Abstract
BACKGROUND
Single embryo transfer (SET) provides the most certain means to reduce the risk of multiple gestation. Regrettably, prospective trials of SET have demonstrated reductions in per-cycle delivery rates. A validated method of comprehensive chromosome screening (CCS) has the potential to optimize SET by transferring only euploid embryos. This retrospective study evaluates the efficacy of SET with CCS in an infertile population.
METHODS
Overall and age-controlled ongoing pregnancy rates (OPR) were compared between women undergoing SET following CCS (CCS-SET, n= 140) and those undergoing SET without aneuploidy screening (control SET, n= 182). All transfers were at the blastocyst stage, with CCS performed after trophectoderm biopsy of expanded blastocysts and analysis with rapid PCR allowing for fresh transfer.
RESULTS
In the CCS-SET and control SET groups, an OPR of 55.0 and 41.8%, respectively, was obtained. The OPR was lower for the control group (P< 0.01) despite a younger age than the CCS group (37.3 ± 3.4 versus 34.2 ± 3.9 years; P< 0.001). Birthweight and gestational age at delivery were equivalent. The proportion of clinical pregnancies resulting in miscarriage was higher in the control group (24.8 versus 10.5%, P< 0.01), with more patients requiring surgical interventions for aneuploid pregnancies. There was one monozygotic twin delivery in the CCS group and none in the control group.
CONCLUSIONS
Compared with traditional blastocyst SET, SET after trophectoderm biopsy and rapid PCR-based CCS increases OPR and reduces the miscarriage rate. The enhanced selection empowered by CCS with SET may provide a practical way to eliminate multi-zygotic multiple gestation without compromising clinical outcomes per cycle.
Keywords: single embryo transfer, IVF, comprehensive chromosome screening, multiple gestation, pregnancy rate
Introduction
Multiple gestation is the principal complication of IVF, causing increased maternal and neonatal morbidity compared with singleton pregnancies (Reddy et al., 2007). Though single embryo transfer (SET) has been recommended as the most certain way to minimize the risk of twins, the practice is still rare, accounting for only 12% of embryo transfers in the USA (Centers for Disease Control and Prevention, 2009). Furthermore, the recommendation for SET applies to young, good-prognosis patients, generally <35 years old (Practice Committee of the Society for Assisted Reproductive Technology and Practice Committee of the American Society for Reproductive Medicine, 2009). A recent meta-analysis of prospective studies comparing SET with double embryo transfer (DET) showed a significant decrease in live birth rates per transfer from SET (42.7 versus 26.3%, P< 0.00001) (Pandian et al., 2009) among good-prognosis patients. There is a paucity of data on the efficacy of SET in an older, poorer prognosis IVF population but the diminution in outcomes is likely to persist. Women over 35 years of age still have a significant risk of twin pregnancy when they do conceive after IVF or ICSI and they also stand to benefit from singleton pregnancies resulting from SET.
Aneuploidy is the leading cause of implantation failure and early miscarriage (Nasseri et al., 1999; Bettio et al., 2008; Scott et al., 2008); therefore, it is logical to propose that preimplantation genetic screening (PGS) can improve selection for SET. It is of paramount importance that the method of PGS is accurate and that the technique itself does not diminish the developmental potential of the individual embryo tested (Scott and Treff, 2010). Experience has shown that fluorescence in situ hybridization (FISH)-based PGS is inaccurate and not beneficial (Practice Committee of the Society for Assisted Reproductive Technology and Practice Committee of the American Society for Reproductive Medicine, 2007; Fritz, 2008). Using aneuploidy screening to aid in selection for SET has not been studied using the newer, validated methods of comprehensive chromosome screening (CCS) that can be performed at the blastocyst stage after trophectoderm biopsy, testing all 24 chromosomes (Treff et al., 2010). In this study, we compared outcomes from traditional blastocyst-stage SET (control SET) with outcomes after SET of a euploid blastocyst screened with PCR-based CCS (CCS-SET).
Materials and Methods
All SETs performed at Reproductive Medicine Associates of New Jersey, NJ, USA, between 1 January 2010 and 30 June 2011 were included in the analysis. Transfers of embryos originating from oocyte donation cycles were excluded. Patients who required preimplantation genetic diagnosis for single-gene defects were also excluded as this introduces an additional selection bias. Cycles in which previously frozen embryos were thawed for rapid PCR and same-day transfer were also excluded. Both elective and non-elective SETs were included, as were fresh and frozen SETs. Only the first SET during the time period was included for patients who had more than one SET performed.
