1422–1431. doi: 10.1016/j.freeradbiomed.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Li YC, Ge LS, Yang PL, et al. Carvedilol treatment ameliorates acute coxsackievirus B3-induced myocarditis associated with oxidative stress reduction. Eur J Pharmacol. 2010;640:112–116. doi: 10.1016/j.ejphar.2010.04.037. [DOI] [PubMed] [Google Scholar]
- 18.Higuchi H, Hara M, Yamamoto K, et al. Mast cells play a critical role in the pathogenesis of viral myocarditis. Circulation. 2008;118:363–372. doi: 10.1161/CIRCULATIONAHA.107.741595. [DOI] [PubMed] [Google Scholar]
- 19.Park HH, Lee S, Son HY, et al. Flavonoids inhibit histamine release and expression of proinflammatory cytokines in mast cells. Arch Pharm Res. 2008;31:1303–1311. doi: 10.1007/s12272-001-2110-5. [DOI] [PubMed] [Google Scholar]
- 20.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H Oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 21.Shih PH, Chan YC, Liao JW, Wang MF, Yen GC. Antioxidant and cognitive promotion effects of anthocyanin-rich mulberry (Morus atropurpurea L.) on senescence-accelerated mice and prevention of Alzheimer’s disease. J Nutr Biochem. 2010;21:598–605. doi: 10.1016/j.jnutbio.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Sugimoto M, Arai H, Tamura Y, et al. Mulberry leaf ameliorates the expression profile of adipocytokines by inhibiting oxidative stress in white adipose tissue in db/db mice. Atherosclerosis. 2009;204:388–394. doi: 10.1016/j.atherosclerosis.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 23.Boldyrev AA. Protection of proteins from oxidative stress: a new illusion or a novel strategy? Ann NY Acad Sci. 2005;1057:193–205. doi: 10.1196/annals.1356.013. [DOI] [PubMed] [Google Scholar]
- 24.Herold K, Moser B, Chen Y, et al. Receptor for advanced glycation end products (RAGE) in a dash to the rescue: inflammatory signals gone awry in the primal response to stress. J Leukoc Biol. 2007;82:204–212. doi: 10.1189/jlb.1206751. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka K, Honda M, Takabatake T. Redox regulation of MAPK pathways and cardiac hypertrophy in adult rat cardiac myocyte. J Am Coll Cardiol. 2001;37:676–685. doi: 10.1016/s0735-1097(00)01123-2. [DOI] [PubMed] [Google Scholar]
- 26.Kang YJ. Cardiac hypertrophy: a risk factor for QT-prolongation and cardiac sudden death. Toxicol Pathol. 2006;34:58–66. doi: 10.1080/01926230500419421. [DOI] [PubMed] [Google Scholar]
- 27.Kyaw M, Yoshizumi M, Tsuchiya K, Izawa Y, Kanematsu Y, Tamaki T. Atheroprotective effects of antioxidants through inhibition of mitogen-activated protein kinases. Acta Pharmacol Sin. 2004;25:977–985. [PubMed] [Google Scholar]
- 28.Sun Y, Liu G, Song T, et al. Upregulation of GRP78 and caspase-12 in diastolic failing heart. Acta Biochimi Pol. 2008;55:511–516. [PubMed] [Google Scholar]
- 29.Nakagawa T, Zhu H, Morishima N, et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-β. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 30.Natsume Y, Ito S, Satsu H, Shimizu M. Protective effect of quercetin on ER stress caused by calcium dynamics dysregulation in intestinal epithelial cells. Toxicology. 2009;258:164–175. doi: 10.1016/j.tox.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 31.Saraste A, Arola A, Vuorinen T, et al. Cardiomyocyte apoptosis in experimental coxsackievirus B3 myocarditis. Cardiovasc Pathol. 2003;12:255–262. doi: 10.1016/s1054-8807(03)00077-2. [DOI] [PubMed] [Google Scholar]
- 32.Gill C, Mestril R, Samali A. Losing heart: the role of apoptosis in heart disease—a novel therapeutic target? FASEB J. 2002;16:135–146. doi: 10.1096/fj.01-0629com. [DOI] [PubMed] [Google Scholar]
- 33.Sharaf AR, Narula J, Nicol PD, Southern JF, Khaw BA. Cardiac sarcoplasmic reticulum calcium ATPase, an autoimmune antigen in experimental cardiomyopathy. Circulation. 1994;89:1217–1228. doi: 10.1161/01.cir.89.3.1217. [DOI] [PubMed] [Google Scholar]
- 34.Ho PD, Zechner DK, He H, Dillmann WH, Glembotski CC, McDonough PM. The Raf-MEK-ERK cascade represents a common pathway for alteration of intracellular calcium by Ras and protein kinase C in cardiac myocytes. J Biol Chem. 1998;273:21730–21735. doi: 10.1074/jbc.273.34.21730. [DOI] [PubMed] [Google Scholar]
- 35.Shin T, Ahn M, Kim H, Kim HM, Matsumoto Y. Increased expression of osteopontin in the heart tissue of Lewis rats with experimental autoimmune myocarditis. J Vet Med Sci. 2006;68:379–382. doi: 10.1292/jvms.68.379. [DOI] [PubMed] [Google Scholar]
- 36.Szalay G, Sauter M, Haberland M, et al. Osteopontin: a fibrosis-related marker molecule in cardiac remodeling of enterovirus myocarditis in the susceptible host. Circ Res. 2009;104:851–859. doi: 10.1161/CIRCRESAHA.109.193805. [DOI] [PubMed] [Google Scholar]
- 37.Soga M, Kamal FA, Watanabe K, et al. Effects of angiotensin II receptor blocker (candesartan) in daunorubicin-induced cardiomyopathic rats. Int J Cardiol. 2006;110:378–385. doi: 10.1016/j.ijcard.2005.08.061. [DOI] [PubMed] [Google Scholar]
- 38.Rehsia NS, Dhalla NS. Mechanisms of the beneficial effects of beta-adrenoceptor antagonists in congestive heart failure. Exp Clin Cardiol. 2010;15:e86–e95. [PMC free article] [PubMed] [Google Scholar]