Abstract
Metastatic renal cell carcinoma (RCC) is a molecularly heterogeneous disease that is intrinsically resistant to chemotherapy and radiotherapy. While VEGF and mTOR targeted therapies have shown clinical activity, their effects are variable and short-lived, underscoring the need for improved treatment strategies for RCC. Here, we used quantitative phosphoproteomics and immunohistochemical profiling of 346 RCC specimens to determine that Src kinase signaling is elevated in RCC cells that retain wild type (WT) von Hippel-Lindau (VHL) protein expression. Correspondingly, VHL-WT RCC cell lines and xenografts were sensitized to the Src inhibitor dasatinib compared to VHL null cells. Forced expression of hypoxia inducible factor (HIF) in VHL-WT RCC cells diminished Src signaling output by repressing transcription of the Src activator protein tyrosine phosphatase 1B (PTP1B) and conferred resistance to dasatinib. Our results suggest that a HIF-regulated VHL-PTP1B-Src signaling axis determines sensitivity of RCC to Src inhibitors and that stratification of RCC patients using antibody-based biomarker profiling may identify patients likely to respond to Src inhibitors in RCC clinical trials.
Renal cell carcinoma (RCC) is the most lethal genitourinary cancer, accounting for approximately 209,000 new cancer occurrences and 102,000 deaths per year worldwide (1). Cure rates in RCC are modest since more than a quarter of patients have metastatic disease at presentation and patients treated surgically for localized cancers frequently relapse with metastatic disease (2, 3).
RCC is histologically heterogeneous. While ~75% of RCC are clear cell carcinomas, other RCC cell types include papillary, chromophobe, sarcomatoid, collecting duct and medullary carcinomas (4). Inactivation of the VHL tumor suppressor gene is the most prevalent driver mutation, accounting for ~60% of all RCC tumors, occurring primarily in the clear cell subtype (5, 6). VHL loss stabilizes HIF-1α and -2α, leading to increased expression of HIF-responsive genes, including VEGF-A, PDGF-B and TGF-A (6). HIF-dependent gene expression is further elevated by mTOR, thereby identifying several therapeutic targets in VHL-negative RCC (7). Indeed, drugs that target VEGF and mTOR show clinical activity in largely unselected patients with metastatic RCC, though these responses are often variable and short-lived (8, 9).
Unfortunately, despite the fact that VHL-positive cancers account for ~40% of RCC, these patients suffer from a lack of biologically rational treatment options due to a paucity of identified molecular drivers. Furthermore, patients with papillary RCC and other “non-clear cell” RCC are often excluded from many registration trials (10, 11), suggesting that identification of predictive biomarkers that stratify patients for rational treatment strategies are urgently required. Indeed, the profound ability of the Bcr-Abl inhibitor imatinib to successfully treat CML supports such an approach and has led to the development of targeted therapies for other cancers (12–14). But whereas targeted therapies are most effective in treating homogenous cancers driven by a single activating oncogene, they are much less effective in treating molecularly heterogeneous cancers such as RCC (8). Indeed, recent quantitative phosphoproteomic studies have revealed cancer to be a disease driven by aberrant networks rather than discrete signaling pathways (15, 16). This observation is exemplified by Src kinase, which despite its pivotal role in tumor growth, angiogenesis and metastasis, is rarely mutated in cancer. Rather, Src's signaling output is controlled posttranslationally by the convergent action of the lipid raft-localized inhibitory receptor tyrosine kinase Csk and by the activating tyrosine phosphatase PTP1B (17).
Here we report a personalized medicine approach for stratifying RCC patients based on the identification of a HIF-regulated VHL-PTP1B-Src signaling axis in patients with VHL-positive RCC. We performed a comprehensive phosphoproteomics analysis of genetically paired VHL-WT and VHL-null RCC cell lines and immunohistochemistry of 346 RCC tumors and identified a positive correlation between VHL, PTP1B and Src signaling output. Treatment with the tyrosine kinase inhibitor dasatinib blocked tumor growth in vivo, supporting the importance of this Src signaling axis in VHL-positive RCC. Our data suggest that stratifying RCC patients using biomarker profiling for expression of VHL and Src, as well as downstream effector molecules may identify patients likely to respond to Src inhibitors as a co- or mono-therapy in future RCC clinical trials.