In both groups, gonadotrophin dosing, stimulation and monitoring of the IVF cycle were per practice routine. Patients underwent controlled ovarian hyperstimulation (COH) generally using a step-down protocol, with a typical starting dose of 150–450 IU/day of recombinant FSH combined with either 75–150 IU/day of human menopausal gonadotrophins or 10–20 IU/day of low-dose hCG. The main methods of COH were GnRH agonist down-regulation during the luteal phase of the prior menstrual cycle or intracycle GnRH antagonist administration.
Day 3 FSH was determined on all patients prior to cycling. Although a value of 17 mIU/l or greater is considered abnormal in the Reproductive Medicine Associates of New Jersey laboratory, patients were not prevented from cycling if they had values above this range. Patients were only taken to oocyte retrieval if they had four or more mature follicles (≥14 mm diameter) on the day of hCG administration. Per laboratory routine, the entire cohort of embryos was placed in extended culture if there were at least four embryos on Day 3 of development with ≥4 cells and ≤30% embryo fragmentation. Patients who elected to perform CCS or elected to have SET had all embryos placed in extended culture, regardless of the number available on Day 3.
Patients were offered CCS if they were of advanced age (>35 years old), had a previous failed IVF cycle, had a history of recurrent pregnancy loss or they wanted to optimize outcomes with SET. The CCS technology was developed and offered clinically using an Institutional Review Board-approved protocol. Patients signed an informed consent form describing the methodology and limitations of CCS.
In the CCS group, all oocytes were fertilized with ICSI. On Day 3 of development, an infrared diode laser was used to create a small breech in the zona pellucida prior to placing the embryos in extended culture. Laser biopsy was then performed on all expanded blastocysts (Gardner grades 3–6) of sufficient quality by removing ∼5 trophectoderm cells that had herniated through the breech in the zona (Gardner and Schoolcraft, 1999). After lysis of the trophectoderm cells as previously described (Treff et al., 2009a,b), DNA was amplified and characterized using quantitative real-time PCR (qPCR) (Treff et al., 2012). Preamplification of 96 loci from embryo biopsy lysates was conducted in a 50 µl reaction volume containing 10 µl of lysate, 25 µl of TaqMan PreAmp Master Mix (Applied Biosystems, Foster City, CA, USA), 12.5 µl of primer pool (10 µM of each primer pair) and 2.5 µl of molecular biology grade water, and the following conditions; 10 min at 95°C, 18 cycles of 95°C for 15 s and 60°C for 4 min and held at 4°C, using an ABI 2700 thermalcycler. The PreAmp reaction was then diluted in 1.25 ml of Gene Expression Master Mix (Applied Biosystems) and 1.2 ml of molecular biology grade water. Four replicate 5 µl qPCR using each individual primer pair were then performed on an ABI 7900 thermalcycler as recommended by the manufacturer for relative quantification (Applied Biosystems). The copy number for all 22 pairs of autosomes and two sex chromosomes was assigned using standard methods of relative quantification (Schmittgen and Livak, 2008) where the average threshold cycle (CT) of all of the autosomes (excluding the chromosome under evaluation) served as the endogenous control data for the average CT of the four assays of each individual chromosome under evaluation (ΔCT), and known normal male sample results were used as the reference data to normalize ΔCT data from each embryo biopsy (ΔΔCT).
This PCR methodology demonstrated 98.6% accuracy in preclinical studies (Treff et al., 2009a,b) and improvement in clinical outcomes in preliminary results of an RCT (Scott et al., 2010a,b). Only embryos diagnosed as 46XX or 46XY with sufficient confidence were suitable for transfer or cryopreservation. The best euploid embryo, in terms of morphology, was selected for transfer in the CCS-SET group. Supernumerary euploid embryos were vitrified and warmed for subsequent frozen transfers. In the control SET group, the morphologically best quality embryo available was selected for transfer.
