Abstract
Peroxiredoxins possess thioredoxin or glutathione peroxidase and chaperone-like activities and thereby protect cells from oxidative insults. Recent studies, however, reveal additional functions of peroxiredoxins in gene expression and inflammation-related biological reactions such as tissue repair, parasite infection and tumor progression. Notably, peroxiredoxin 1, the major mammalian peroxiredoxin family protein, directly interacts with transcription factors such as c-Myc and NF-κB in the nucleus. Additionally, peroxiredoxin 1 is secreted from some cells following stimulation with TGF-β and other cytokines and is thus present in plasma and body fluids. Peroxiredoxin 1 is now recognized as one of the pro-inflammatory factors interacting with toll-like receptor 4, which triggers NF-κB activation and other signaling pathways to evoke inflammatory reactions. Some cancer cells release peroxiredoxin 1 to stimulate toll-like receptor 4-mediated signaling for their progression. Interestingly, peroxiredoxins expressed in protozoa and helminth may modulate host immune responses partly through toll-like receptor 4 for their survival and progression in host. Extracellular peroxiredoxin 1 and peroxiredoxin 2 are known to enhance natural killer cell activity and suppress virus-replication in cells. Peroxiredoxin 1-deficient mice show reduced antioxidant activities but also exhibit restrained tissue inflammatory reactions under some patho-physiological conditions. Novel functions of peroxiredoxins in inflammation, cancer and innate immunity are the focus of this review.
Keywords: peroxiredoxin, toll-like receptor 4, NF-κB, inflammation, cancer
Contents
1. Introduction
-
2. Prxs Protect Cells from Stress-induced Death
2.1. Prx1 suppresses ASK1/JNK signaling
2.2. Prx2 suppresses ASK1 activation in neurons
2.3. Prx1 inhibits p66Shc activation
2.4. Prx1 inhibits c-Abl activation
2.5. Upregulation of Prxs expression levels by stress
-
3. Roles of Nuclear Prx1 and Prx2
3.1. Prx1 binds with c-Myc and modulates gene expression
3.2. Nuclear Prx1 enhances NF-κB activity
3.3. Effects of Prx1 and Prx2 on activation of androgen receptor
3.4. Nuclear Prx2 protects cancer cells from DNA-damaging agents
-
4. Biological Functions of Extracellular Prxs
4.1. Presence of Prxs in body fluids
4.2. Prx1 secretion from mammalian cells
4.3. Binding of Prx1 and malarial Prx to TLR4
4.4. Interaction of Prx1 with MIF
4.5. Interaction of Prxs with cyclophilin A
4.6. Effects of Prx1 and Prx2 on virus replication
4.7. NK cell enhancing activity of Prx1 and Prx2
-
5. Parasite Prxs in Infection and Immunity
5.1. Malaria plasmodium Prxs
5.2. Host immune reaction to malaria plasmodium
5.3. Incorporation of erythrocyte Prx2 into plasmodium
5.4. Prxs in helminth infection
-
6. Roles of Prxs in Tissue Inflammation
6.1. Roles of Prxs in lung protection
6.2. Roles of Prx1 in ozone-induced lung inflammation
6.3. Role of Prx1 in sensitivity to cisplatin
-
7. Promotion of Tumor Growth by Prxs
7.1. Prx1 enhances tumor progression
7.2. Prx4 enhances tumor growth and metastasis
7.3. Prx6 promotes tumor metastasis
8. Prevention of Oncogenesis by Prx1
9. Highlights and Future Perspectives
Acknowledgments
Abbreviations
References
1. Introduction
Roughly two decades have passed since the cloning of peroxiredoxins (Prxs).(1–4) Yamamoto et al.(1) selected a cDNA encoding mouse Prx3 initially termed MER5 as a preferentially expressed gene in erythroleukemia cells. Chae et al.(2) cloned a yeast Saccharomyces cerevisiae peroxiredoxin termed thiol-specific antioxidant (TSA), a previously characterized 25-kDa antioxidant enzyme. Ishii et al.(3) cloned Prx1 initially termed MSP23 as one of the major electophile- and oxidative stress-inducible proteins from mouse peritoneal macrophages. In the same year, Prosperi et al.(4) cloned human Prx1, designated PAG gene, as a highly expressed gene in ras-transformed mammary epithelial cell line HBL100. Additional researches in 1990s established the presense of TSA-like antioxidant family proteins among wide variety of organisms, and it was proposed to call the family as peroxiredoxin by Rhee and his group (reviewed in Rhee et al.(5)). Since then, numerous studies have described unique properties of Prx family proteins in various animals and plants. Previous studies established that Prxs or thioredoxin peroxidases are important endogenous antioxidants, protecting cells from oxidative damage by reducing H2O2 and peroxynitrite and scavenging thiyl radicals.(5–7) Upregulation of some prxs in cells and tissues by various stress agents is important biological response to cope with tissue damages.(8) As H2O2 generated in cells serves as an effector molecule following binding of ligands to their membrane receptors, Prx’s peroxidase activity is reversibly and effectively inactivated by protein modification to transduce signals within cells.(9,10) It is also known that some Prxs play a role as a chaperone in the form of oligomers, the formation of which is enhanced under oxidative stress conditions.(5,6,11) Godoy et al.(12) recently have studied a detailed tissue localization of peroxiredoxin and thioredoxin (Trx) family proteins in mouse by immunohistochemistry. A detailed overview on the Prxs distribution will help to fully comprehend their potential role in the various tissues.
The functions of Prxs are not restricted to their antioxidant activities. Recent exciting discoveries include Prx1 (also termed Prdx1 or PrxI) secretion from cancer or virus-infected cells(13) and binding of both malarial parasite Prx(14) and Prx1(15) to the cell surface alarm signal receptor toll-like receptor 4 (TLR4). These reports have opened a door to explore the roles of Prxs in inflammation and innate immunity. They also shed light on previous findings that extracellular Prx1 and Prx2 enhance natural killer cell enhancing activity,(16,17) transforming growth factor-β1 (TGF-β1) enhances Prx1 secretion,(18) Prx1 binds to macrophage migration inhibitory factor (MIF)(19) and Prxs bind cyclophilins.(20,21) These and other recent studies highlight extracellular Prxs as modulators of inflammation in relation to pathogen infection, tissue repair after damage and tumor progression. In most cases extracellular Prxs function independently of their peroxidase activity. The interaction of Prxs with alarm signal receptors TLRs will be the most important aspects for the study of biological functions of extracellular Prxs.
It is noteworthy that Prx1 and Prx2 function in nucleus and to modulate gene expression. Prx1 associates with transcription factors such as NF-κB,(22) c-Myc(23) and androgen receptor (AR),(24,25) affecting their activities to regulate gene expression. In addition to its peroxidase activity, Prx1 in cytoplasm has anti-apoptotic functions through direct or indirect interactions with the key apoptosis regulators, ASK1,(26) p66Shc,(27) and GSTpi/JNK.(28)
Thus, in this review we highlight the recent discoveries of the biological functions of Prxs, focusing particularly on the novel roles of Prx1 and Prx2 in protection against cell death, infection, immunity and cancer.
2. Prxs Protect Cells from Stress-induced Death
Prxs protect cells and tissues from oxidative damage through their peroxidase activities. Furthermore, Prx1 suppresses oxidative stress-induced cell death through direct/indirect interactions with different types of kinases and enzymes that play key roles in regulation of cell death and/or apoptosis depending on cell type and stimuli (Fig. 1). Prx2 also suppresses apoptosis signal-regulating kinase 1 (ASK1) activation by reactive oxygen species (ROS) through its peroxidase activity maintaining Trx in a reduced state in neurons (Fig. 1).(29) Both electrophile- and oxidative-stress activate expression of Prx genes.
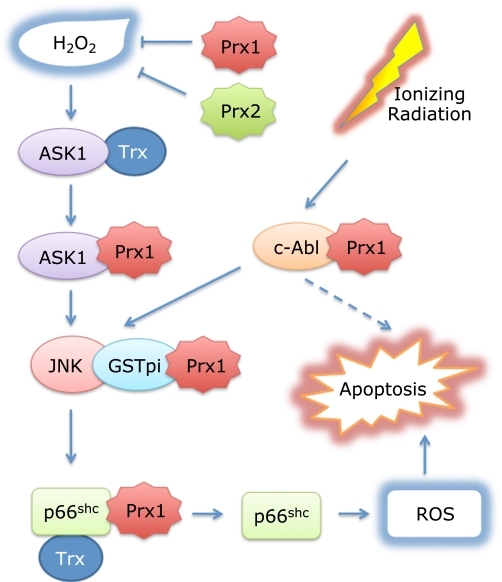
Fig. 1.
Multiple roles of Prx1 in suppression of oxidative stress-induced apoptosis. In addition to the Trx peroxidase activity to eliminate H2O2, Prx1 cooperates with Trx in suppression of H2O2-induced activation of the two apoptosis signal regulators, ASK1(26) and p66Shc,(27) through direct interactions. The activation of ASK1 by oxidant stress is inhibited by reduced form of Trx under low stress conditions. When human embryonic kidney 293 cells were treated with 5 mM H2O2 for 20 min, oxidized Trx was replaced by oxidized Prx1 to further suppress ASK1 activation.(26) Under low stress conditions, Trx and Prx1 maintain p66Shc inactive, by keeping it reduced and dimeric form. Under high stress conditions (1.47 mM H2O2), Prx1 is oxidized forming a decameric chaperone and separated from p66Shc, which is phosphorylated at Ser-36. JNK phosphorylates p66Shc at Ser-36,(38,39) inducing tetramer formation and translocation of p66Shc to mitochondria to generate ROS.(27,37) On the other hand, ionizing radiation activates tyrosine kinase c-Abl(41) and JNK.(28) We speculate Prx1 may inhibit activation of c-Abl following ioning radiation. Prx1 indirectly binds JNK through GSTpi, which suppress activation of JNK.(28) Prx2 like Prx1 suppresses ASK1 activation in neuronal cells in which Prx2 is highly expressed.(29)
2.1. Prx1 suppresses ASK1-JNK signaling
ASK1 is a member of the mitogen-activated protein kinase family that activates both JNK and p38 MAPK signaling cascades.(30) Under non-stressed conditions, ASK1 is associated with its physiological inhibitor, Trx.(31) Trx in its reduced form binds directly to the N-terminal non-catalytic region of ASK1. The association of Prx1 with ASK1 was observed after the treatment of human embryonic kidney 293 cells with 5 mM H2O2 for 20 min.(31) Prx1 interacts with ASK1 via the thioredoxin-binding domain of ASK1, suggesting that oxidized Prx1 exchanges with reduced Trx as the binding partner after H2O2 treatment. Overexpression of Prx1 significantly inhibits the phosphorylation/activation of ASK1 following H2O2 treatment. In Prx1 knockdown cells, ASK1, p38 and JNK are rapidly activated, leading to apoptosis following exposure of cells to 0.2 mM H2O2.(26) However, it is difficult based on these results to estimate how much Prx1 binding to ASK1 contributes to suppression of its activation following H2O2 treatment, since Prx1 can eliminate H2O2 in cooperation with Trx.
Prx1 also suppresses JNK activation through an indirect interaction with JNK in a peroxidase-independent manner.(28) Glutathione S-transferase pi (GSTpi) is known to bind JNK and to inhibit its activation induced by various stresses.(32) Interestingly, Prx1 physically interacts with GSTpi and inhibits JNK release from the GSTpi-JNK complex following treatment of human cancer 1170i cells with γ-ray radiation.(28) Prx1 overexpression in cancer cells suppresses radiation-induced JNK activation and apoptosis.(28) Since GSTpi catalyzes S-conjugation of glutathione by electrophilic compounds, including carcinogen and anticancer drugs, tumors overexpressing GSTpi seem to be drug-resistance in chemotherapy in two ways. In case of Schizosaccharomyces pombe, another role of Prx was shown in the activation of p38/JNK homolog (Sty1) by peroxide treatment, where the formation of a disulfide bond between Prx and Sty1 activated the kinase.(33)
2.2. Prx2 suppresses ASK1 activation in neurons
Among prxs, Prx2 is the most abundant in mammalian neurons and plays a protective role under oxidative stress.(29) Since Prx2 expression levels are significantly elevated in Parkinson’s disease (PD) patient brains, Hu et al.(29) examined its protective role against dopaminergic toxin 6-hydroxydopamine on neurons. It is widely recognized in PD that dopaminergic neurodegeneration occurres in the substantia nigra pars compacta due to activation of the mitochondrial apoptotic pathways. Inactivation of Prx2 peroxidase activity either by S-nitrosylation through reaction with nitric oxide at two critical cysteine residues(34) or by Cdk5-mediated phosphorylation at Thr-89(35) are observed in human PD brains and in PD models. Lentivirus-mediated Prx2 overexpression conferred marked neuroprotection against 6-hydroxydopamine toxicity in dopaminergic neuron cells and in mouse brain.(29) Prx2 exhibited anti-apoptotic effects in dopaminergic neurons via suppression of ASK1 activation. Prx2 overexpression maintained Trx in a reduced state thereby suppressing ASK1 activation (Fig. 1).
2.3. Prx1 inhibits p66shc activation
An adaptor protein p66Shc is the only known pro-apoptotic member of the Shc protein family.(36) It is a central player in stress-induced apoptosis through induction of the respiratory burst and mitochondrial rupture.(36,37) Prx1 was identified as one of the negative modulators of p66Shc activation. Pull-down assays using resin-bound p66Shc identified Prx1 as a binding partner with a high affinity in mitochondrial extracts, which contained low levels of Prx1.(27) Prx3, the major Prx in the mitochondrial matrix, did not exhibit affinity for p66Shc. Prx1 associated with p66Shc and suppressed H2O2-generation via p66Shc independent of its peroxidase activity. When cells were treated with 5 mM H2O2, p66Shc was phosphorylated at Ser-36 inducing disassembly of the p66Shc-Prx1 complex, resulting in the formation of a Prx1 oligomer. Subsequently, p66Shc translocates to the mitochondrial compartment where it forms a tetramer, leading to ROS generation through its interaction with cytochrome c and induces apoptosis(37) (Fig. 1).
The phosphorylation of p66Shc at Ser-36 is key for its activation. Phosphorylation depends on JNK activation induced by UV irradiation in human neuroblastoma cells(38) or H2O2 treatment (0.1 mM, 1 h) in bovine aortic endothelial cells.(39) In addition to JNK, PKC-β phosphorylates p66Shc in embryonic fibroblasts after treatment with H2O2 (0.5 mM, 10 min).(40) Thus, Prx1 could suppress oxidant stress-induced activation of ASK1-JNK-p66Shc signal pathway via multiple mechanisms (Fig. 1). It is worth noting, however, that Trx inhibits both ASK1 and p66Shc activation under basal conditions and that Prx1 plays auxiliary inhibitory role under oxidative stress.