RESULTS
Src is expressed in RCC and correlates with VHL expression
To identify cellular signaling networks differentially regulated in RCC subgroups, we performed quantitative phosphoproteomics on SN12C clear cell carcinoma cells, which retain VHL protein expression, and its isogenic subline, SN12C-shVHL, which have reduced VHL by shRNA knockdown (7, 18–20). Lysates from parallel cultures of serum-stimulated SN12C and SN12C-shVHL cells were labeled with iTRAQ 8-plex reagent and phosphotyrosine-containing peptides were subjected to immobilized metal affinity chromatography-tandem MS analysis (15). Quantitative phosphorylation profiles were generated for 22 phosphorylation sites while cluster analysis revealed a >50% reduction of pTyr at numerous phosphorylation sites in SN12C-shVHL lysates (Fig. 1A and table S1). Specifically, the proportion of pY419 autophosphorylated Src as well as several Src substrates, including annexin II, paxillin and inositol polyphosphate phosphatase-like 1 (INPPL1) were diminished in SN12C-shVHL lysates. The ability of serum to increase pTyr levels of Src substrates in SN12C cells but not SN12C-shVHL cells suggested VHL expression is a key determinant of Src kinase activity. Consistent with this possibility, in vitro kinase assays showed SN12C cells contained approximately twice as much dasatinib-sensitive Src kinase activity as did SN12C-shVHL cells (Fig. 1B). Together, these data suggest VHL may regulate Src kinase activity as well as its downstream signaling.
Figure 1. Src is expressed in RCC and is associated with poor outcome.
A. Lysates from parallel cultures of serum-stimulated SN12C and SN12C-shVHL cells were labeled with iTRAQ 8-plex reagent and phosphotyrosine-containing peptides were subjected to immobilized metal affinity chromatography-tandem MS analysis. Quantitative phosphorylation profiles were generated for 22 phosphorylation sites. Mean ratios to SN12C control were log transformed and partitioned according to similarity of phosphorylation status by unsupervised, hierarchical clustering using Cluster 3.0 (54) and visualized with TreeView (55). Heatmap is pseudo-colored to indicate direction and magnitude of mean ratios relative to SN12C control cells. SF, serum free; FBS, fetal bovine serum). See also table S1 and Methods.
B. Src was immunoprecipitated from SN12C and SN12C shVHL cells and Src kinase activity was measured in the absence or presence of 50 nM dasatinib as described in methods. Data are presented as the mean CPM ± S.D. from three independent experiments assayed in duplicate. (Lower panel) Corresponding western blot showing control (no primary antibody) or Src immunoprecipitates and relative Src expression in SN12C and SN12C-shVHL cells. The amount of Src was quantified with Image J and presented numerically as the fold-change.
C. Immunohistochemistry for Src from samples from 3 representative RCC patients with strong (left and center panels) or weak expression (right panel). Arrowheads indicate membranous localization. Scale bar, 20 μm.
D. Kaplan-Meier survival analysis of clear cell RCC patients with tumors expressing weak or strong Src immunohistochemical staining (n = 117, p = 0.0367).
As both the amount of total Src protein as well as its enzyme activity are implicated in cancer development (17, 21, 22), we analyzed a human RCC tissue microarray with samples from 215 patients for Src protein expression by immunohistochemistry (Fig. 1C). We found a significant positive association between total Src protein, which correlates with cytoplasmic staining, and active Src, which correlates with membranous staining (p=0.0185, table S2a). RCC patient samples with strong Src immunostaining had reduced survival when compared to those with weak expression (p=0.0367; Fig. 1D). In addition, multivariate analysis with stage (grouped as organ confined (pT1, 2) or advanced (pT3, 4)) and Fuhrman grade revealed that strong Src levels independently predicted against survival (p=0.02, table S2b). Since VHL loss is a dominant feature in RCC pathogenesis, we tested whether Src and VHL protein levels were associated. Indeed, Src positively correlated with the presence of VHL protein (p= 0.04; table S2c).