All transfers occurred at the blastocyst stage either in the afternoon of the fifth day or the morning of the sixth day of embryo development after oocyte retrieval. Fresh CCS-SETs all occurred on the morning of Day 6, as trophectoderm biopsies were performed on Day 5 with rapid PCR performed overnight. Frozen embryo transfers (FETs) included both natural and programmed cycles and were performed 5 days after ovulation or the initiation of a vaginal progesterone suppository (Endometrin, Ferring Pharmaceuticals; Parsippany, NJ, USA) or i.m. progesterone in oil. All transfers were performed in the same manner, using a soft Wallace transfer catheter and abdominal ultrasound guidance.
A pregnancy test was performed 8 or 9 days after transfer. Serial ultrasound monitoring was performed until 8–9 weeks gestation, at which time patients were discharged to obstetrical care. Follow up of all pregnancies was performed to determine live birth and ongoing pregnancy rates (OPR). A clinical pregnancy was defined as the presence of an intrauterine gestational sac on ultrasound. The OPR was defined as live births plus sustained pregnancies (beyond the first trimester) per embryo transferred.
Statistical analysis was performed using Open Epi Version 2.3.1 (www.openepi.com). Chi-square and Fisher's exact test was applied for categorical variables and unpaired t-test was applied for interval variables. Statistical significance was set at P < 0.05.
Results
In the CCS-SET group, there were 140 patients. In the control SET group, there were 182 patients. Patient demographics are summarized in Table I. Of note, the CCS-SET group was older (37.3 versus 34.2 years old, P< 0.001) and had more prior miscarriages and IVF cycles. Cycle-specific embryology outcomes are summarized in Table II.
Table I.
Patient demographics according to treatment group.
| Number of patients | Control SET | CCS-SET | |
|---|---|---|---|
| 182 | 140 | ||
| Age at retrieval (mean ± SD) | 34.2 ± 3.9 years | 37.3 ± 3.4 years | P< 0.001* |
| Maximal Day 3 FSH (mean ± SD) | 8.2 ± 2.8 mIU/ml | 8.1 ± 3.3 mIU/ml | P= 0.8* |
| Prior pregnancies (mean ± SD) | 1.4 ± 1.3 | 1.9 ± 1.7 | P= 0.003* |
| Prior deliveries (mean ± SD) | 0.7 ± 0.7 | 0.7 ± 0.9 | P> 0.9* |
| Prior miscarriages (mean ± SD) | 0.5 ± 0.8 | 0.8 ± 1.7 | P= 0.04* |
| Prior COH/IUI cycles (mean ± SD) | 0.7 ± 1.3 | 0.7 ± 1.5 | P> 0.9* |
| Prior IVF cycles (mean ± SD) | 1.1 ± 1.1 | 1.8 ± 1.6 | P< 0.001* |
| Prior FETs (mean ± SD) | 0.3 ± 0.7 | 0.3 ± 0.7 | P> 0.9* |
COH, controlled ovarian hyperstimulation; IUI, intrauterine insemination;
FET, frozen embryo transfer.
*t-test.
Table II.
Cycle outcomes according to treatment group.
| Control SET | CCS-SET | ||
|---|---|---|---|
| Estradiol on day of hCG (mean ± SD) | 2049 ± 1151 pg/ml | 2187 ± 1246 pg/ml | P= 0.3* |
| Oocytes retrieved (mean ± SD) | 14.9 ± 9.6 | 15.9 ± 10.0 | P= 0.4* |
| Number of 2PNs (mean ± SD) | 9.0 ± 6.5 | 9.9 ± 6.4 | P= 0.2* |
| Number of blastocysts (mean ± SD) | 3.9 ± 3.6 | 5.6 ± 3.8 | P< 0.01* |
| Cryopreserved blastocysts (mean ± SD) | 2.1 ± 2.8 | 3.1 ± 2.6 | P< 0.01* |
2PN, two pronuclei.
*t-test.
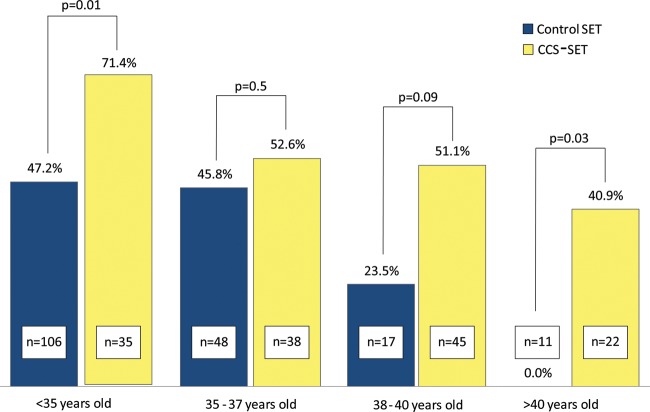
Pregnancy outcomes are summarized in Table III. The CCS-SET group demonstrated a higher OPR than the control SET group. Figure 1 shows OPR stratified by age group with the CCS-SET group demonstrating a higher OPR in the <35 year old and >40 year old categories and a trend toward higher OPR for age 38–40 years.