2.4. Prx1 inhibits c-Abl activation
Prx1 is known as a physiological inhibitor of c-Abl tyrosine kinase activity.(41) The c-abl gene was originally identified as the cellular homolog of the transforming gene of the Abelson murine leukemia virus. This kinase is one of the nonreceptor tyrosine kinases of the Src family and ubiquitously expressed among tissues, with particularly high levels in thymus, spleen, and testes.(42) Prx1 associates with the SH3 domain of c-Abl and inhibits its kinase activity.(41) Although c-Abl has many functions as cell cycle regulation,(42,43) an important role of the kinase is regulation of double-strand breaks repair and promotion of the cell death response following DNA damage.(44) As c-Abl plays a crucial role in the apoptotic response to ionizing radiation and other DNA-damaging agents and is known to function as an upstream effector of the JNK and p38 MAPK pathways,(45) we speculate that Prx1 may play a role in inhibiting this cell death signaling pathway (Fig. 1).
2.5. Upregulation of Prxs levels by stress
Induction of prx1 gene activation is an important adaptive response to cope with elecrtophiles and oxidative stress agents. Transcription factor Nrf2 is the major positive regulator of prx1 gene expression by wide variety of stress agents.(46) In addition to Prx1, Nrf2 regulates upregulation of group of antioxidative enzymes and proteins such as heme oxygenase-1 (HO-1), A170/sequestosome1/p62 and a cystine transporter in mouse peritoneal macrophages.(46) Prx1 cooperates with these gene products to protect cells from insulting agents. Nrf2 plays crucial roles in induction of the group of detoxification enzymes by electrophiles through antioxidant/electrophile responsive element in vivo.(47) Dietary 2(3)-tert-butyl-4-hydroxyanisol, a synthetic phenolitic antioxidant and previously used as a food preservative, enhanced Nrf2-dependent expression of both Prx1 and GST in liver and epithelial cells of proximal small intestine.(48)
Shiota et al.(49) showed transcription factors Ets1 and Ets2 regulate upregulation of both Prx1 and Prx5 by H2O2 and hypoxia in human prostate cancer PC3 cells and epidermoid cancer KB cells. They treated cells either with 1 mM H2O2 for 30 min or hypoxia (oxygen-free) for 4 h and afterward monitored expression of Prx1 and Prx5 in the cells. Ets1 and Ets2 were also upregulated by the stress and function through direct interaction with high-mobility group protein 1 (HMGB1).(49) These transcription factors may partly contribute in maintaining high Prx1 and Prx5 levels in tumors.
Wang et al.(50) found that Prx5 is upregulated in degenerative human tendon. Osteoarthritic cartilage expresses higher Prx5 levels compared to normal cartilage.(51) Their results show inflammatory cytokines TNF-α and IL-1β enhance Prx5 expression in human cartilage tissue, suggesting Prx5 may play a protective role against oxidative stress in human cartilage.(50) It is shown by others that LPS-TLR4 signaling upregulates Prx5 expression in bone marrow-derived macrophages.(52) Supplementing LPS (0.01 to 50 ng/ml) and IFN-γ (0.01 to 20 U/ml) respectively enhanced expression of Prx5 mRNA and protein in the macrophages. The prx5 gene activation depended on TLR4 and adaptor protein TRIF but not on MyD88.(52) Experiments using MAPKs inhibitors revealed that p38 and JNK mainly contribute to Prx5 upregulation in immunostimulated macrophages.
3. Roles of Nuclear Prx1 and Prx2
Prx1 is localized in the nucleus as well as in the cytoplasm and has been shown to interact with transcription factors such as c-Myc, NF-κB and AR to modulate their activities (Fig. 2). The effects of nuclear Prx1 on gene expression via c-Myc are especially important for elucidating the phenotype of Prx1-deficient mice as will be discussed in later sections. Prx2 also localizes in both the nucleus and cytoplasm to protect cancer cells from chemotherapeutic agents.(51)
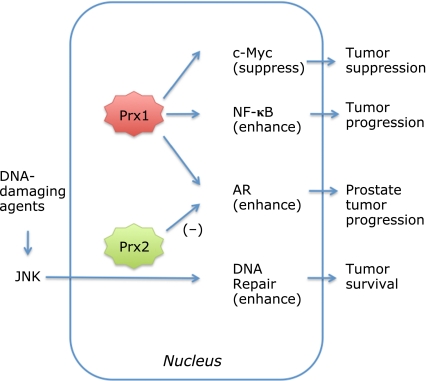
Fig. 2.
Roles of nuclear Prx1 and Prx2. Prx1 is localized in both the cytoplasm and nucleus and interacts directly with the transcription factors, c-Myc,(56) NF-κB(22,59) and AR.(24) Prx1 suppress c-Myc,(23,56) whereas it enhances activities of p50(22) and p65(59) components of NF-κB and helps activation/phosphorylation of AR.(24) Prx2 is also localized in the nucleus and enhances JNK signaling induced by anticancer/DNA-damaging agent resulting in DNA-repair and tumor survival.(53) Cytoplasmic Prx2 increases AR transactivation, whereas nuclear Prx2 suppresses AR transactivation.
3.1. Prx1 binds with c-Myc and modulates gene expression
Myc proteins are bHLH-ZIP transcription factors that bind to their cognate genes in heterodimeric association with Max, another bHLH-ZIP protein.(54,55) The c-Myc oncogene product influences many cellular processes, including growth, cell cycle progression, apoptosis, and differentiation. Prx1 interacts with the c-Myc oncogene product through its highly conserved Myc Box II (MBII) domain, which is critically important for transformation and transcriptional regulation.(56) Their physical interaction was first shown in a yeast two-hybrid screen system and then by immunoprecipitation. The number of both positive and negative target genes of c-Myc is more than 1000, and it was shown that Prx1 apparently suppresses regulation of some c-Myc target genes and thereby suppresses tumor growth.(23,56) Over-expression of Prx1 in 32D myeloid cells down-regulates some but not all expression of the c-Myc MBII domain-dependent genes. In embryonic fibroblasts from Prx1 deficient mice, altered expression of a subset of c-Myc target genes has also been observed.(23) Enhanced expression of c-Myc in Rat1a cells enhanceds cell colony formation in soft agar and tumor formation in explanted mice. However, simultaneous expression of Prx1 and c-Myc in these cells significantly suppresses the action of c-Myc, suggesting a role for Prx1 as a tumor suppressor.(56)
Graves et al.(57) found that Prx5 expression level was roughly 5 times higher in Prx1 deficient embryo fibroblasts. It may due to the fact that the gene expression of Prx5 partly depended on c-Myc, of which activity was suppressed in the presence of Prx1.(23,57)
3.2. Nuclear Prx1 enhances NF-κB activity
Cytoplasmic Prx1 is known to suppress NF-κB activation by eliminating peroxides,(58) but nuclear Prx1 enhances NF-κB activity by at least two ways. In HeLa cells expressing nuclear localizing Prx1 (NLS-Prx1), stimulation with H2O2 (0.1–0.5 mM) enhanced NF-κB activation 1.8–2.8-fold measured using a luciferase reporter assay.(22) It was suggested that increased nuclear Prx1 inhibited oxidation of the redox-sensitive cysteine in the DNA binding domain of p50, the cleaved product of p105 (NF-κB1).(22) Another study reported that nuclear Prx1 enhances p65-mediated cyclooxygenase (COX)-2 gene expression in estrogen receptor (ER) deficient human breast cancer cells (MDA-MB-231).(59) Prx1 expression levels in breast cancer cell lines were higher than in normal or pseudonormal breast cell lines, and interestingly, nuclear Prx1 levels were higher than cytoplasmic levels in these cancer cells. In ER deficient cells, Prx1 was partially phosphorylated at Thr-90, which promoted oligomerization to enhance the chaperone activity. This may due to enhanced Cdk2 activity, which phosphorylates at Thr-90 and inactivates Prx1 peroxidase activity. Under these conditions Prx1 interacted with NF-κB p65 and associated with the COX-2 gene upstream promoter region.(59) In ER-positive MCF7 breast cancer cells, however, Prx1 was neither phosphorylated and nor associated with the promoter region. MCF7 cells may have reduced Cdk2 activity due to higher expression levels of p27 and p21, intrinsic inhibitors of Cdk2, compared to MDA-MB-231 cells.(60) These studies suggest nuclear Prx1 could enhance NF-κB activation in ER deficient tumor cells in which Prx1 phosphorylation is enhanced.
3.3. Effects of Prx1 and Prx2 on activation of androgen receptor
Activation of AR plays a critical role in prostate cancer development. Hypoxia/reoxygenation or synthetic androgen treatment activates the AR in cultured prostate cancer cells, and Prx1 acts as a key mediator as monitored using an AR-dependent luciferase reporter assay.(24) Increased Prx1 expression enhanced the AR action and Prx1 knockdown by short hairpin RNA suppresses AR activity and decreases the growth rate of the androgen-dependent cancer cells.(24) It was shown that hypoxia/reoxygenation treatment induces oxidation of Cys-52 residue in Prx1, which was associated with AR binding to the AR-element of the prostate-specific antigen gene.(24) The antioxidant activity of Prx1 was not necessary for its AR stimulatory function. The association of Prx1 with AR significantly increased the affinity of AR for dihydrotestosterone.(25) These studies suggest that disrupting the interaction between Prx1 and AR may serve as a target in the management of prostate cancer (Fig. 2).
Prx2 also modulates AR transactivation in prostate cancer cells.(61) Compared to human prostate cancer LNCaP cells, the expression levels of Prxs were higher in both castration-resistant derivatives LNCaP-CxR and H2O2-resistant derivative LNCaP-HPR50 cells. Among the prxs, Prx2 expression was markedly increased in the prostate cancer cells. Overexpression of Prx2 increased AR transactivation, whereas overexpression of nuclear-localizing Prx2 suppressed AR transactivation (Fig. 2). The nuclear localization of Prx2 was reduced in LNCaP-CxR cells compared with LNCaP cells. Downregulation of Prx2 with siRNA reduced growth of LNCaP-CxR cells.(61)
3.4. Nuclear Prx2 protects cancer cells from DNA-damaging agents
Treatment of cancer cells with chemotherapeutic or DNA-damaging agents like etoposide induces necrotic cell death. It was shown that down-regulation of Prx2 by siRNA in cancer cells results in increased cell death and sensitivity to these agents, suggesting Prx2 facilitates tumor survival.(53) Further characterization revealed Prx2 is present in nucleus and enhances agent-induced activation of the JNK/c-Jun pathway involved in repair of damaged DNA.(53) Prx1 knockdown in HeLa cells did not show any effect in this assay system. Prx2 knockdown in other cancer cell lines such as HCT116 and U2OS induces similar effects as in HeLa cells, but it did not cause any significant effect in normal fibroblasts. Peroxidase activity of Prx2 is unlikely to be involved in the protection of cancer cells from DNA-damaging agents.
4. Biological Functions of Extracellular Prxs
In addition to Prx4,(7) Prx1 is also secreted from cells. Other Prxs are also detected in body fluids, suggesting they also function in extracellular space. Biological functions of extracellular Prxs are interesting subjects for future studies especially in regulation of inflammation. TLRs may be key molecules in their biological functions. Interaction of parasite Prxs with host TLR4 is important in respect to both self-defense and infection. Physiological importance of interaction between Prx1 and MIF or cyclophilin A has not been established yet.
4.1. Presence of Prxs in body fluids
Prxs are present in various extracellular body fluids. Bronchoalveolar lavage fluid (BALF) contains many proteins, which represents a unique clinically useful sampling of the lower respiratory tract. Guo et al.(62) and Gharib et al.(63) performed proteomic analysis of unique proteins contained in mice and human BALF and detected Prx1, Prx5 and Prx6 among them. Interestingly, the increased level of Prx1 in BALF was observed in many patients with acute lung injury compared with normal subjects.(63)
The interstitial fluid of bone marrow, a pivotal component of the hematopoietic niche, includes all the extracellular cytokines between the cells of bone marrow and constitutes the external microenvironment of the bone marrow stromal cells. Wang et al.(64) analyzed differentially expressed fluid proteins and identified Prx2 as one of the proteins down-regulated during aging. They suggested Prx2 is produced by stromal cells like other soluble factors and might be secreted. They speculated that decrease in Prx2 expression might cause increase in intracellular H2O2 levels in the cells of aged mice.
4.2. Prx1 secretion from mammalian cells
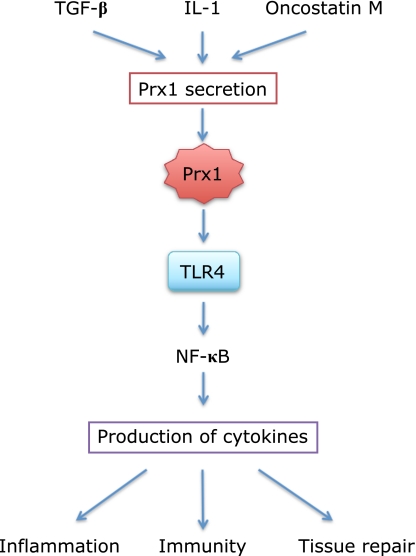
Recent studies have established the secretion of Prx1 from cells. Although the precise mechanism underlying its secretion is not known, different cultured cells have been shown to secrete Prx1 following stimulation with some cytokines such as TGF-β1,(18) IL-1 and oncostatin M,(65) a subfamily of IL-6. Prx1 is detected in plasma and various tissue fluids and seems to play a key role in modulating inflammation, immunity and tissue repairing reactions in vivo partly through its interaction with the cell surface sensor TLR4 (Fig. 3).
Fig. 3.
Prx1 is secreted from cells and activates TLR4-mediated signaling. Cytokines such as TGF-β,(18) IL-1 and oncostatin M(61) enhance Prx1 secretion from cells although precise mechanism is unknown. Prx1 binds TLR4 that regulates intracellular signaling to activate NF-κB, which induces production of various cytokines important in inflammation, immunity and tissue repair reactions.(68)
Chang et al.(66) reported that non-small cell lung cancer tissue expresses high levels of Prx1. Nearly half of the cancer-bearing patients had autoantibodies to Prx1 and about one-third of them had immuno-detectable Prx1 in their sera, suggesting Prx1 was secreted from the lung cancer tissue.(13) These studies suggest the Prx1 secretion could be used as potential lung cancer biomarker or as a criterion for cancer typing. They found that cultured lung adenocarcinoma cells (A549) secrete Prx1, but non-cancer lung cells (BEAS 2B) do not secrete Prx1(13) and that Prx1 secretion from A549 cells was largely dependent on TGF-β1 and was not associated with cell lysis or death.(18) It was shown that A549 cells expressed higher TGF-β1 mRNA levels and converting enzyme activity to produce its active form.(18) Other cancer cells as MCF7, Hep3B and HeLa do not secrete Prx1 via TGF-β1,(18) indicating that Prx1 secretion may be cell-type specific. Since Prx1 lacks a signal peptide for the classical or ER/Golgi-dependent secretory pathway, its secretion from cells may be mediated through the non-classical secretory pathway like other proteins such as IL-1, MIF, Trx and Hsp70. It seems important to further characterize the biological role of Prx1 secretion induced by TGF-β since it is a released from blood platelets and various stromal components (reviewed in Massague et al.(67)).