VHL-WT RCC cells are sensitive to dasatinib
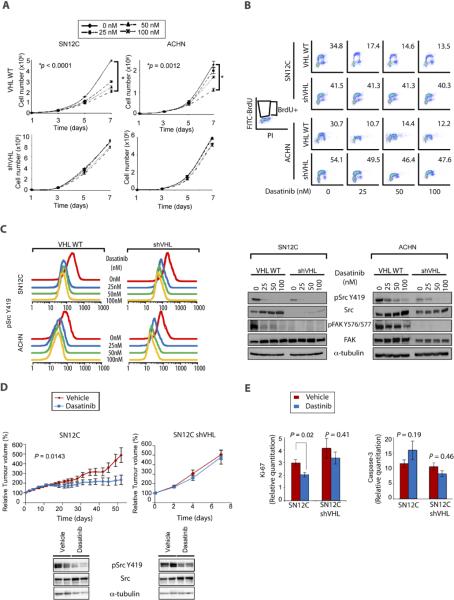
To evaluate whether VHL expression determined sensitivity to Src inhibitors, we treated SN12C and SN12C-shVHL cells as well as ACHN or ACHN-shVHL papillary RCC cells with dasatinib (7, 18–20). We found that dasatinib reduced proliferation of VHL-WT SN12C and ACHN cells but not their shVHL counterparts (Fig. 2A). The inhibition of proliferation by dasatinib correlated with an increase in G1 arrested cells and a corresponding decrease in S-phase cells as determined by propidium iodide (PI) staining (Table 1). Moreover, BrdU staining showed dasatinib caused a dose-dependent decrease in DNA synthesis in VHL-WT SN12C and ACHN cells but not their shVHL counterparts (Fig. 2B). No accumulation of a sub-G1 population was observed, suggesting dasatinib is cytostatic in the cell lines tested. Similar results were obtained using VHL-WT RXF-393 and Caki-1 RCC cells compared to VHL-null 786–0 cells (figs. S1 and S2). Correspondingly, ectopic expression of VHL in 786–0 cells conferred increased sensitivity to dasatinib (fig. S2).
Figure 2. Dasatinib induces growth arrest in VHL-WT RCC cells.
A. 5×104 VHL-WT (VHL+) SN12C and ACHN or shVHL cells were treated with vehicle, 25, 50 or 100 nM dasatinib 24h after-seeding. Effect of dasatinib on cell growth was monitored by cell count at indicated time points (n = 3). Data are presented as the mean ± S.D.
B. Sub-confluent SN12C and ACHN VHL-WT (VHL+) or shVHL cells were treated with the indicated doses of dasatinib for 48h and labeled with 10 μM BrdU for 30 min prior to harvesting. Cells were dual-stained with FITC-BrdU antibody and PI and analyzed by flow cytometry.
C. Sub-confluent SN12C and ACHN VHL-WT (VHL+) or shVHL cells were treated with vehicle, 25, 50 or 100 nM dasatinib. Inhibitory effect of dasatinib on Src kinase activity was assessed by flow cytometry using anti-pY419 Src (left panel). Levels of total and phospho-specific forms of Src and FAK were determined by immunoblotting. α-tubulin (right panel), loading control.
D. Nude mice bearing SN12C and SN12C shVHL xenografts were treated daily with vehicle or 10 mg/kg of dasatinib by oral gavage. Fold increase in tumor volume is plotted against days following tumor injection. Xenografts were analyzed by immunoblot for levels of pSrc Y419 and total Src. α-tubulin, loading control. Data are presented as the mean ± S.E.M. of six mice in each group.
E. Xenograft tumors from (D) were analyzed for cell proliferation and apoptosis by immunohistochemistry against Ki-67 and cleaved-caspase-3, respectively and subjected to quantitative image analysis. Data are presented as the mean ± S.E.M. (n = 11–21).