Table III.
Pregnancy outcomes by treatment group.
| Control SET | CCS-SET | ||
|---|---|---|---|
| Chemical pregnancy rate | 108/182 (59.3%) | 96/140 (68.6%) | P= 0.04* |
| Ongoing pregnancy rate | 76/182 (41.8%) | 77/140 (55.0%) | P< 0.01* |
| Clinical miscarriage rate | 25/101 (24.8%) | 9/86 (10.5%) | P< 0.01* |
| Monozygotic twin rate | 0/76 (0%) | 1/77 (1.3%) | P= 0.5† |
| Gestational age at delivery (weeks ± SD) | 38.9 ± 1.4 | 38.6 ± 2.0 | P= 0.4‡ |
| Birthweight (grams ± SD) | 3286 ± 522 | 3281 ± 525 | P> 0.9‡ |
*Chi-square.
†Fisher's exact test.
‡t-test.
Figure 1.
OPR by age group for control SET versus CCS-SET.
The clinical miscarriage rate was lower after CCS-SET than control SET (P< 0.01). Among patients with clinical miscarriages, 14 out of 25 patients in the control group required dilation and curettage (D&C) with 8 out of 11 villous samples demonstrating aneuploidy by conventional karyotyping. In contrast, three out of nine patients with a clinical miscarriage in the CCS-SET group required D&C. One villous specimen was found to have a de novo balanced translocation. The risk of a clinical pregnancy resulting in a D&C was reduced after CCS (13.9 versus 3.4%, P= 0.02).
To date, there have been 63 deliveries in the control group and 49 in the CCS-SET group. Birthweights between groups were equivalent (Table III). There was one delivery prior to 32 weeks gestation in each group and no major congenital anomalies have been reported to date in either group. There was one case of monozygotic twins delivered in the CCS-SET group and none in the control SET group.
The OPR for fresh transfers and FETs was similar in both groups (control SET 39.1 versus 44.4%, P= 0.4; CCS-SET 59.7 versus 51.8%, respectively, P= 0.4).
Elective SETs were defined based on the presence of additional blastocysts that were deemed suitable for cryopreservation. For the CCS-SET group, an elective SET implies the availability of another euploid blastocyst. In the CCS-SET, there was no difference in OPR between elective and non-elective SETs (54.6 versus 56.5%, respectively, P= 0.8), despite an older average age for patients who had non-elective SETs (36.6 versus 38.1 years, P= 0.01). Among the control group, elective SET resulted in higher OPRs than non-elective SET (54.5 versus 32.4%, respectively, P< 0.01), though the average age was significantly younger in the elective SET group (33.1 versus 34.9 years, P< 0.01).
All CCS-SETs were performed on Day 6 of embryo development because trophectoderm biopsy is only performed at the expanded blastocyst stage. In the control SET group, 62.6% of patients had transfers of blastocysts that had developed in culture to Day 6. There was no difference between groups in the proportion of transferred Day 6 blastocysts that were considered high quality with inner cell mass and trophectoderm grades ≥B (75.4% control SET versus 82.9% CCS-SET, P= 0.2). The CCS-SET group had 667 blastocysts biopsied for CCS, with 56.2% being euploid.
Discussion
In the current study, we have reviewed a consecutive series of 140 patients who had SET after the application of clinically indicated CCS. The OPR of 55.0% surpasses the cumulative live birth rate after DET in a recent Cochrane Review, despite an average age that is older than the maximum age enrolled in five of the six studies included for analysis (Pandian et al., 2009). In an attempt to assess the benefit of CCS-SET while correcting for variations in laboratory and other variables, we compared CCS-SET to all unscreened blastocyst SETs performed in the same programme during the same time period. The OPR was higher in the CCS-SET than the control SET group (55.0 versus 41.8%, respectively, P< 0.01) despite an older age and a history of more prior IVF cycles and miscarriages.