Prx1 secretion was also observed from primary human articular chondrocytes.(65) To analyze the secretion, the chondrocytes were cultured to pre-confluence and changed to serum-free medium, and stimulated with either IL-1 (1 ng/ml) or oncostatin M (10 ng/ml) for 24 h. The proteins in the medium were separated by 2D gel electrophoresis and the spot of Prx1 was identified. Prx1 was one of several major proteins found in the medium after treatment with these cytokines.(65) This result suggests a role of Prx1 in the maintenance of cartilage tissue.
4.3. Binding of Prx1 and malarial Prx to TLR4
The findings that both Prx1(15) and malaria parasite derived Prx(14) bind cell surface danger signal receptor TLR4 opens a new field to investigate novel roles of extracellular Prxs in inflammation and innate immunity. Riddell et al.(15) found extracellular Prx1 binds to TLR4 and acts as a pro-inflammatory factor. Supplementing Prx1 (about 50 µg/ml) to culture medium stimulated TNF-α and IL6 secretion from thioglycollate-elicited murine macrophages or immature bone marrow-derived dendritic cells.(15) This reaction is mediated through TLR4, a major cell surface receptor resulting in inflammatory response in an MyD88-dependent fashion. Cell surface TLRs recognize foreign pathogens as LPS and also various endogenous host molecules that trigger inflammatory and repair responses. Most of these host molecules are produced either as a result of cell death or injury. These include degradation products of the extracellular matrix, heat-shock proteins and HMGB1 proteins (reviewed in Kawai and Araki(65)).
4.4. Interaction of Prx1 with MIF
Association of Prx1 with MIF has been reported in transformed human kidney embryonic fibroblasts, 293T cells.(19) The direct binding was first found in a two-hybrid system and further characterized by immunoprecipitation using cell extracts and their recombinant proteins.(19) MIF forms a homotrimer (3 × 12.5-kDa) and possesses a tautomerase activity for which physiological substrates have not been identified. Prx1 binds MIF through a disulfide bond using its conserved Cys-173 and partially inhibits the tautomerase activity of human recombinant MIF. It is also evident that MIF inhibits the peroxidase activity of Prx1 in the associated form. However, the biological role of Prx1 association with MIF remains to be elucidated.
MIF is a pro-inflammatory factor originally identified more than 40 years ago as a T-cell-derived factor responsible for the inhibition of macrophage migration in delayed-type hypersensitivity.(69,70) Various studies have established that it helps tumor growth and plays a central role in the host immune and inflammatory response (see recent reviews by Conroy et al.(71) and Noels et al.(72)). It is expressed in various cells including lymphocytes, macrophages, epithelial cells and endothelial cells.(71,72) Intracellular MIF affects signaling to suppress apoptosis but secreted MIF from cells acts like cytokines through its receptors. MIF acts to modulate and amplify the response to LPS at least partly by upregulation of TLR4 expression.(73,74) LPS administration in vivo promotes secretion of MIF into plasma causing enhanced inflammation. Many other factors as H2O2 (100 µM) and hypoxia also enhance MIF expression and secretion.(75) Although there is no published evidence, it is reasonable to assume extracellular Prx1 could associate with secreted MIF causing suppression of MIF-mediated enhancement of inflammation.
4.5. Interaction of Prxs with cyclophilin A
Cyclophilin A (CyPA) is a regulator of inflammation and bind to immunosuppressant cyclosporine A like other cyclophilin family proteins. CyPA is a ubiquitously and abundantly expressed protein having peptidyl-prolyl cis-trans isomerase activity. Lee et al.(21) first identified CyPA as Prx6 binding protein using rat lung crude extracts. They found that CyPA binds to all Prx1 to Prx6 mammalian Prxs and enhance their thiol-specific antioxidant activity. The reaction mixture of the assay system contains 10 mM dithiothleitol (DTT) and 3 µM FeCl2, which produces thiyl radicals by iron-catalyzed autoxidation of DTT causing inactivation of glutamine synthetase. Prxs protect the target protein by scavenging thiyl radicals, and oxidized Prxs are reduced to reactive form by excess DTT in the absence of intrinsic Prx reducing system, Trx/Trx reductase/NADPH. CyPA seems to facilitates reduction of Prxs in the presence of DTT, although precise mechanism is not known. Jaschke et al.(20) also showed that human T cell cyclophilin18 binds to yeast Prx and enhanced its thiol-specific antioxidant activity.
CyPA is secreted from vascular smooth muscle cells (VSMCs) by a vesicular pathway under oxidative stress and mediates vascular remodeling by promoting inflammation and VSMC proliferation.(76) Extracellular CyPA binds its receptor CD147 and activates ERK1/2, Akt and JAK, which promotes chemotactic activity towards a variety of immune cells.(77) CyPA is upregulated in a variety of inflammatory conditions, such as rheumatoid arthritis, autoimmune disease, and cancer. Interestingly, both CyPA and Prx1 are secreted from human articular chondrocytes by stimulation with either IL-1 or oncostatin M.(65) CyPA coexsists with Prx1, Prx5 and Prx6 in bronchoalveolar lavage fluids (BALFs) of mice.(62,63) These results suggest both Prxs and CyPA may mutually affect their functions and modulate inflammatory reactions in extracellular space.
4.6. Effects of Prx1 and Prx2 on virus replication
Geiben-Lynn et al.(78) found Prx1 and Prx2 were preferentially upregulated in bulk CD8+ T-cells from HIV seropositive indivisuals compared to with seronegative individuals. They found an interesting phenomenon that plasma levels of the NKEKs were elevated (up to 500 ng/ml) in 3 of 13 HIV-infected but untreated persons and that exogenous Prx1 and Prx2 inhibited virus replication in human T-cells.(78) Supplementing recombinant Prx1 or Prx2 to culture medium respectively inhibited HIV-1 replication in the cells at an ID50 (dose inhibiting HIV-1 replication by 50%) of –130 nM (3 µg/ml). Supplemented Prx1 (3 µg/ml) also inhibited replication of dual-tropic simian immunodeficiency virus and dual-tropic simian-human immunodeficiency virus. Both Prx1 and Prx2 were detected in the culture medium of CD8+ T-cells at 15–40 ng/ml after 4 h, regardless of whether the cells were stimulated with anti-CD3 and whether the cells were from HIV-1 infected or uninfected individuals. The concentration of Prx1 in the medium reached up to –125 ng/ml at 16 h, however, this concentration was below that causing significant inhibition of HIV-1 replication. When Prx1 or Prx2 were over-expressed –10-fold in Jurkat CD4+ T-cells, the HIV-1 replication was inhibited after 5 to 8 days by 80–98%. Notably, the inhibitory effect appeared only several days after virus infection, which meant stably expressed high levels of intracellular Prxs could not inhibit virus replication at the early infection stage. Although these authors suggested that intracellular Prx1 and Prx2 blocks virus replication through down-regulation of the NF-κB pathway,(78) it is more likely that secreted Prx1 and Prx2 gradually accumulated in the medium and inhibit virus replication within cells.
Another study shows cytoplasmic Prx1 enhances the RNA-dependent RNA polymerase activity (i.e., transcription and replication) of the lymphotropic measles virus in HEK293-SLAM cells.(79) It was shown Prx1 binds to the C-terminal region of the nucleoprotein (NTAIL) of the virus and enhance RNA synthesis of the virus in the cells.
4.7. NK cell enhancing activity of Prx1 and Prx2
Natural killer cell enhancing factor (NKEF) was first identified in erythrocyte cytosol.(16) This study initiated from the observation that intact red blood cells (RBCs) supplemented to assay medium significantly enhanced natural killer cell-mediated cytotoxicity to cancer cells. Since cytosol proteins from RBCs also augmented the NK activity to kill human erythroleukemic cell K562, the active protein component was isolated and partially sequenced. Two cDNA clones encoding NKEF-A and -B, respectively corresponding to Prx1 and Prx2, were isolated from K562 cells. Both NKEF-A and -B recombinant proteins augmented NK cytotoxicity, but they did not enhance lymphokine-activated killer cytotoxicity.(17) Active RBC NKEF (NKEF-B/Prx2) has an apparent molecular mass of between 300 and 400 kDa,(16) suggesting it exists as oligomers. The recombinant Prx2, pre-treated with dithiothreitol, which might inhibit oligomerization, hardly enhanced cytotoxicity of NK cells.(17) Blocking sulfhydryl residues in NKEFs with N-ethylmaleimide, inhibits either dimer or oligomer formation and diminishes activity. These results show exogenously added Prx1 and Prx2 in the form of oligomer could enhance NK activity, although the underlying mechanisms remain to be elucidated. The NK cell enhancing activity of Prx1 and Prx2 is now regarded as an important immunological activity, since Prx1 can be secreted from some tumors and both Prx1 and Prx2 are released from virus-infected CD8+ T cells(78) and Prx2 can be released from RBC following hemolysis.
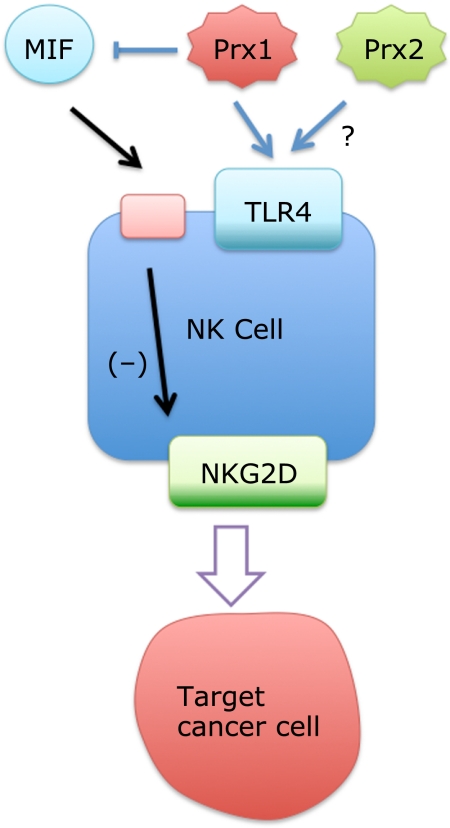
We propose a potential mechanism by which exogenous Prx1 and Prx2 could enhance NK cell killing activity (Fig. 4). Prx1 and Prx2 may act through TLR4 in NK cells, since these cells also express a functional TLR4/MD2 complex and can produce type 1 chemokines and IFN-γ upon stimulation,(80) which may result in enhancement of killing activity. It is reasonable to assume that Prx2 also binds TLR4 similar to Prx1 due to their sequence homology. Another possibility worth examining is the inhibition of MIF activity by Prx1. MIF is known to suppress NK cell activity through down-regulation of NK cell receptor NKG2D, a key player for signaling of the perforin-mediated cytolytic response,(81) which results in inhibition of release of perforin granules from NK cells.(82) MIF is also important for maintaining TLR4 expression levels.(74) MIF is ubiquitously expressed, and it is released from both NK cells(83) and their target corneal endothelial cells(59) and human uveal melanoma cells.(84) MIF is also contained in serum, which is an essential component of the NK cell activity assay system.(16,17) These results suggest that extracellular Prx1 could enhance NK cell-mediated lytic activity through inhibition of MIF activity, a concept consistent with the fact that Prx1 has stronger NKEF activity compared to Prx2,(17) for which binding to MIF has not been shown.
Fig. 4.
A hypothetical mechanism how NKEF-A and -B (Prx1 and 2) could enhance NK cell activity. Prx1 has stronger NK cell enhancing activity than Prx2.(17) Prx1 and presumably Prx2 bind TLR4 expressed on NK cells inducing activation of NK cells through production of chemokine and IFN-γ.(80) It is also possible that Prx1 binds to MIF and inhibits its activity. MIF can be released from target cells and is contained in serum, resulting in down-regulation of NKG2D expression,(81) a receptor important for perforin-mediated cytolytic response. This effect might be restricted to Prx1 since binding of Prx2 with MIF is not shown.
5. Parasite Prxs in Infection and Immunity
Parasitic protozoa and helminths have different strategies to cope with host immune attack for their survival. Parasitic peroxiredoxins play important roles for their protection against oxidative damages and also for interaction with host immune systems.
5.1. Malarial plasmodium Prxs
Protozoan parasite plasmodium faciparum, that cause tropical malaria, is a rapidly multiplying unicellular organism undergoing a complex developmental cycle in man and mosquito. Malaria parasites are subjected to high levels of oxidative stress during their development inside erythrocytes. Malaria plasmodium, which lacks genuine catalase and glutathione peroxidase, depends largely on Prxs for their survival in erythrocytes.(85–87) Kawazu et al.(88) first cloned a Prx6-like 1-Cys peroxiredoxin in plasmodium (called Pf-1-Cys-Prx), of which content is roughly 0.5% of the total proteins in the late trophozoites. Malaria plasmodium also expresses a Prx2-like 2-Cys Prx (PfTPx-1)(89–91) and another 2-Cys Prx (PfTPx-2), of which peroxidase activity, however, has not been proven.(87) Additionally, it has a Prx5-like atypical Prx termed PfAOP,(91) and a glutathione peroxidase-like thioredoxin peroxidase (TPxG1).(85,93) TPx-1 and 1-Cys-Prx mainly localize in cytoplasm, TPx-2 in mitochondrial, and AOP in apicoplasm.(85,87,93) TPxG1 localizes in both cytoplasm and apicoplast.(93)
In addition to these 5 peroxiredoxins, plasmodium has an unusual peroxiredoxin renamed PfnPrx (earlier called MCP1) which has a broad substrate specificity using both thioredoxin and glutaredoxin as reductants.(94) It localizes to the nucleus and is associated with chromatin, suggesting a role in the protection of nuclear DNA against oxidative damages.(94)
5.2. Host immune reaction to malaria plasmodium
Malaria parasites have devised many strategies to escape the host’s immune response and to survive in the host. It has been reported that malarial parasite infection increases production of IgE, which plays a protective role against malarial infection.(14) In the infection experiments of Plasmodium berghei ANKA to mice, IgE produced in mouse sera detected the parasite Prx as a major antigen among 65 separated parasite’s proteins.(14) The infection of Plasmodium falciparum induces elevation in immune mediators such as inflammatory interleukins, TNF and NO in human and animal. Among the mediators, high concentrations of TNF, mainly produced by mast cells, were shown to be associated with disease severity.(95) They searched parasite-produced TLR4 agonists, since TNF production mainly depends on TLR4/MD-2 mediated activation of the transcription factor NF-κB, and identified the malarial parasite PfTPx-1, a Prx2-like peroxiredoxin, as a TLR4 agonist leading NF-κB activation.(14) These studies first established the novel function of parasite-derived Prx in innate and acquired immune responses in malarial parasite infection.