Combining CCS with SET resulted in higher OPRs for every age group, though owing to the sample size the difference was not statistically significant in the 35–37 and 38–40-year-old age groups. The miscarriage rate was significantly reduced by transferring euploid embryos, despite an older average age in the CCS-SET group. Furthermore, CCS-SET appears to reduce the risk of a clinical pregnancy resulting in a D&C for an abnormal gestation. Preventing abnormal pregnancies allows patients to resume treatment more rapidly and decreases the emotional and financial burden of IVF. Performing trophectoderm biopsy for CCS does not have an effect on the birthweight of singletons at delivery, regardless of maternal age. Transferring only euploid embryos appears to attenuate the age-related decline in live birth rates and maintains high pregnancy rates even when there are no other embryos from which to select.
With a retrospective analysis, it is difficult to determine the impact of trophectoderm biopsy with CCS on delivery rates per cycle initiated. Most patients utilizing CCS have a poor prognosis and would plan for a DET if two euploid blastocysts were available. However, during the same time period at the Reproductive Medicine Associates of New Jersey, 47 patients had cycles in which all blastocysts biopsied were aneuploid and thus a transfer could not be performed. This represented 8.3% of cycles with biopsy for CCS. These patients were 40.9 ± 2.9 years old, had 10.7 ± 5.8 oocytes retrieved and had 1.9 ± 1.0 blastocysts tested. If these 47 patients were included with the CCS-SET group, the OPR per biopsy cycle initiated would be 41.2% (77/187). While this is equivalent to the OPR for the control SET group of 41.8%, the age difference is accentuated between the groups (34.2 versus 38.2 years, P< 0.001).
It also must be noted that while low responders were included in this study, both groups demonstrated a good response in terms of oocytes retrieved. The number of oocytes retrieved and basal FSH were similar between groups, so differences in oocyte quantity or quality cannot explain the improvement in per-transfer OPR with CCS-SET. As expected given the average age in the CCS group, 43.8% of blastocysts tested with CCS were aneuploid. The transfer of untested blastocysts that were aneuploid likely explains the decreased OPR in the control SET group.
The last decade has seen a dramatic reduction in the incidence of higher-order multiple pregnancies, in large part related to the introduction of extended culture and a reduction in the number of embryos transferred per cycle. Nevertheless, the rate of IVF twin pregnancies in the USA remains essentially unchanged at ∼30% (Centers for Disease Control and Prevention, 2009). The cost of caring for preterm babies resulting from IVF multiple gestation is estimated at $1 billion annually in the USA (Bromer et al., 2011).
SET holds the promise of providing patients with a single healthy child as a result of each IVF cycle. However, there has been a persistent gap between live birth rates from SET and multiple embryo transfer. Prospective studies in good-prognosis patients have shown a lower pregnancy rate per transfer when employing SET. Although nearly equivalent pregnancy rates have been achieved when including an additional FET (Thurin et al., 2004), this introduces additional cost and time to achieve pregnancy. Many patients drop out of care because of the burden of treatment required by multiple cycles (Verberg et al., 2008). Owing to the difference in outcomes, most reproductive endocrinologists do not routinely offer SET to all patients (Jungheim et al., 2010). Through education and outreach a majority of good-prognosis patients can be persuaded to accept SET (Ryan et al., 2007) but many are reluctant to compromise outcomes.
Significant improvements in embryo selection—allowing the ability to better assess the reproductive potential of an individual embryo—are required before SET will be considered an acceptable option for all patients undergoing IVF. An RCT showed that extended culture improves SET live birth rates compared with cleavage-stage SET (Papanikolaou et al., 2006). With increasing female age, however, the rate of embryonic aneuploidy increases and a substantial proportion of morphologically normal blastocysts are chromosomally abnormal (Scott et al., 2010a,b). An additional selection criteria—beyond blastocyst morphology—is necessary to improve outcomes from SET and make it a more acceptable option to patients and physicians.
The concept of using PGS to optimize SET selection was previously attempted with FISH-based aneuploidy screening (Jansen et al., 2008; Staessen et al., 2008). Both studies were halted prematurely as there was no benefit in using FISH to optimize SET in patients <36 or 38 years old. These outcomes likely relate to inherent flaws in FISH-based PGS and do not necessarily invalidate the concept of using aneuploidy screening to improve selection for SET.
Over the past few years several new PGS methods have been introduced that offer CCS. In retrospective studies, these technologies have yielded excellent results in poor-prognosis patients (Schoolcraft et al., 2010, 2011). An ongoing prospective trial of PCR-based CCS revealed increased implantation rates after an interim analysis (Scott et al., 2010b).
Few studies have previously addressed SET in women of advanced reproductive age. One retrospective study found an OPR of 51.1% in 45 patients over age 35 years (Davis et al., 2008). This older population of women stands to benefit from singleton gestation and prospective data are lacking. Given the age-related increased incidence of aneuploidy, this group may benefit most from SET with CCS.
The present study, while suggestive of a benefit from CCS, has several limitations. Given the retrospective nature of this study, the two groups were not equivalent. Though older, the patients who utilized CCS had more embryos available for biopsy and cryopreservation. Patients in the control SET group who received non-elective SETs may represent a poor-prognosis group as they had only one embryo of sufficient quality to transfer. The groups being compared may have had different indications for performing SET. On average, both groups tended to be good responders with good-quality blastocysts from which to select. Patients without any blastocysts available for transfer would not be able to benefit from CCS-SET. Ongoing research is aimed at determining the impact of applying this treatment strategy more broadly to the infertility population.
It is becoming evident that increased application of SET will be required to reduce the incidence of multiple pregnancy and its complications. While some countries have instituted mandatory SET policies, pregnancy rates per transfer are reduced with this strategy and patients who shoulder the cost of their care remain reluctant to choose this option. The next major improvement in assisted reproduction technology (ART) will be to optimize SET so that high pregnancy rates are maintained and the option can be recommended to patients regardless of their age or previous reproductive history. While the results of this study are promising, a definite answer awaits a prospective comparison of CCS-SET versus contemporary transfer practice. Such a study is currently underway at the Reproductive Medicine Associates of New Jersey (NCT01408433). Should level I evidence demonstrate the efficacy of this approach, the result would be a paradigm shift in ART, with CCS-SET allowing for greater patient safety by avoiding multiple gestation while maintaining excellent outcomes and reducing the burden of care for patients and society.
Authors’ roles
E.J.F.: analyzed data and wrote majority of manuscript. N.R.T.: interpreted CCS data, assisted with writing and editing of manuscript. X.T.: assisted with CCS data. K.M.F.: performed embryo biopsies for CCS and selected embryos for transfer. D.T.: assisted with CCS data. R.T.S.: oversaw entire project, assisted with writing and editing of manuscript.
Funding
Funding to pay the Open Access publication charges for this article was provided by Reproductive Medicine Associates of New Jersey.
Conflict of interest
For R.T.S. grant/research support and speakers bureau: EMD Serono, Merck and Ferring Pharmaceutical Scientific Advisory Board, EMD Serono and Ferring Pharmaceuticals.
Acknowledgements
The authors acknowledge Carol A. Steinhart for compiling SART data, and the RMA Genetics Team for SART data entry and CCS data support.
References
- Bettio D, Venci A, Levi Setti PE. Chromosomal abnormalities in miscarriages after different assisted reproduction procedures. Placenta. 2008;29(Suppl. B):126–128. doi: 10.1016/j.placenta.2008.08.015. doi:10.1016/j.placenta.2008.08.015. [DOI] [PubMed] [Google Scholar]
- Bromer JG, Ata B, Seli M, Lockwood CJ, Seli E. Preterm deliveries that result from multiple pregnancies associated with assisted reproductive technologies in the USA: a cost analysis. Curr Opin Obstet Gynecol. 2011;23:168–173. doi: 10.1097/GCO.0b013e32834551cd. doi:10.1097/GCO.0b013e32834551cd. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. National Summary Report: Assisted Reproductive Technology (ART) Report. 2009. Atlanta: U.S. Department of Health and Human Services.