5.3. Incorporation of erythrocyte Prx2 into plasmodium
Koncarevic et al.(96) recently reported an interesting finding that malaria Plasmodium imports erythrocyte Prx2, thereby using the host protein for its protection. Notably, human Prx2 accounts for roughly 50% of thioredoxin peroxidase activity in parasite extracts. Treatment of plasmodium-bearing erythrocytes with chloroquine, a drug promoting oxidative stress, increased the content of human Prx2 in parasite cytoplasm.(96) They denied a possibility that transported Prx2 is directed to the food vacuole, where erythrocyte cytoplasmic proteins including hemoglobin are digested. The specific-incorporation of Prx2 and three other rafts-localized host proteins into intracellular parasitophorous vacuolar membrane formed by merozoite was previously characterized.(97) Interestingly, Prx2 is also localized in Mauer’s clefts,(96) which are membranous structures used by the parasite for protein sorting and protein export.(98) In the case of malaria parasite infection, we speculate that host Prx2 may be released from either erythrocyte upon hemolysis or protozoa through Murer’s clefts resulting in competition between Prx2 and malarial Prx for modulating host immune reaction. Further studies are warranted to clarify the functional differences, if any, among Prx1, Prx2 and parasites Prxs in modulation of host immune reactions against parasites.
5.4. Prxs in helminth infection
Parasitic nematodes express high levels Prxs that are assumed to play a pivotal role in dealing with both internal and external oxidative stress since they lack selenium-containing glutathione peroxidase (see review by Henkle-Duhrsen and Kampkotter(99)). The 2-Cys Prxs have only 10% sequence identity with mammalian 2-Cys Prxs.(99) Both 1-Cys and 2-Cys Prxs are released from nematodes.(99–101)
Parasitic helminths have a striking feature for their ability to elicit protective immunity and to persist within the host for long-term. Helminths are known to secrete a variety of molecules such as Prx, proteinases and their inhibitors, and cytokine homologues that help them to penetrate the defensive barriers and avoid the immune attack of the host.(101,102) Recent studies have established that secreted helminth Prx modulates the immune responses of their hosts in a manner independent of its antioxidant activity.(100,103–105) The recombinant parasite Prx directly stimulates macrophages to develop into alternatively activated phenotype, of which macrophages are assumed to induce the differentiation of naïve T cells to Th2 phenotype resulting production of high levels of IL-10 and IL-4.(100,105) The macrophages activated by helminth Prx suppressed the development of Th1 cells (IFNγ production) without affecting the promotion of the differentiation of Th2 cells (IL-4 production) from naïve CD4+ T cells in the development of antigen-specific Th2 immune responses.(105) Interestingly, recombinant murine Prx2 also induces the marker gene Ym1 expression in macrophages and promotes polarized Th2 immune responses,(105) an effect is apparently different from that of Prx.(15) It is not clear at present how nematode Prx act through macrophages and whether or not they interact with TLRs.
Although further studies are required, above results suggest that murine Prx1 and malarial Prx (PfTPx-1) promote polarized Th1 immune responses to produce inflammatory cytokines, and Prx2 and helminth Prx promote polarized Th2 immune responses to produce anti-inflammatory cytokines or induce M2/wound-healing macrophages under the employed experimental conditions. Based on these results we suggest that the helminth parasites secrete Prx to counteract host Prx1 function, thereby shifting the Th1/Th2 balance in the surrounding microenvironment beneficial for their survival.
6. Roles of Prxs in Tissue Inflammation
Inflammation reactions are self defense response and provoked following tissue injury and infection. Oxidative stress is involved in the reactions and Prxs play important and complex roles in the process of inflammation. Since different type of cells and various regulatory factors are involved in a time-dependent manner during the complex inflammation reaction, model mice deficient in each Prx provide their physiological roles, which may not be observed in single cell culture system.
6.1. Role of Prxs in lung protection
Prxs play important roles in protection of lung, which is always facing danger of infection and tissue oxidative damages by oxygen and various insulting agents in the air. The pulmonary system has acquired endogenous defenses such as antioxidant system, innate immunity, drug detoxification and tissue repair systems to cope against these attacks. Prx family proteins are expressed in a cell-type specific manner in pulmonary tissue.(106) Bronchial and alveolar epithelial cells express Prx1, 2, 3, 5 and 6 and alveolar macrophages express Prx1, 3, 4, 5 and 6 at relatively high levels, respectively.(106) Prx6, having non-selenium glutathione peroxidase activity, is also known as a lysosomal-type acidic, Ca2+-independent phospholipase A2 and plays important role in lung surfactant dipalmitoylphosphatidylcholine degradation and synthesis.(107) Prx6-deficient mice have increased sensitivity to hyperoxia,(108) while transgenic mice overexpressing Prx6 have an increased defense to hyperoxia.(109) Additionally, Prx1, 2, 4, 5 and 6 have been detected in bronchoalveolar lavage fluid, suggesting their roles in airway space in lung protection.(62,63)
Bleomycins, products of Streptomyces verticillis, have potent tumor killing properties. However, a major adverse effect is pulmonary toxicity. The toxicity of bleomycin is not restricted to its effect on DNA but production of reactive oxygen species through interaction with iron.(110,111) We recently compared the bleomycin-induced acute lung injury and pulmonary fibrosis between Prx1-deficient and wild type mice.(112) We showed administration of bleomycin induced enhanced inflammation and fibrosis in Prx1-deficient mice, suggesting Prx1 protects cells from the acute oxidative damage by reactive oxygen species. The enhanced pulmonary inflammation and fibrosis in Prx1-deficient mice was partly inhibited by administration of N-acetyl-L-cysteine.(112)
In another study, we have obtained results suggesting Prx1 is involved in regulation of allergen-related airway inflammation. Prx1 deficient mice exhibited an enhanced response to Th2 adjuvant (ovalbumin + aluminum potassium sulfate) but a suppressed response to Th1 adjuvant (ovalbumin + complete Freund’s adjuvant).(113) The Th1 adjuvant challenge in Prx1 deficient mice lung caused reduced infiltration of eosinophils and neutrophils into bronchoalveolar lavage fluid, suggesting a positive role of Prx1 in the LPS-induced inflammatory reaction.(113)
6.2. Role of Prx1 in ozone-induced lung inflammation
Ambient O3 is a commonly encountered environmental air pollutant. Since O3 is chemically reactive gas, it preferentially induces oxidation of endogenous unsaturated lipids present in pulmonary surfactant.(114,115) It is assumed that the secondary ozonation products, such as lysophospholipids, aldehydes and epoxycholesterol, mediate various cellular responses induced by O3.(114,116,117) We recently found that O3 exposure (6 h, 2 ppm) induced significantly less lung inflammation in Prx1 deficient mice compared to WT mice monitored after 24 h. In bronchoalveolar lavage fluids (BALF), infiltrated immune cell numbers and protein levels of proinflammatory cytokines were significantly less in Prx1 deficient mice compared to WT mice (Yanagisawa et al., unpublished results).
Recent studies established that the biological response to O3 is dependent on complex interaction with innate immune signaling (reviewed in Al-Hegelan et al.(118) and Hollingsworth et al.(119)). The endotoxin receptor, TLR4 significantly contributes O3-induced lung hyperpermeability(120) and airway hyperresponsiveness (AHR).(117) It was suggested that TLR4-dependent signaling could lead to MyD88-dependent activation of NF-κB and generation of downstream proinflammatory factors leading to AHR.(121,122) TLRs 2 and 4 play important role in initiation of inflammation and following tissue repair reaction.(123) We detected Prx1 in BALF from WT mice both before and after O3 inhalation, suggesting Prx1 is steadily secreted into the alveolar space even without any injury.(114)
Our results suggest that alveolar Prx1 may be one of the intrinsic activators of TLR4 signaling upon airway injury by O3 inhalation. Notably, low molecular weight hyaluronan (LM-HA) fragments are released from the extracellular matrix in the early O3-induced biological responses in mice lung and has been identified as one of the TLR4 ligands.(118,123–126) We speculate LM-HA and other factors such as platelet-activating factor(127) produced under the tissue-damaging conditions may cooperate with Prx1 in activation of TLR4 following ozone inhalation. Since, pulmonary innate immunity is dependent on a complex signaling network between many cell types,(128) further careful studies are required to clarify the exact roles of Prx1 in ozone-induced lung inflammation.
6.3. Role of Prx1 in sensitivity to cisplatin
A major toxicity of the cancer chemotherapeutic agent cis-diamminedichloroplatinum (II) (cisplatin) is acute renal failure. The toxicity of cisplatin is partially associated with increased oxidative stress and the protective role of Prx1 against its toxicity can be observed in cultured cells. Mouse embryo fibroblasts (MEFs) derived from Prx1-deficient mice showed increased cisplatin-induced apoptosis compared with wild-type MEFs.(129) Cisplatin treatment led to increased activation of p38 MAPK and JNK in Prx1-deficient MEFs compared with wild-type MEFs. A JNK-specific inhibitor protected the Prx1-deficient MEFs from cisplatin-induced apoptosis, suggesting a role of JNK activation in the induction of apoptosis.(129) The above cell protection activity of Prx1 against cisplatin/ROS-mediated apoptosis is in accord with the anti-apoptotic activity of cytoplasmic Prx1 as shown above (Fig. 1).
However, in contrast to an in vitro cell study, Prx1 deficient mice show significant resistance to acute renal damage caused by cisplatin administration (Okada et al., unpublished data). We suggest two possible reasons to elucidate the cisplatin resistance in Prx1 deficient mice. The first reason is that the Prx1 deficient mice have a higher clearance rate of cisplatin from blood compared to wild-type mice. We found that Prx1 deficient mice had higher expression levels of membrane transporters for metabolites such as Mrp4 in kidney. Enhanced renal cisplatin clearance through Mrp2 and Mrp4 across the brush border membrane into the urine reduces the accumulation of cisplatin in the plasma and proximal tubule cells, and results in reduced nephrotoxicity.(130) It is shown the upregulation of c-Myc activity induces increase in expression of membrane transporter genes in human epithelial cells.(131) The enhanced expression of membrane transporters in kidney may be due to enhanced c-Myc activity in the Prx1 deficient mice.(23)
The second reason for cisplatin resistance in Prx1 deficient mice may be defects in Prx1-TLR4 signaling. This idea is consistent with the fact that cisplatin nephrotoxicity in mice is largely mediated through parenchymal TLR4 signaling and that in TLR4 deficient kidney, neutrophil infiltration, increase in IL-6, keratinocyte chemoattractant and TNF-α were minimal after cisplatin administration.(132)
7. Promotion of Tumor Growth by Prxs
Recent studies show Prxs expressed in tumor cells play positive roles in their progression and/or metastasis in transplanted animals. Different functions of Prxs are required for their progression/metastasis in vivo depending on tumor types.
7.1. Prx1 enhances tumor progression
Human Prx1 or Pag (proliferation associated gene) was originally cloned from ras oncogene-transformed mammary-epithelial cells.(4) Prx1 expression is elevated in various cancer tissues and cancer cell lines that are apparently linked with poor clinical outcomes and diminished overall patient survival.(133–137)
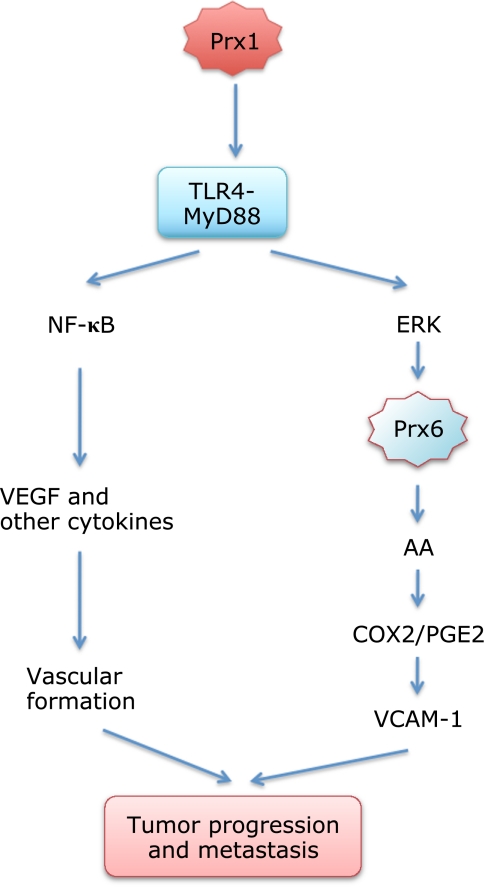
A recent study shows Prx1 enhances growth of prostate cancer cells through TLR4 signaling.(138) The Prx1 expression levels increased in prostate cancer tissues during progression in model mouse in vivo. Prostate cancer cells, human PC-3M and murine C2H, were respectively inoculated into mice and time-dependent increase in tumor volume was measured with calipers.(138) Down-regulation of cellular Prx1 expression levels with shRNA treatment before inoculation caused significant delay in PC-3M and no growth in C2H tumors in vivo. The results suggest Prx1 expression within the tumor cells plays a role in its progression in vivo. When Prx1-expressing C2H cells were inoculated into mice deficient in TLR4 signaling, tumor did not grow. These results show both Prx1 in the cancer cells and TLR4 in the host are required for the tumor growth in vivo. Their results further suggested that the Prx1-TLR4-MyD88 signaling, which actually occurs in both tumor and host, contributes to expression of VEGF, TNFα, IL-6, TGF-α and TGF-β within solid tumors and to vasculature formation and function. They concluded that the Prx1-TLR4 system plays a critical role in tumor growth by enhancing vascular endothelial cell recruitment or migration through VEGF production(138) (Fig. 5).
Fig. 5.
Roles Prx1 and Prx6 in tumor progression and metastasis through TLR4-MyD88 signaling. Cancer cells secrete Prx1 and activate TLR4-MyD88 signaling in cancer and normal cells to help progression in host.(138) Prx6 also helps growth and metastasis of cancer cells through its GSH peroxidase and phospholidase A2 activities.(145,152) We suggest a cooperation of extracellular Prx1 and cytoplasmic Prx6 in tumor progression through TLR4-MyD88 signaling (see text).
It is noteworthy that functional TLR4 is expressed in various tumors and its activation promotes production of immunosuppressive cytokines and chemokines such as TGF-β, VEGF and IL-8.(139) The TLR4-mediated signaling makes tumor cells more resistance to lysis mediated by NK cells,(140) and to apoptosis by anti-cancer drug,(141) TNF-α and TRAIL.(139) The study by Riddle et al.(138) first showed that Prx1 is one of the TLR4 ligands utilized by cancer cells for their progression through TLR4 signaling. It is important to note, however, that small hyaluronan fragments are also known to contribute tumor development through TLR4 mediated signaling (see reviews by Lokeshwar and Selzer(142) and Girish and Kemparaju(143)). In human cancers, hyaluronan contents are usually higher in tumors than in normal tissues and hyaluronidase-1 is the main degrading enzyme expressed in tumors. We suggest it is an interesting research target to study functional interactions between Prx1 and hyaluronan oligomers in production of inflammatory and anti-inflammatory mediators through TLR4 in respect to tumor growth and metastasis (Fig. 5 and 6).
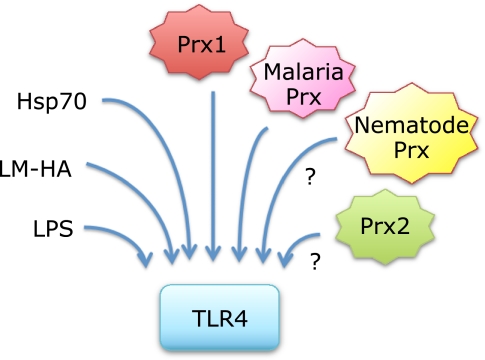
Fig. 6.