- Davis LB, Lathi RB, Westphal LM, Milki AA. Elective single blastocyst transfer in women older than 35. Fertil Steril. 2008;89:230–231. doi: 10.1016/j.fertnstert.2007.02.047. doi:10.1016/j.fertnstert.2007.02.047. [DOI] [PubMed] [Google Scholar]
- Fritz MA. Perspectives on the efficacy and indications for preimplantation genetic screening: where are we now? Hum Reprod. 2008;23:2617–2621. doi: 10.1093/humrep/den400. doi:10.1093/humrep/den400. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Schoolcraft WB. In vitro culture of human blastocysts. In: Jansen R, Mortimer D, editors. Towards Reproductive Certainty: Infertility and Genetics Beyond. Carnforth: Parthenon Press; 1999. [Google Scholar]
- Jansen RP, Bowman MC, de Boer KA, Leigh DA, Lieberman DB, McArthur SJ. What next for preimplantation genetic screening (PGS)? Experience with blastocyst biopsy and testing for aneuploidy. Hum Reprod. 2008;23:1476–1478. doi: 10.1093/humrep/den129. doi:10.1093/humrep/den129. [DOI] [PubMed] [Google Scholar]
- Jungheim ES, Ryan GL, Levens ED, Cunningham AF, Macones GA, Carson KR, Beltsos AN, Odem RR. Embryo transfer practices in the United States: a survey of clinics registered with the Society for Assisted Reproductive Technology. Fertil Steril. 2010;94:1432–1436. doi: 10.1016/j.fertnstert.2009.07.987. doi:10.1016/j.fertnstert.2009.07.987. [DOI] [PubMed] [Google Scholar]
- Nasseri A, Mukherjee T, Grifo JA, Noyes N, Krey L, Copperman AB. Elevated day 3 serum follicle stimulating hormone and/or estradiol may predict fetal aneuploidy. Fertil Steril. 1999;71:715–718. doi: 10.1016/s0015-0282(98)00525-1. doi:10.1016/S0015-0282(98)00525-1. [DOI] [PubMed] [Google Scholar]
- Pandian Z, Bhattacharya S, Ozturk O, Serour G, Templeton A. Number of embryos for transfer following in-vitro fertilisation or intra-cytoplasmic sperm injection. Cochrane Database Syst Rev (Online) 2009;2:CD003416. doi: 10.1002/14651858.CD003416.pub3. [DOI] [PubMed] [Google Scholar]
- Papanikolaou EG, Camus M, Kolibianakis EM, Van Landuyt L, Van Steirteghem A, Devroey P. In vitro fertilization with single blastocyst-stage versus single cleavage-stage embryos. N Engl J Med. 2006;354:1139–1146. doi: 10.1056/NEJMoa053524. doi:10.1056/NEJMoa053524. [DOI] [PubMed] [Google Scholar]
- Practice Committee of the Society for Assisted Reproductive Technology and Practice Committee of the American Society for Reproductive Medicine. Preimplantation genetic testing: a Practice Committee opinion. Fertil Steril. 2007;88:1497–1504. doi: 10.1016/j.fertnstert.2007.10.010. doi:10.1016/j.fertnstert.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Practice Committee of the Society for Assisted Reproductive Technology and Practice Committee of the American Society for Reproductive Medicine. Guidelines on number of embryos transferred. Fertil Steril. 2009;92:1518–1519. doi: 10.1016/j.fertnstert.2009.08.059. doi:10.1016/j.fertnstert.2009.08.059. [DOI] [PubMed] [Google Scholar]
- Reddy UM, Wapner RJ, Rebar RW, Tasca RJ. Infertility, assisted reproductive technology, and adverse pregnancy outcomes: executive summary of a National Institute of Child Health and Human Development workshop. Obstet Gynecol. 2007;109:967–977. doi: 10.1097/01.AOG.0000259316.04136.30. doi:10.1097/01.AOG.0000259316.04136.30. [DOI] [PubMed] [Google Scholar]
- Ryan GL, Sparks AE, Sipe CS, Syrop CH, Dokras A, Van Voorhis BJ. A mandatory single blastocyst transfer policy with educational campaign in a United States IVF program reduces multiple gestation rates without sacrificing pregnancy rates. Fertil Steril. 2007;88:354–360. doi: 10.1016/j.fertnstert.2007.03.001. doi:10.1016/j.fertnstert.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. NatProtocols. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. doi:10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Schoolcraft WB, Fragouli E, Stevens J, Munne S, Katz-Jaffe M, Wells D. Clinical application of comprehensive chromosomal screening at the blastocyst stage. Fertil Steril. 2010;94:1700–1706. doi: 10.1016/j.fertnstert.2009.10.015. doi:10.1016/j.fertnstert.2009.10.015. [DOI] [PubMed] [Google Scholar]
- Schoolcraft WB, Treff NR, Stevens JM, Ferry K, Katz-Jaffe M, Scott RT., Jr Live birth outcome with trophectoderm biopsy, blastocyst vitrification, and single-nucleotide polymorphism microarray-based comprehensive chromosome screening in infertile patients. Fertil Steril. 2011;96:638–640. doi: 10.1016/j.fertnstert.2011.06.049. [DOI] [PubMed] [Google Scholar]
- Scott RT, Jr, Treff NR. Assessing the reproductive competence of individual embryos: a proposal for the validation of new ‘-omics’ technologies. Fertil Steril. 2010;94:791–794. doi: 10.1016/j.fertnstert.2010.03.041. [DOI] [PubMed] [Google Scholar]
- Scott RT, Jr, Miller KA, Olivares R, Su J, Fratterelli J, Treff NR. Microarray based 24 chromosome preimplantation genetic diagnosis (mPGD) is highly predictive of the reproductive potential of human embryos:a prospective blinded non-selection trial. Fertil Steril. 2008;90:22. doi:10.1016/j.fertnstert.2008.07.438. [Google Scholar]
- Scott KL, Taylor D, Ferry KM, Treff NR, Scott RT., Jr Characterizing the relationship between morphologic embryonic development and ploidy status as assessed by 24 chromosome microarray PGD. Fertil Steril. 2010a;94:S122–S123. doi:10.1016/j.fertnstert.2010.07.498. [Google Scholar]
- Scott RT, Jr, Tao X, Taylor D, Ferry K, Treff N. A prospective randomized controlled trial demonstrating significantly increased clinical pregnancy rates following 24 chromosome aneuploidy screening: biopsy and analysis on day 5 with fresh transfer. Fertil Steril. 2010b;94:S2. doi:10.1016/j.fertnstert.2010.07.007. [Google Scholar]
- Staessen C, Verpoest W, Donoso P, Haentjens P, Van der Elst J, Liebaers I, Devroey P. Preimplantation genetic screening does not improve delivery rate in women under the age of 36 following single-embryo transfer. Hum Reprod. 2008;23:2818–2825. doi: 10.1093/humrep/den367. doi:10.1093/humrep/den367. [DOI] [PubMed] [Google Scholar]
- Thurin A, Hausken J, Hillensjo T, Jablonowska B, Pinborg A, Strandell A, Bergh C. Elective single-embryo transfer versus double-embryo transfer in in vitro fertilization. N Engl J Med. 2004;351:2392–2402. doi: 10.1056/NEJMoa041032. doi:10.1056/NEJMoa041032. [DOI] [PubMed] [Google Scholar]
- Treff N, Su J, Tao X, Miller K, Scott RT., Jr First IVF babies born after rapid 24 chromosome embryo aneuploidy screening and fresh embryo transfer. Fertil Steril. 2009a;92:S49. [Google Scholar]
- Treff NR, Su J, Tao X, Miller KA, Levy B, Scott RT., Jr A novel single-cell DNA fingerprinting method successfully distinguishes sibling human embryos. Fertil Steril. 2009b;94:477–484. doi: 10.1016/j.fertnstert.2009.03.067. doi:10.1016/j.fertnstert.2009.03.067. [DOI] [PubMed] [Google Scholar]
- Treff NR, Su J, Tao X, Levy B, Scott RT., Jr Accurate single cell 24 chromosome aneuploidy screening using whole genome amplification and single nucleotide polymorphism microarrays. Fertil Steril. 2010;94:2017–2021. doi: 10.1016/j.fertnstert.2010.01.052. doi:10.1016/j.fertnstert.2010.01.052. [DOI] [PubMed] [Google Scholar]
- Treff NR, Tao X, Ferry KM, Su J, Taylor D, Scott RT. Development and validation of an accurate quantitative real-time PCR based assay for human blastocyst comprehensive chromosomal aneuploidy screening. Fertil Steril. 2012 doi: 10.1016/j.fertnstert.2012.01.115. (in press) doi:10.1016/j.fertnstert.2010.01.052. [DOI] [PubMed] [Google Scholar]
- Verberg MF, Eijkemans MJ, Heijnen EM, Broekmans FJ, de Klerk C, Fauser BC, Macklon NS. Why do couples drop-out from IVF treatment? A prospective cohort study. Hum Reprod. 2008;23:2050–2055. doi: 10.1093/humrep/den219. doi:10.1093/humrep/den219. [DOI] [PubMed] [Google Scholar]