Prx1-TLR4 signaling may be affected by other ligands of TLR4. Recent studies have established that Prx1(15) and malaria parasite Prx(14) are ligands of TLR4. Although there is no evidence, Prx2 may also bind TLR4. Nematode Prx, having a low structural similarity to mammalian 2-Cys Prxs, might also bind to TLR4. Functional interactions through TLR4 among Prxs and Prxs with other key inflammatory modulators including LM-HA, Hsp70 and LPS will be important subjects for future research (see text).
7.2. Prx4 enhances tumor growth and metastasis
A recent report shows that Prx4 is also important for human lung cancer progression and metastasis.(144) This study used a microarray assay to compare gene expression patterns between normal and tumor tissues. They first found that sulfiredoxin (Srx), which catalyzes the reaction of hyperoxidized Prxs to the reduced form, was highly expressed in tumor samples from patients with aquamous cell carcinoma or adenocarcinoma. To explore the role of Srx in lung tumors, they used human lung cancer cell lines A549 and HEK293T as models. They found Srx knockdown reduced anchorage-independence proliferation of the cells in soft agar.(144) Then, Prx4 was identified as the most abundant Srx-interacting protein in both A549 and HEK293T cells. Interestingly, Prx4 knockdown recapitulated the phenotypic changes induced by Srx knockdown in A549 cells. Inoculation of Srx knocked-down cells into SCID immuno-deficient mice caused reduced tumor growth and lung metastasis formation. In contrast, inoculation of Srx plus Prx4 overexpressed cells caused an opposite effect. They suggested that Srx and Prx4 play role in intracellular phosphokinase signaling including AP-1-MMP9 and MAPK cascades and that enhanced Srx and Prx4 expressions in squamous cell carcinoma or adenocarcinoma contribute to tumor progression and metastasis formation.(144)
It is noted, however, this study did not examine whether Srx knockdown may also have caused functional defects of other intracellular Prxs, and that Prx4 secreted from cells may act either through yet unidentified cell receptor(s) or as an extracellular antioxidant. It is also possible that Prx4 may protect tumor cells from oxidative stress through facilitating degradation of thromboxane A2 receptor, which is predominantly localized in the endoplasmic reticulum.(145) Additionally, a possible cooperation of Prx4 with Prx1 and Prx6 in modulating tumor progression should be considered since A549 cells secrete Prx1(18) and their proliferation also depends on Prx6(146) as will be shown below.
7.3. Prx6 promotes tumor metastasis
Other groups showed that Prx6 is also upregulated in some malignant human tissues including lung(137,147) and breast.(148,149) Prx6 is the only mammalian 1-Cys peroxiredoxin and contributes in lung phospholipid metabolism through GSH peroxidase and phospholipase A2 (PLA2) activities.(150–152) Recent studies have shown Prx6 promotes progression and metastasis of cancer cells.(146,153) Chang et al.(146) showed that upregulation of Prx6 levels in human breast cancer cell lines, MDA-MB-435 and MDA-MB-231, increased their growth rates and adhesion to dishes in serum-free conditions. Down-regulation of Prx6 expression caused opposite effects. Parallel results to those in vitro assays were obtained in in vivo inoculation of the Prx6-modified cancer cells, indicating Prx6 in tumors positively regulates tumor growth and pulmonary metastasis in mice.(146) They observed that Prx6 expression levels affects several gene expressions in both cultured cancer cells and inoculated tumors.(146)
In contrast, Ho et al.(153) provided evidence that both peroxidase and PLA2 activities of Prx6 are required for metastasis of human lung cancer cells A549 and H460. They showed that enhanced Prx6 expression in A549 cells significantly increased pulmonary tumor nodule formation in the implanted nude mice compared to control cells. This tumor enhancing effect was not observed when the cells expressing Prx6 mutant lacking either peroxidase or PLA2 activity was injected, respectively. Further analysis using cultured cells and their implantation to hind legs, they concluded that the endogenous Prx6 peroxidase activity was sufficient for growth of the cells both in culture and in vivo leg inoculation. Transfection of the peroxidase mutant C47S-Prx6 suppressed the cell/tumor growth in a dominant-negative manner.(153) They suggested that Prx6 peroxidase activity might contribute to metastatic colony formation by facilitating cancer cell growth. They further showed that arachidonic acid (AA) produced by the PLA2 activity promotes cell invasion by stimulating the signaling pathway involving p38 kinase, PI3K, Akt and urokinase-type plasminogen activator.
Concerning tumor progression, we suggest Prx1-mediated TLR4 signaling may link with Prx6 phospholipase activation (Fig. 5). Previous studies have established that LPS treatment of cells such as macrophages and synovial fibroblasts induce TLR4-mediated MyD88- and TRIF-dependent ERK activation leading cytosolic PLA2 activation and AA-COX-2-PGE2 synthesis.(154–156) In a cell-free system, ERK and p38 phosphorylated Prx6 at Thr-177, resulting in an 11-fold increase in PLA2 activity without changing its GSH peroxidase activity.(157) Therefore, Prx1 instead of LPS could also induce Prx6 phospholipase activation through TLR4 signaling. Although there are several isoforms of PLA2 that can be activated through TLR4, Prx6 could play a major role in tumor progression if the tumor expresses high Prx6 levels.
8. Prevention of Oncogenesis by Prx1
It is an interesting question whether or not host Prx1 is important for suppressing spontaneous oncogenesis in vivo. The above results that some inoculated tumor cells utilize their Prxs for their progression in mice do not answer this question. Neumann et al.(158) first proposed that Prx1 prevents spontaneous tumor generation by reducing ROS levels and oxidative DNA damage. They showed Prx1 deficient mice have a shortened lifespan owing to development of severe hemolytic anaemia and several malignant cancers over 9 months of age. It is important to note that lack of Prx1 causes various effects in cells since it directly binds with several key molecules of the transformation such as PTEN and c-Myc resulting modification of their activities.
Prx1 binds tumor suppressor PTEN to protect its lipid phosphatase activity from inactivation under mild oxidative stress (25 µM H2O2).(159) PTEN is a nonredundant phosphatase, counteracting one of the most critical cancer-promoting pathways: PI3K-Akt signaling pathway (see recent review by Zhang and Yu(160)). It was shown that Akt is highly activated in Prx1 deficient fibroblasts and mammary epithelial cells, under which condition Ras or ErbB expression enhanced cell transformation.(159) Interestingly, on the other hand PTEN deficiency in fibroblasts causes 60–80% decrease in Prx1, Prx2, Prx5, Prx6 and Cu/Zn-SOD expression levels.(161) These results suggest Prx1 and PTEN cooperate to maintain cellular antioxidant levels and to suppress PI3K-Akt signaling.
Interaction of Prx1 with c-Myc to inhibit its transcriptional activities is also important to protect cells from transformation.(23) In Prx1 deficient fibroblasts and mice, some c-Myc functions, e.g. suppression or enhancement of target genes, are altered. As the results, the fibroblasts were transformed by a ras oncogene alone.(23) Another study showed that activation of PI3K/Akt signaling pathway by loss of PTEN function induces stabilization of c-Myc protein in pancreatic cancer cells.(162) Overexpression of c-Myc in focal prostate luminal epithelial cells and prostate-specific deletion of PTEN induces high-grade prostatic intraepithelial neoplasia lesions.(163)
These studies suggest that Prx1 deficient cells are more susceptible to transformation at least due to reduced tumor suppressor PTEN activity and also enhanced c-Myc activity. It is important to note that Prx1 deficient embryonic fibroblasts grow more slowly compared to wild type cells and do not spontaneously transform during culture in vitro.(23,158) Additionally tumorigenesis in vivo was not observed in other Prx1 single gene deficient mice like the mouse strain used by Neumann et al.(158) We could not detect tumor formation in our Prx1 deficient mice over one year breeding under germ-free conditions.(164) This discrepancy may be due to differences of mouse strain and/or breeding conditions. However, there are good reasons to believe as discussed above that presence of Prx1 in cells actually functions against tumorigenesis through different manners in addition to its antioxidant activity.
9. Highlights and Future Perspectives
Recent evidence highlights novel functions of Prxs independently of their peroxidase activity. One of the highlights is the function of Prxs in extracellular space as modulators in inflammation-related biological reactions as discussed above. Fig. 7 summarizes the multi-function of Prx1, the most characterized Prx family protein. Prx1 is a unique protein that could interact with many different types of proteins. Since inflammation reaction is controlled by many different types of factors and cells, it is important in the future studies to resolve fine roles of Prx1 and other Prx family proteins in each reaction systems.
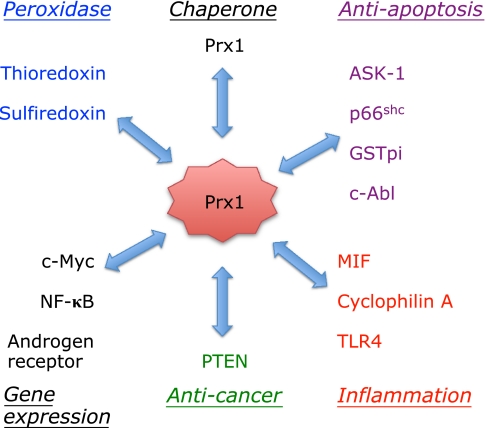
Fig. 7.
Prx1 interacts with many proteins and exhibits many biological functions. Prx1 is the major Prx family protein and plays many functions through direct interaction with different types of proteins. Recent studies have established that Prx1 is not just an antioxidant in the cytoplasm, but it also regulates gene expression in nucleus and inflammatory reactions in extracellular space. In addition to these proteins, some kinases and phosphatases modify Prx1 and regulate its biological functions.
Functions of malarial and helminth parasite Prxs as immuno-modulators(14,100,105) and their functional interaction with host Prx1 and Prx2 will be an important subject of future research to defend against parasite infections. Studies on the role of Prx1 and other Prxs in tumor progression are also important to establish novel strategies to suppress tumor growth and metastasis. Although there are no reports to date, Prx1 may also interact with TLR2 and other unknown cell surface receptors, and Prx2 may also interact with TLR2 and TLR4. Their precise signaling pathways through the receptors and their adaptors require further characterization.
We would like to emphasize the importance of functional interactions of Prx1 with low molecular weight hyaluronan, LM-HA through TLRs (Fig. 3 and 6). Both Prx1 and LM-HA interact with TLR4 but activate respectively different intracellular signaling.(126,138) HA is the major matrix component and LM-HA can be released upon tissue injury,(123) infection of nematode(165) and tumor progression.(166,167) Functional interaction or signaling cross talk between Prx1 and other stress proteins such as Hsp70 and Hsp60 may also be important. Hsp70 and Prx1 are abundant intracellular molecules and they complement one another to make cells resistant against various stresses such as heat shock and oxidative stress. Hsp70 is also known to play dual roles in apoptosis and innate immunity.(168,169) Both Hsp70 and Hsp60 are secreted from cells and functions as immune modulators through interaction with TLR2/4 and other cell surface receptors.(169–172) Extracellular Hsp70 derived from stressed and damaged cells can elicit a proinflammatory (Th1) immune response and enhance NK cell activity like Prx1 and Prx2.(17)
Trx is also excreted from cells under oxidative stress and its plasma levels are good markers for oxidative stress in variety of disorders.(173) Trx in the circulation exhibits anti-apoptotic and anti-inflammatory effects. Trx expression is enhanced in cancer tissues like Prx1 and it stimulates growth of virus-transformed B cells.(173) It will be an interesting in future studies to characterize possible functional interactions of PrxI with Trx and Trx family protein MIF in the extracellular space. Additionally, interaction of extracellular Prxs with CyPA will be another future interesting subject to study regulation of inflammatory reactions.
In summary, recent studies have opened a new era of Prx research that is not restricted to its antioxidant activities. These studies have revealed novel functions of Prxs in self-defense against infection, tissue damages and tumors through the regulation of inflammation. Further characterization of the roles of Prx1/2 in TLR2/4-mediated signaling and on direct or functional interaction with other inflammatory modulators are required to unravel the physiological functions of Prxs in the complex biological responses. Further studies using Prxs animal models are required to clarify the in vivo roles of Prxs, which can not be characterized in simple cell culture systems. These studies will help to establish strategies for treatment of stress- and inflammation-related wide variety of disorders.
Acknowledgments
We thank Prof. G.E. Mann and Dr. R.C.M. Siow (BHF Centre of Research Excellence, King’s College London) for their helpful suggestions and critical reading of the manuscript. We thank the Japanese Society for Promotion of Science and Great Britain Sasakawa Foundation (G.E. Mann and T. Ishii) for their financial support. We also apologize to the authors of many interesting studies that were omitted due to limited space.
Abbreviations
- AP-1
activator protein-1
- AR
androgen receptor
- ASK1
apoptosis signal-regulating kinase 1
- bHLH-ZIP
basic helix-loop-helix leucine zipper
- CD
cluster of differentiation
- COX-2
cyclooxygenase-2
- CyPA
cyclophilin A
- HMGB1
high-mobility group protein 1
- HIV-1
human immunodeficiency virus, type 1
- Hsp
heat shock protein
- JNK
jun N-terminal kinase
- LM-HA
low molecular weight hyaluronon
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- MIF
macrophage migration inhibitory factor
- MyD88
myeloid differentiation factor 88
- NF-κB
nuclear factor κB
- NKEF
natural killer cell enhancing factor
- NO
nitric oxide
- PD
Parkinson’s disease
- PLA2
phospholipase A2
- Prx
peroxiredoxin
- RBC
red blood cell
- ROS
reactive oxygen species
- SHIV
simian-human immunodeficiency virus
- SIV
simian immunodeficiency virus
- TGF-β
transforming growth factor-β
- TLR
toll-like receptor
- TNF
tumor necrosis factor
- Trx
thioredoxin
- TSA
thiol-specific antioxidant
- VEGF
vascular endothelial growth factor
References
- 1.Yamamoto T, Matsui Y, Natori S, Obinata M. Cloning of a housekeeping-type gene (MER5) preferentially expressed in murine erythroleukemia cells. Gene. 1989;80:337–343. doi: 10.1016/0378-1119(89)90297-7. [DOI] [PubMed] [Google Scholar]
- 2.Chae HZ, Kim IH, Kim K, Rhee SG. Cloning, sequencing, and mutation of thiol-specific antioxidant gene of Saccharomyces cerevisiae. J Biol Chem. 1993;268:16815–16821. [PubMed] [Google Scholar]
- 3.Ishii T, Yamada M, Sato S, et al. Cloning and characterization of a 23-kDa stress-induced mouse peritoneal macrophage protein. J Biol Chem. 1993;268:18633–18636. [PubMed] [Google Scholar]
- 4.Prospéri MT, Ferbus D, Karczinski I, Goubin G. A human cDNA corresponding to a gene overexpressed during cell proliferation encodes a product sharing homology with amoebic and bacterial proteins. J Biol Chem. 1993;268:11050–11056. [PubMed] [Google Scholar]
- 5.Rhee SG, Chae HZ, Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med. 2005;38:1543–1552. doi: 10.1016/j.freeradbiomed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 6.Flohé L, Harris JR, editors. Structure and Functions Subcellular Biochemistry. Vol. 44. New York: Springer, Inc.; 2007. Peroxiredoxin Systems; pp. 1–389. [Google Scholar]
- 7.Fujii J, Ikeda Y. Adavances in our understanding of peroxiredoxin, a multifuncational, mammalian redox protein. Redox Rep. 2002;7:123–130. doi: 10.1179/135100002125000352. [DOI] [PubMed] [Google Scholar]
- 8.Ishii T, Yanagawa T. Stress-induced peroxiredoxins. In: Flohe L, Harris JR, editors. Structure and Functions Subcellular Biochemistry. Vol. 44. New York: Springer, Inc.; 2007. pp. 375–384. [DOI] [PubMed] [Google Scholar]
- 9.Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 10.Woo HA, Yim SH, Shin DH, Kang D, Yu DY, Rhee SG. Inactivation of peroxiredoxin I by phosphorylation allows localized H2O2 accumulation for cell signaling. Cell. 2010;140:517–528. doi: 10.1016/j.cell.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Barranco-Medina S, Lázaro JJ, Dietz KJ. The oligomeric conformation of peroxiredoxins links redox state to function. FEBS Lett. 2009;583:1809–1816. doi: 10.1016/j.febslet.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 12.Godoy JR, Funke M, Ackermann W, et al. Redox atlas of the mouse: Immunohistochemical detection of glutaredoxin-, peroxiredoxin-, and thioredoxin-family proteins in various tissues of the laboratory mouse. Biochem Biophys Acta. 2011;1810:2–92. doi: 10.1016/j.bbagen.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Chang JW, Lee SH, Jeong JY, et al. Peroxiredoxin-I is an autoimmunogenic tumor antigen in non-small cell lung cancer. FEBS Lett. 2005;579:2873–2877. doi: 10.1016/j.febslet.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 14.Furuta T, Imajo-Ohmi S, Fukuda H, Kano S, Miyake K, Watanabe N. Mast cell-mediated immune responses through IgE antibody and Toll-like receptor 4 by malarial peroxiredoxin. Eur J Immunol. 2008;38:1341–1350. doi: 10.1002/eji.200738059. [DOI] [PubMed] [Google Scholar]
- 15.Riddell JR, Wang XY, Minderman H, Gollnick SO. Peroxiredoxin 1 stimulates secretion of proinflammatory cytokines by binding to TLR4. J Immunol. 2010;184:1022–1030. doi: 10.4049/jimmunol.0901945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shau H, Gupta RK, Golub SH. Identification of a natural killer enhancing factor (NKEF) from human erythroid cells. Cell Immunol. 1993;147:1–11. doi: 10.1006/cimm.1993.1043. [DOI] [PubMed] [Google Scholar]
- 17.Sauri H, Ashjian PH, Kim AT, Shau H. Recombinant natural killer enhancing factor auguments natural killer cytotoxicity. J Leukoc Biol. 1996;59:925–931. doi: 10.1002/jlb.59.6.925. [DOI] [PubMed] [Google Scholar]
- 18.Chang JW, Lee SH, Lu Y, Yoo YJ. Transforming growth factor-β1 induces the non-classical secretion of peroxiredoxin-I in A549 cells. Biochem Biophys Res Commun. 2006;345:118–123. doi: 10.1016/j.bbrc.2006.04.073. [DOI] [PubMed] [Google Scholar]
- 19.Jung H, Kim T, Chae HZ, Kim KT, Ha H. Regulation of macrophage migration inhibitory factor and thiol-specific antioxidant protein PAG by direct interaction. J Biol Chem. 2001;276:15504–15510. doi: 10.1074/jbc.M009620200. [DOI] [PubMed] [Google Scholar]
- 20.Jäschke A, Mi H, Tropschug M. Human T cell cyclophilin 18 binds to thiol-specific antioxidant protein Aop1 and stimulates its activity. J Mol Biol. 1998;277:763–769. doi: 10.1006/jmbi.1998.1644. [DOI] [PubMed] [Google Scholar]
- 21.Lee SP, Hwang YS, Kim YJ, et al. Cyclophilin A binds to perosiredoxins and activates its peroxidase activity. J Biol Chem. 2001;276:29826–29832. doi: 10.1074/jbc.M101822200. [DOI] [PubMed] [Google Scholar]
- 22.Hansen JM, Moriarty-Craige S, Jones DP. Nuclear and cytoplasmic peroxiredoxin-1 differentlly regulate NF-kappaB activities. Free Radic Biol Med. 2007;43:282–288. doi: 10.1016/j.freeradbiomed.2007.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Egler RA, Fernandes E, Rothermund K, et al. Regulation of reactive oxygen species, DNA damage, and c-Myc function by peroxiredoxin 1. Oncogene. 2005;24:8038–8050. doi: 10.1038/sj.onc.1208821. [DOI] [PubMed] [Google Scholar]
- 24.Park SY, Yu X, Ip C, Mohler JL, Bogner PN, Park YM. Peroxiredoxin 1 interacts with androgen receptor and enhances its transactivation. Cancer Res. 2007;67:9294–9303. doi: 10.1158/0008-5472.CAN-07-0651. [DOI] [PubMed] [Google Scholar]
- 25.Chhipa RR, Lee KS, Onate S, Wu Y, Ip C. Prx1 enhances androgen receptor function in prostate cancer cells by increasing receptor affinity to dihydrotestosterone. Mol Cancer Res. 2009;7:1543–1552. doi: 10.1158/1541-7786.MCR-08-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim SY, Kim TJ, Lee KY. A novel function of peroxiredoxin 1 (Prx-1) in apoptosis signal-regulating kinase 1 (ASK1)-mediated signaling pathway. FEBS Lett. 2008;582:1913–1918. doi: 10.1016/j.febslet.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 27.Gertz M, Fischer F, Leipelt M, Wolters D, Steegborn C. Identification of peroxiredoxin 1 as a novel interaction partner for the lifespan regulator protein p66Shc. Aging (Ablany NY) 2009;1:254–265. doi: 10.18632/aging.100017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim YJ, Lee WS, Ip C, Chae HZ, Park EM, Park YM. Prx1 suppresses radiation-induced c-Jun NH2-terminal kinase signaling in lung cancer cells through interaction with the glutathione S-transferase Pi/c-Jun NH2-terminal kinase complex. Cancer Res. 2006;66:7136–7142. doi: 10.1158/0008-5472.CAN-05-4446. [DOI] [PubMed] [Google Scholar]
- 29.Hu X, Weng Z, Chu CT, et al. Peroxiredoxin-2 protects against 6-hydroxydopamine-induced dopaminergic neurodegeneration via attenuation of the apoptosis signal-regulating kinase (ASK1) signaling cascade. J Neurosci. 2011;31:247–261. doi: 10.1523/JNEUROSCI.4589-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ichijo H, Nishida E, Irie K, et al. Induction of apoptosis by ASK1, a mammalian MAP-KKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 31.Saitoh M, Nishitoh H, Fujii M, et al. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adler V, Yin Z, Fuchs SY, et al. Regulation of JNK signaling by GSTp. EMBO J. 1999;18:1321–1334. doi: 10.1093/emboj/18.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veal EA, Findlay VJ, Day AM, et al. A 2-Cys peroxiredoxin regulates peroxide-induced oxidation and activation of a stress-activated MAP kinase. Mol Cell. 2004;15:129–139. doi: 10.1016/j.molcel.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 34.Fang J, Nakamura T, Cho DH, Gu Z, Lipton SA. S-nitrosylation of perosiredoxin 2 promotes oxidative stress-indiced neuronal cell death in Parkinson’s disease. Proc Natl Acad Sci USA. 2007;104:18742–18747. doi: 10.1073/pnas.0705904104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qu D, Rashidian J, Mount MP, et al. Role of Cdk5-mediated phosphorylation of Prx2 in MPTP toxicity and Parkinson’s disease. Neuron. 2007;55:37–52. doi: 10.1016/j.neuron.2007.05.033. [DOI] [PubMed] [Google Scholar]
- 36.Finetti F, Savino MT, Baldari CT. Positive and negative regulation of antigen receptor signaling by the Shc family of protein adapters. Immuno Rev. 2009;232:115–134. doi: 10.1111/j.1600-065X.2009.00826.x. [DOI] [PubMed] [Google Scholar]
- 37.Giorgio M, Migliaccio E, Orsini F, et al. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221–233. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 38.Le S, Connors TJ, Maroney AC. c-Jun N-terminal kinase specifically phosphprylates p66Shc A at serine 36 in response to ultraviolet irradiation. J Biol Chem. 2001;276:48332–48336. doi: 10.1074/jbc.M106612200. [DOI] [PubMed] [Google Scholar]
- 39.Li M, Chiou K-R, Kass DA. Shear stress inhibition of H2O2 induced p66Shc phosphorylation by ASK1-JNK inactivation in endothelium. Heart Vessels. 2007;22:423–427. doi: 10.1007/s00380-007-0994-9. [DOI] [PubMed] [Google Scholar]
- 40.Pinton P, Rimessi A, Marchi S, et al. Protein kinase Cβ and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant p66Shc. Science. 2007;315:659–663. doi: 10.1126/science.1135380. [DOI] [PubMed] [Google Scholar]
- 41.Wen ST, Van Etten RA. The PAG gene product, a stress-induced protein with antioxidant properties, is an Abl SH3-binding protein and a physiological inhibitor of c-Abl tyrosine kinase activity. Genes Dev. 1997;11:2456–67. doi: 10.1101/gad.11.19.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tybulewicz VL, Crawford CE, Jackson PK, Bronson RT, Mulligan RC. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell. 1991;65:1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- 43.Schwartzberg PL, Stall AM, Hardin JD, et al. Mice homozygous for the ablm1 mutation show poor viability and depletion of selected B and T cell populations. Cell. 1991;65:1165–1175. doi: 10.1016/0092-8674(91)90012-n. [DOI] [PubMed] [Google Scholar]
- 44.Gonfloni S. DNA damage stress response in germ cells: role of c-Abl and clinical implications. Oncogene. 2010;29:6193–6202. doi: 10.1038/onc.2010.410. [DOI] [PubMed] [Google Scholar]
- 45.Kharbanda S, Yuan ZM, Weichselbaum R, Kufe D. Determination of cell fate by c-Abl activation in the response to DNA damage. Oncogene. 1998;17:3309–3318. doi: 10.1038/sj.onc.1202571. [DOI] [PubMed] [Google Scholar]
- 46.Ishii T, Itoh K, Takahashi S, et al. Transcription factor Nrf2 coodinately regulates a group of oxidative stress-inducible genes in macrophages. J Biol Chem. 2000;275:16023–16029. doi: 10.1074/jbc.275.21.16023. [DOI] [PubMed] [Google Scholar]
- 47.Itoh K, Chiba T, Takahashi S, et al. An Nrf2/small maf heterodimer mediates the induction of phase II detoxyfying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 48.Ishii T, Itoh K, Akasaka J, et al. Induction of murine intestinal and hepatic peroxiredoxin MSP23 by dietary butylated hydroxyanisole. Carcinogenesis. 2000;21:1013–1016. doi: 10.1093/carcin/21.5.1013. [DOI] [PubMed] [Google Scholar]
- 49.Shiota M, Izumi H, Miyamoto N, et al. Ets regulates peroxiredoxin1 and 5 expressions through their interaction with the high-mobility group protein B1. Cancer Sci. 2008;99:1950–1959. doi: 10.1111/j.1349-7006.2008.00912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang MX, Wei A, Yuan J, Trickett A, Knoops B, Murrell GA. Expression and regulation of peroxiredoxin 5 in human osteoarthritis. FEBS Lett. 2002;531:359–362. doi: 10.1016/s0014-5793(02)03511-1. [DOI] [PubMed] [Google Scholar]
- 51.Wang MX, Wei A, Yuan J, et al. Antioxidant enzyme peroxiredoxin 5 is upregulated in degenerative human tendon. Biochem Biophys Res Commun. 2001;284:667–673. doi: 10.1006/bbrc.2001.4991. [DOI] [PubMed] [Google Scholar]
- 52.Abbas K, Breton J, Picot CR, Quesniaux V, Bouton C, Drapier JC. Signaling events leading to peroxiredoxin 5 up-regulation in immunostimulated macrophages. Free Rad Biol Med. 2009;47:794–802. doi: 10.1016/j.freeradbiomed.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 53.Lee KW, Lee DJ, Lee JY, Kang DH, Kwon J, Kang SW. Peroxiredoxin II restrains DNA damage-induced death in cancer cells by positively regulating JNK-dependent DNA repair. J Biol Chem. 2011;286:8394–8404. doi: 10.1074/jbc.M110.179416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blackwood EM, Eisenman RN. Max: a helix-loop-helix zipper protein that forms a sequence-specific DNA-binding complex with Myc. Science. 1991;251:1211–1217. doi: 10.1126/science.2006410. [DOI] [PubMed] [Google Scholar]
- 55.Prendergast GC, Lawe D, Ziff EB. Association of Myn, the murine homolog of max, with c-Myc stimulates methylation-sensitive DNA binding and ras cotransformation. Cell. 1991;65:395–407. doi: 10.1016/0092-8674(91)90457-a. [DOI] [PubMed] [Google Scholar]
- 56.Mu ZM, Yin XY, Prochownik EV. Pag, a putative tumor suppressor, interacts with the Myc Box II domain of c-Myc and selectively alters its biological function and target gene expression. J Biol Chem. 2002;277:43175–43184. doi: 10.1074/jbc.M206066200. [DOI] [PubMed] [Google Scholar]
- 57.Graves JA, Metukuri M, Scott D, Rothermund K, Prochownik EV. Regulation of reactive oxygen species homeostasis by peroxiredoxins and c-Myc. J Biol Chem. 2009;284:6520–6529. doi: 10.1074/jbc.M807564200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kang SW, Chae HZ, Seo MS, Kim K, Baines IC, Rhee SG. Mammalian peroxiredoxin isoforms can reduce hydrogen peroxide generated in response to growth factors and tumor necrosis factor-α. J Biol Chem. 1998;273:6297–6302. doi: 10.1074/jbc.273.11.6297. [DOI] [PubMed] [Google Scholar]
- 59.Wang X, He S, Sun JM, Delcuve GP, Davie JR. Selective association of peroxiredoxin 1 with genomic DNA and COX-2 upstream promoter elements in estrogen receptor negative breast cancer cells. Mol Biol Cell. 2010;21:2987–2995. doi: 10.1091/mbc.E10-02-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhuang Y, Miskimins WK. Cell cycle arrest in Metformin treated breast cancer cells involves activation of AMPK, downregulation of cyclin D1, and requires p27Kip1 or p21Cip1. J Mol Signal. 2008;3:18. doi: 10.1186/1750-2187-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shiota M, Yokomizo A, Kashiwagi E, et al. Peroxiredoxin 2 in the nucleus and cytoplasm distinctly regulates androgen receptor activity in prostate cancer cells. Free Rad Biol Med. 2011;51:78–87. doi: 10.1016/j.freeradbiomed.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 62.Guo Y, Ma SF, Grigoryev D, Van Eyk J, Barcia JG. 1-DE MS and 2-D LC-MS analysis of the mouse bronchoalveolar lavage proteome. Proteomics. 2005;5:4608–4624. doi: 10.1002/pmic.200500052. [DOI] [PubMed] [Google Scholar]
- 63.Gharib SA, Nguyen E, Altemeier WA, et al. Of mice and men: comparative proteomics of bronchoalveolar fluid. Eur Respir J. 2010;35:1388–1395. doi: 10.1183/09031936.00089409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang W, Gou L, Xie G, et al. Proteomic analysis of interstitial fluid in bone marrow identified that peroxiredoxin 2 regulates H2O2 level of bone marrow during aging. J Proteome Res. 2010;9:3812–3819. doi: 10.1021/pr901180w. [DOI] [PubMed] [Google Scholar]
- 65.Catterall JB, Rowan AD, Sarsfield S, Saklatvala J, Wait R, Cawston TE. Development of a novel 2D proteomics approach for the identification of proteins secreted by primary chondrocytes after stimulation by IL-1 and oncostatin M. Rheumatol (Oxford) 2006;45:1101–1109. doi: 10.1093/rheumatology/kel060. [DOI] [PubMed] [Google Scholar]
- 66.Chang JW, Jeon HB, Lee JH, et al. Augmented expression of peroxiredoxin I in lung cancer. Biochem Biophys Res Commun. 2001;289:507–512. doi: 10.1006/bbrc.2001.5989. [DOI] [PubMed] [Google Scholar]
- 67.Massagué J. TGFβ in cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 69.Bloom BR, Bennett B. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science. 1966;153:80–82. doi: 10.1126/science.153.3731.80. [DOI] [PubMed] [Google Scholar]
- 70.David JR. Delayed hypertensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc Natl Acad Sci USA. 1966;56:72–77. doi: 10.1073/pnas.56.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Conroy H, Mawhinney L, Donnelly SC. Inflammation and cancer: macrophage migration inhibitory factor (MIF)—the potential missing link. QJM. 2010;103:831–836. doi: 10.1093/qjmed/hcq148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Noels H, Bernhagen J, Weber C. Macrophage migration inhibitory factor: a noncanonical chemokine important in atherosclerosis. Trends Cardiovasc Med. 2009;19:76–86. doi: 10.1016/j.tcm.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 73.Kudrin A, Scott M, Martin S, et al. Human macrophage migration inhibitory factor: a proven immunomodulatory cytokine? J Biol Chem. 2006;281:29641–29651. doi: 10.1074/jbc.M601103200. [DOI] [PubMed] [Google Scholar]
- 74.Roger T, David J, Glauser MP, Clandra T. MIF regulates innate immune responses through modulation of Toll-like receptor 4. Nature. 2001;414:920–924. doi: 10.1038/414920a. [DOI] [PubMed] [Google Scholar]
- 75.Takahashi M, Nishihira J, Shimpo M, et al. Macrophage migration inhibitory factor as a redox-sensitive cytokine in cardiac myocytes. Cardiovasc Res. 2001;52:438–445. doi: 10.1016/s0008-6363(01)00408-4. [DOI] [PubMed] [Google Scholar]
- 76.Suzuki J, Jin ZG, Meoli DF, Matoba T, Berk BC. Cyclophilin A is secreted by a vesiclar pathway in vascular smooth muscle cells. Circ Res. 2006;98:811–817. doi: 10.1161/01.RES.0000216405.85080.a6. [DOI] [PubMed] [Google Scholar]
- 77.Satoh K, Shimokawa H, Berk BC. Cyclophilin A: promising new target in cardiovascular therapy. Circ J. 2010;74:2249–2256. doi: 10.1253/circj.cj-10-0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Geiben-Lynn R, Kursar M, Brown NV, et al. HIV-1 antiviral activity of recombinant natural killer cell enhancing factors, NKEF-A and NKEF-B, members of the peroxiredoxin family. J Biol Chem. 2003;278:1569–1574. doi: 10.1074/jbc.M209964200. [DOI] [PubMed] [Google Scholar]
- 79.Watanabe A, Yoneda M, Ikeda F, Sugai A, Sato H, Kai C. Peroxiredoxin 1 is required for the efficient transcription and replication of measles virus. J Virol. 2011;85:2247–2253. doi: 10.1128/JVI.01796-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sawaki J, Tsutsui H, Hayashi N, et al. Type 1 cytokine/chemokine production by mouse NK cells following activation of their TLR/MyD88-mediated pathways. Int Immunol. 2007;19:311–320. doi: 10.1093/intimm/dxl148. [DOI] [PubMed] [Google Scholar]
- 81.Krockenberger M, Domrowski Y, Weidler C, et al. Macrophage migration inhibitory factor contributes to the immune escape of ovarian cancer by down-regulating NKG2D. J Immunol. 2008;180:7338–7348. doi: 10.4049/jimmunol.180.11.7338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Apte RS, Sinha D, Mayhew E, Wistow GJ, Niederkorn JY. Cutting edge: role of macrophage migration inhibitory factor in inhibiting NK cell activity and preserving immune privilege. J Immunol. 1998;160:5693–5696. [PubMed] [Google Scholar]
- 83.Arcuri F, Cintorino M, Carducci A, et al. Human decidual natural killer cells as a source and target of macrophage migration inhibitory factor. Reproduction. 2006;131:175–182. doi: 10.1530/rep.1.00857. [DOI] [PubMed] [Google Scholar]
- 84.Repp AC, Mayhew ES, Apte S, Niederkorn JY. Human unveal melanoma cells produce macrophage migration-inhibitory factor to prevent lysis by NK cells. J Immunol. 2000;165:710–715. doi: 10.4049/jimmunol.165.2.710. [DOI] [PubMed] [Google Scholar]
- 85.Müller S. Redox and antioxidant systems of the malaria parasite Plasmodium falciparum. Mol Microbiol. 2004;53:1291–1305. doi: 10.1111/j.1365-2958.2004.04257.x. [DOI] [PubMed] [Google Scholar]
- 86.Deponte M, Rahlfs S, Becker K. Peroxiredoxin systems of protozoal parasites. Subcell Biochem. 2007;44:219–229. doi: 10.1007/978-1-4020-6051-9_10. [DOI] [PubMed] [Google Scholar]
- 87.Kehr S, Sturm N, Rahlfs S, Przyborski JM, Becker K. Compartmentation of redox metabolism in malaria parasites. PLoS Pathog. 2010;6:e1001242. doi: 10.1371/journal.ppat.1001242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kawazu S, Tsuji N, Hatabu T, Kawai S, Matsumoto Y, Kano S. Molecular cloning and characterization of a peroxiredoxin from the human malaria parasite Plasmodium faciparum. Mol Biochem Parasitol. 2000;109:165–169. doi: 10.1016/s0166-6851(00)00243-7. [DOI] [PubMed] [Google Scholar]
- 89.Kawazu S, Komaki K, Tsuji N, et al. Molecular characterization of a 2-Cys peroxiredoxin from the human malaria parasite Plasmodium falciparum. Mol Biochem Parasitol. 2001;116:73–79. doi: 10.1016/s0166-6851(01)00308-5. [DOI] [PubMed] [Google Scholar]
- 90.Rahlfs S, Becker K. Thioredoxin peroxidases of the malarial parasite Plasmodium falciparum. Eur J Biochem. 2001;268:1404–1409. doi: 10.1046/j.1432-1327.2001.02005.x. [DOI] [PubMed] [Google Scholar]
- 91.Sarma GN, Nickel C, Rahlfs S, Fischer M, Becker K, Karplus PA. Crystal structure of a novel Plasmodium falciparum 1-Cys peroxiredoxin. J Mol Biol. 2005;346:1021–1034. doi: 10.1016/j.jmb.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 92.Sztajer H, Gamain B, Aumann KD, et al. The putative glutathione peroxidase gene of Plasmodium falciparum codes for a thioredoxin peroxidase. J Biol Chem. 2001;276:7397–7403. doi: 10.1074/jbc.M008631200. [DOI] [PubMed] [Google Scholar]
- 93.Kawazu S, Komaki-Yasuda K, Oku H, Kano S. Peroxiredoxins in malaria parasites: parasitologic aspects. Parasitol Int. 2008;57:1–7. doi: 10.1016/j.parint.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 94.Richard D, Bartfai R, Volz J, et al. A genome-wide chromatin-associated nuclear peroxiredoxin from the malaria parasite Plasmodium falciparum. J Biol Chem. 2011;286:11746–11755. doi: 10.1074/jbc.M110.198499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grau GE, Taylor TE, Molyneux ME, et al. Tumor necrosis factor and disease severity in children with falciparum malaria. New Engl J Med. 1989;320:1586–1591. doi: 10.1056/NEJM198906153202404. [DOI] [PubMed] [Google Scholar]
- 96.Koncarevic S, Rohrbach P, Deponte M, et al. The malarial parasite Plasmodium falciparum imports the human protein peroxiredoxin 2 for peroxide detoxification. Proc Natl Acad Sci USA. 2009;106:13323–13328. doi: 10.1073/pnas.0905387106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Murphy SC, Samuel BU, Harrison T, et al. Erythrocyte detergent-resistant membrane proteins: their characterization and selective uptake during malarial infection. Blood. 2004;103:1920–1928. doi: 10.1182/blood-2003-09-3165. [DOI] [PubMed] [Google Scholar]
- 98.Sam-Yellowe TY. The role of the Maurer’s clefts in protein transport in Plasmodium falciparum. Trends Parasitol. 2009;25:277–284. doi: 10.1016/j.pt.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 99.Henkle-Duhrsekn K, Kampkötter A. Antioxidant enzyme families in parasitic nematodes. Mol Biochem Parasitol. 2001;114:129–142. doi: 10.1016/s0166-6851(01)00252-3. [DOI] [PubMed] [Google Scholar]
- 100.Donnelly S, O’Neil SM, Sekiya M, Mulcahy G, Dalton JP. Thioredoxin peroxidase secreted by Fasciola hepatica induces the alternative activation of macrophages. Infect Immun. 2005;73:166–173. doi: 10.1128/IAI.73.1.166-173.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dzik JM. Molecules released by helminth parasites involved in host colonization. Acta Biochim Pol. 2006;53:33–64. [PubMed] [Google Scholar]
- 102.Robinson MW, Menon R, Donnelly SM, Dalton JP, Ranganathan S. An integrated transcriptomics and proteomics analysis of the secretome of the helminth pathogen Fasciola hepatica: proteins associated with invasion and infection of the mammalian host. Mol Cell Proteomics. 2009;8:1891–1907. doi: 10.1074/mcp.M900045-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Siracusano A, Riganó R, Ortona E, et al. Immunomodulatory mechanisms during Echinococcus granulosus infection. Exp Parasitol. 2008;119:483–489. doi: 10.1016/j.exppara.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 104.Robinson MW, Hutchison AT, Dalton JP, Donnelly S. Peroxiredoxin: a central player in immune modulation. Parasite Immunol. 2010;32:305–313. doi: 10.1111/j.1365-3024.2010.01201.x. [DOI] [PubMed] [Google Scholar]
- 105.Donnelly S, Stack CM, O’Neil SM, Sayed AA, Williams DL, Dalton JP. Helminth 2-Cys peroxiredoxin drives Th2 responses through a mechanism involving alternatively activated macrophages. FASEB J. 2008;22:4022–4032. doi: 10.1096/fj.08-106278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kinnula VL, Lehtonen S, Kaarteenaho-Wiik R, et al. Cell specific expression of peroxiredoxins in human lung and pulmonary sarcoidosis. Thorax. 2002;57:157–164. doi: 10.1136/thorax.57.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fisher AB, Dodia C, Feinstein SI, Ho YS. Altered lung phospholipid metabolism in mice with targeted deletion of lysosomal-type phospholipase A2. J Lipid Res. 2005;46:1248–1256. doi: 10.1194/jlr.M400499-JLR200. [DOI] [PubMed] [Google Scholar]
- 108.Wang Y, Feinstein SI, Manevich Y, Ho YS, Fisher AB. Lung injury and mortality with hyperoxia are increased in peroxiredoxin 6 gene-targeted mice. Free Rad Biol Med. 2004;1:1736–1743. doi: 10.1016/j.freeradbiomed.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 109.Wang Y, Phelan SA, Manevich Y, Feinstein SI, Fisher AB. Transgenic mice overexpressing peroxiredoxin 6 show increased resistance to lung injury in hyperoxia. Am J Respir Cell Mol Biol. 2006;34:481–486. doi: 10.1165/rcmb.2005-0333OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hay J, Shahzeidi S, Laurent G. Mechanisms of bleomycin-induced lung damage. Arch Toxicol. 1991;65:81–94. doi: 10.1007/BF02034932. [DOI] [PubMed] [Google Scholar]
- 111.Wallach-Dayan SB, Izbicki G, Cohen PY, Gerstl-Golan R, Fine A, Breuer R. Bleomycin initiates apoptosis of lung epithelial cells by ROS but not by Fas/FasL pathway. Am J Physiol Lung Cell Mol Physiol. 2006;290:L790–L796. doi: 10.1152/ajplung.00300.2004. [DOI] [PubMed] [Google Scholar]
- 112.Kikuchi N, Ishii Y, Morishima Y, et al. Aggravation of bleomycin-induced pulmonary inflammation and fibrosis in mice lacking peroxiredoxin I. Am J Respir Cell Mol Biol. 2011;45:600–609. doi: 10.1165/rcmb.2010-0137OC. [DOI] [PubMed] [Google Scholar]
- 113.Inoue K, Takano H, Koike E, et al. Peroxiredoxin I is a negative regulator of Th2-dominant allergic asthma. Int Immunopharmacol. 2009;9:1281–1288. doi: 10.1016/j.intimp.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 114.Wynalda KM, Murphy RC. Low-concentration ozone reacts with plasmalogen glycerophosphoethanolamine lipids in lung surfactant. Chem Res Toxicol. 2010;23:108–117. doi: 10.1021/tx900306p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Thompson KC, Rennie AR, King MD, et al. Reaction of a phospholipid monolayer with gas-phase ozone at the air-water interface: measurement of surface excess and surface pressure in real time. Langmuir. 2010;26:17295–17303. doi: 10.1021/la1022714. [DOI] [PubMed] [Google Scholar]
- 116.Pulfer MK, Murphy RC. Formation of biologically active oxysterols during ozonolysis of cholesterol present in lung surfactant. J Biol Chem. 2004;279:26331–26338. doi: 10.1074/jbc.M403581200. [DOI] [PubMed] [Google Scholar]
- 117.Pulfer MK, Taube C, Gelfand E, Murphy RC. Ozone exposure in vivo and formation of biologically active oxysterols in the lung. J Pharmacol Exp Ther. 2005;312:256–264. doi: 10.1124/jpet.104.073437. [DOI] [PubMed] [Google Scholar]
- 118.Al-Hegelan M, Tighe RM, Castillo C, Hollingsworth JW. Ambient ozone and pulmonary innate immunity. Immunol Res. 2011;49:173–191. doi: 10.1007/s12026-010-8180-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hollingsworth JW, Kleeberger SR, Foster WM. Ozone and pulmonary innate immunity. Proc Am Thorac Soc. 2007;4:240–246. doi: 10.1513/pats.200701-023AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kleeberger SR, Reddy SP, Zhang LY, Cho HY, Jedlicka AE. Toll-like receptor 4 mediates ozone-induced murine lung hyperpermeability via inducible nitric oxide synthase. Am J Physiol Lung Cell Mol Physiol. 2001;280:L326–L333. doi: 10.1152/ajplung.2001.280.2.L326. [DOI] [PubMed] [Google Scholar]
- 121.Hollingsworth JW, 2nd, Cook DN, Brass DM, et al. The role of Toll-like receptor 4 in environmental airway injury in mice. Am J Respir Crit Care Med. 2004;170:126–132. doi: 10.1164/rccm.200311-1499OC. [DOI] [PubMed] [Google Scholar]
- 122.Williams AS, Leung SY, Nath P, et al. Role of TLR2, TLR4 and MyD88 in murine ozone-induced airway hyperresponsiveness and neutrophilia. J Appl Physiol. 2007;103:1189–1195. doi: 10.1152/japplphysiol.00172.2007. [DOI] [PubMed] [Google Scholar]
- 123.Jiang D, Liang J, Fan J, et al. Regulation of lung injury and repair by Toll-like receptor and hyaluronan. Nature Med. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 124.Li Z, Potts EN, Piantadosi CA, Foster WM, Hollingsworth JW. Hyaluronan fragments contribute to the ozone-primed immune response to lipopolysaccharide. J Immunol. 2010;185:6891–6898. doi: 10.4049/jimmunol.1000283. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 125.Taylor KR, Yamasaki K, Radek KA, et al. Recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on Toll-like receptor 4, CD44 and MD-2. J Biol Chem. 2007;282:18265–18275. doi: 10.1074/jbc.M606352200. [DOI] [PubMed] [Google Scholar]
- 126.Garantziotis S, Li Z, Potts EN, et al. TLR4 is necessary for hyaluronan-mediated airway hyperresponsiveness after ozone inhalation. Am J Respir Crit Care Med. 2010;181:666–675. doi: 10.1164/rccm.200903-0381OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 127.Samet JM, Noah TL, Devlin RB, et al. Effect of ozone on platelet-activating factor production in phorbol-differentiated HL60 cells, a human bronchial epithelial cell line (BEAS S6), and primary human bronchial epithelial cells. Am J Respir Cell Mol Biol. 1992;7:514–522. doi: 10.1165/ajrcmb/7.5.514. [DOI] [PubMed] [Google Scholar]
- 128.Morris GE, Parker LC, Ward JR, et al. Cooperative molecular and cellular networks regulate Toll-like receptor-dependent inflammatory responses. FASEB J. 2006;20:2153–2155. doi: 10.1096/fj.06-5910fje. [DOI] [PubMed] [Google Scholar]
- 129.Ma D, Warabi E, Yanagawa T, et al. Peroxiredoxin I plays a protective role against cisplatin cytotoxicity through mitogen activated kinase signals. Oral Oncol. 2009;45:1037–1043. doi: 10.1016/j.oraloncology.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 130.Aleksunes LM, Augustine LM, Scheffer GL, Cherrington NJ, Manautou JE. Renal xenobiotic transporters are differently expressed in mice following cisplatin treatment. Toxicology. 2008;250:82–88. doi: 10.1016/j.tox.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kang KW, Im YB, Go WJ, Han HK. C-Myc amplification altered the gene expression of ABC- and SLC-transporters in human breast epithelial cells. Mol Pharm. 2009;6:627–633. doi: 10.1021/mp800116f. [DOI] [PubMed] [Google Scholar]
- 132.Zhang B, Ramesh G, Uematsu S, Akira S, Reeves WB. TLR4 signaling mediates inflammation and tissue injury in nephrotoxicity. J Am Soc Nephrol. 2008;19:923–932. doi: 10.1681/ASN.2007090982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yanagawa T, Iwasa S, Ishii T, et al. Peroxiredoxin I expression in human thyroid tumors. Cancer Lett. 1999;145:127–132. doi: 10.1016/s0304-3835(99)00243-8. [DOI] [PubMed] [Google Scholar]
- 134.Yanagawa T, Iwasa S, Ishii T, et al. Peroxiredoxin I expression in oral cancer: a potential new tumor marker. Cancer Lett. 2000;156:27–35. doi: 10.1016/s0304-3835(00)00434-1. [DOI] [PubMed] [Google Scholar]
- 135.Yanagawa T, Omura K, Harada H, et al. Peroxiredoxin I expression in tongue squamous cell carcinoma as involved in tumor recurrence. Int J Oral Maxillofac Surg. 2005;34:915–920. doi: 10.1016/j.ijom.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 136.Kim HJ, Chae HZ, Kim YJ, et al. Preferential elevation of Prx I and Trx expression in lung cancer cells following hypoxia and in human lung cancer tissues. Cell Biol Toxicol. 2003;19:285–298. doi: 10.1023/b:cbto.0000004952.07979.3d. [DOI] [PubMed] [Google Scholar]
- 137.Kinnula VL, Lehtonen S, Sormunen R, et al. Overexpression of peroxiredoxins I, II, III, V and VI in malignant mesothelioma. J Pathol. 2002;196:316–323. doi: 10.1002/path.1042. [DOI] [PubMed] [Google Scholar]
- 138.Riddell JR, Bshara W, Moser MT, Spernyak JA, Foster BA, Gollnick SO. Peroxiredoxin 1 controls prostate cancer growth through Toll-like receptor 4-dependent regulation of tumor vasculature. Cancer Res. 2011;71:1–10. doi: 10.1158/0008-5472.CAN-10-3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.He W, Liu Q, Wang L, Chen W, Li N, Cao X. TLR4 signaling promotes immune escape of human cancer cells by inducing immunosuppressive cytokines and apoptosis resistance. Mol Immunol. 2007;44:2850–2859. doi: 10.1016/j.molimm.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 140.Szczepanski MJ, Czystowska M, Szajnik M, et al. Triggering of Toll-like receptor 4 expressed on human head and neck squamous cell carcinoma promotes tumor development and protects the tumor from immune attack. Cancer Res. 2009;69:3105–3113. doi: 10.1158/0008-5472.CAN-08-3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Szajnik M, Szczepanski MJ, Czystowska M, et al. TLR4 signaling induced by lipopolysaccharide or paclitaxel regulates tumor survival and chemoresistance in ovarian cancer. Oncogene. 2009;28:4353–4363. doi: 10.1038/onc.2009.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lokeshwar VB, Selzer MG. Hyaluronidase: both a tumor promoter and suppressor. Semin Cancer Biol. 2008;18:281–287. doi: 10.1016/j.semcancer.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Girish KS, Kemparaju K. The magic glue hyaluronan and its eraser hyaluronidase: a biological overview. Life Sci. 2007;80:1921–1943. doi: 10.1016/j.lfs.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 144.Wei Q, Jiang H, Xiao Z, et al. Sulfiredoxin-Peroxiredoxin IV axia promotes human lung cancer progression through modulation of specific phosphokinase signaling. Proc Natl Acad Sci USA. 2011;108:7004–7009. doi: 10.1073/pnas.1013012108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Giguère P, Turcotte ME, Hamelin E, et al. Peroxiredoxin-4 interacts with and regulates the thromboxane A2 receptor. FEBS Lett. 2007;581:3863–3868. doi: 10.1016/j.febslet.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 146.Chang XZ, Li DQ, Hou YF, et al. Identification of the functional role of peroxiredoxin 6 in the progression of breast cancer. Breast Cancer Res. 2007;9:R76. doi: 10.1186/bcr1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lehtonen ST, Svensk AM, Soini Y, et al. Peroxiredoxins, a novel protein family in lung cancer. Int J Cancer. 2004;111:514–521. doi: 10.1002/ijc.20294. [DOI] [PubMed] [Google Scholar]
- 148.Karihtala P, Mäntyniemi A, Kang SW, Kinnula VL, Soini Y. Peroxiredoxins in breast carcinoma. Clin Cancer Res. 2003;9:3418–3424. [PubMed] [Google Scholar]
- 149.Li DQ, Wang L, Fei F, et al. Identification of breast cancer metastasis-associated proteins in an isogenic tumor metastasis model using two-dimesional gel electrophoresis and liquid chromatography-ion trap-mass spectrometry. Proteomics. 2006;6:3352–3368. doi: 10.1002/pmic.200500617. [DOI] [PubMed] [Google Scholar]
- 150.Manevich Y, Fisher AB. Peroxiredoxin 6, a 1-Cys peroxiredoxin, functions in antioxidant defense and lung phospholipid metabolism. Free Radic Biol Med. 2005;38:1422–1432. doi: 10.1016/j.freeradbiomed.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 151.Nevalainen TJ. 1-Cysteine peroxiredoxin: a dual-function enzyme with peroxidase and acidic Ca2+-independent phospholipase A2 activities. Biochimie. 2010;92:638–644. doi: 10.1016/j.biochi.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 152.Liu G, Feinstein SI, Wang Y, et al. Comparison of glutathione peroxidase 1 and peroxiredoxin 6 in protection against oxidative stress in the mouse lung. Free Radic Bio Med. 2010;49:1172–1181. doi: 10.1016/j.freeradbiomed.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ho JN, Lee SB, Lee SS, et al. Phospholipase A2 activity of peroxiredoxin 6 promotes invasion and metastasis of lung cancer cells. Mol Cancer Ther. 2010;9:825–832. doi: 10.1158/1535-7163.MCT-09-0904. [DOI] [PubMed] [Google Scholar]
- 154.Qi HY, Shelhamer JH. Toll-like receptor 4 signaling regulates cytosolic phospholipase A2 activation and lipid generation in lipopolysaccharide-stimulated macrophages. J Biol Chem. 2005;280:38969–38975. doi: 10.1074/jbc.M509352200. [DOI] [PubMed] [Google Scholar]
- 155.Ruipèrez V, Astudillo AM, Balboa MA, Balsinde J. Coordinate regulation of TLR-mediated arachidonic acid mobilization in macrophages by group IVA and group V phospholipase A2s. J Immunol. 2009;182:3877–3883. doi: 10.4049/jimmunol.0804003. [DOI] [PubMed] [Google Scholar]
- 156.Wu CY, Chi PL, Hsieh HL, Luo SF, Yang CM. TLR4-dependent induction of vascular adhesion molecule-1 in rheumatoid arthritis synovial fibroblasts: roles of cytosolic phospholipase A(2)alpha/cyclooxygenase-2. J Cell Physiol. 2010;223:480–491. doi: 10.1002/jcp.22059. [DOI] [PubMed] [Google Scholar]
- 157.Wu Y, Feinstein SI, Manevich Y, et al. Mitogen-activated protein kinase-mediated phosphorylation of peroxiredoxin 6 regulates its phospholipase A2 activity. Biochem J. 2009;19:669–679. doi: 10.1042/BJ20082061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Neumann CA, Krause DS, Carman CV, et al. Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Nature. 2003;424:561–565. doi: 10.1038/nature01819. [DOI] [PubMed] [Google Scholar]
- 159.Cao J, Schulte J, Knight A, et al. Prdx1 inhibits tumorigenesis via regulating PTEN/AKT activity. EMBO J. 2009;28:1505–1517. doi: 10.1038/emboj.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang S, Yu D. PI(3)king apart PTEN’s role in cancer. Clin Cancer Res. 2010;16:4325–4330. doi: 10.1158/1078-0432.CCR-09-2990. [DOI] [PubMed] [Google Scholar]
- 161.Huo YY, Li G, Duan RF, et al. PTEN deletion leads to deregulation of antioxidants and increased oxidative damage in mouse embryonic fibroblasts. Free Radic Biol Med. 2008;44:1578–1591. doi: 10.1016/j.freeradbiomed.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 162.Asano T, Yao Y, Zhu J, Li D, Abbruzzese JL, Reddy SA. The PI 3-kinase/Akt signaling pathway is activated due to aberrant Pten expression and targets transcription factors NF-kB and c-Myc in pancreatic cancer cells. Oncogene. 2004;23:8571–8580. doi: 10.1038/sj.onc.1207902. [DOI] [PubMed] [Google Scholar]
- 163.Kim J, Eltoum IE, Roh M, Wang J, Abdulkadir SA. Interactions between cells with distinct mutations in c-Myc and Pten in prostate cancer. PLoS Genet. 2009;5:e1000542. doi: 10.1371/journal.pgen.1000542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Uwayama J, Hirayama A, Yanagawa T, et al. Tissue Prx I in the protection against Fe-NTA and the reduction of nitroxyl radicals. Biochem Biophys Res Commun. 2006;339:226–231. doi: 10.1016/j.bbrc.2005.10.192. [DOI] [PubMed] [Google Scholar]
- 165.Hotez PJ, Narasimhan S, Haggerty J, et al. Hyaluronidase from infective Ancylostoma hookworm larvae and its possible function as a virulence factor in tissue invasion and in cutaneous larva migrans. Infect Immun. 1992;60:1018–1023. doi: 10.1128/iai.60.3.1018-1023.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sironen RK, Tammi M, Tammi R, Auvinen PK, Anttila M, Kosma VM. Hyaluronan in human malignancies. Exp Cell Res. 2011;317:383–391. doi: 10.1016/j.yexcr.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 167.Itano N, Zhuo L, Kimata K. Impact of the hyaluronan-rich tumor microenvironment on cancer initiation and progression. Cancer Sci. 2008;99:1720–1725. doi: 10.1111/j.1349-7006.2008.00885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Joly AL, Wettstein G, Mignot G, Ghringhelli F, Garrido C. Dual role of heat shock proteins as regulators of apoptosis and innate immunity. J Innate Immun. 2010;2:238–247. doi: 10.1159/000296508. [DOI] [PubMed] [Google Scholar]
- 169.Pockley AG, Muthana M, Calderwood SK. The dual immunoregulatory roles of stress proteins. Trends Biochem Sci. 2008;33:71–79. doi: 10.1016/j.tibs.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 170.Calderwood SK, Mambula SS, Gray PJ, Jr., Theriault JR. Extracellular heat shock proteins in cell signaling. FEBS Lett. 2007;581:3689–3694. doi: 10.1016/j.febslet.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 171.Schmitt E, Gehrmann M, Brunet M, Multhoff G, Garrido C. Intracellular and extracellular functions of heat shock proteins: repercussions in cancer therapy. J Leukoc Biol. 2007;81:15–27. doi: 10.1189/jlb.0306167. [DOI] [PubMed] [Google Scholar]
- 172.Molvarec A, Tamasi L, Losonczy G, Madách K, Prohászka Z, Rigó J., Jr Circulating heat shock protein 70 (HSPA1A) in normal and pathological pregnancies. Cell Stress Chaperones. 2010;15:237–247. doi: 10.1007/s12192-009-0146-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nakamura H, Masutani H, Yodoi J. Extracellular thioredoxin and thoredoxin-binding protein 2 in control of cancer. Semin Cancer Biol. 2006;16:444–451. doi: 10.1016/j.semcancer.2006.09.001. [DOI] [PubMed] [Google Scholar]