Table 1.
Cell cycle analysis of shVHL lines
| VHL WT | Dasatinib (nM) | shVHL | Dasatinib (nM) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 25 | 50 | 100 | 0 | 25 | 50 | 100 | |||
| SN12C | G1 | 52.4 | 59.5 | 63.6 | 70.0 | G1 | 45.0 | 45.2 | 45.4 | 46.1 |
| S | 30.9 | 23.9 | 22.8 | 16.3 | S | 35.6 | 36.9 | 38.5 | 35.3 | |
| G2/M | 15.4 | 15.0 | 11.7 | 11.7 | G2M | 19.1 | 17.5 | 15.7 | 18.5 | |
| VHL WT | Dasatinib (nM) | shVHL | Dasatinib (nM) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 25 | 50 | 100 | 0 | 25 | 50 | 100 | |||
| ACHN | G1 | 54.7 | 59.7 | 62.1 | 64.5 | G1 | 51.4 | 50.2 | 47.6 | 46.1 |
| S | 26.5 | 25.8 | 17.6 | 15.7 | S | 33.0 | 37.4 | 36.7 | 40.8 | |
| G2/M | 16.8 | 12.6 | 16.0 | 14.9 | G2/M | 13.9 | 9.8 | 14.2 | 10.9 | |
VHL-WT or shVHL SN12C and ACHN cell lines were treated with the indicated concentration of dasatinib and cell cycle profiles were examined by flow cytometry. Cell population (%) in each cell cycle phase was quantified.
Our determination that dasatinib inhibited proliferation of VHL-WT RCC cells prompted us to test whether dasatinib inhibited Src kinase activation and the phosphorylation of Src substrates. Indeed, flow cytometric and immunoblot analyses showed dasatinib reduced pY419 Src levels irrespective of VHL status (Fig. 2C). In agreement with the in vitro kinase assay (Fig. 1B), western blot analysis showed pY419 Src levels were higher in VHL-WT SN12C or ACHN cells compared to their shVHL counterparts. Dasatinib also caused total Src protein levels to increase regardless of VHL status (Fig. 2C and fig. S2). This dasatinib-induced increase in total Src protein has been observed in other tumor types as well as with other classes of Src inhibitors and is consistent with the increased stability of dephosphorylated Src in vivo (23–26). In addition to inhibiting pY419 Src, dasatinib inhibited phosphorylation of the Src substrate Fak in VHL-WT cells (Fig. 2C). Surprisingly, the levels of pY576, 577 Fak in VHL knockdown cells were undetectable despite the presence of total Fak protein. These results suggest how dasatinib may selectively inhibit proliferation of VHL-WT cells compared to their VHL-null or VHL-low counterparts.
To evaluate the effect of dasatinib on tumor growth in vivo, we implanted SN12C and SN12C-shVHL cells subcutaneously into the flanks of nude mice. Daily treatment with dasatinib significantly reduced the growth of VHL-WT SN12C cells but had no effect on SN12C-shVHL cells, recapitulating our in vitro findings (compare Figs. 2A and 2D). Dasatinib had no statistically significant effect on apoptosis in the xenograft tumors. Notably, administration of dasatinib resulted in a statistically significant reduction of Ki-67 positive proliferating SN12C cells but not SN12C-shVHL cells (Fig. 2E). Together, these results demonstrate that VHL-WT RCC cells are more sensitive than shVHL cells to dasatinib in xenograft tumors as well as in vitro and that this sensitivity is mediated through a blockade on proliferation.
Next, we asked whether the dasatinib-induced growth inhibitory effects on VHL-WT RCC cells were due to Src inhibition. Indeed, SN12C cells knocked down for Src (SN12C-shSrc) were resistant to dasatinib treatment (Fig. 3A). By contrast, rescue of SN12C-shSrc cells by expression of chicken Src, which is resistant to the human-specific shRNA (27), restored dasatinib sensitivity. Moreover, stable expression of a dasatinib-resistant Src encoding a T388I gatekeeper mutation, which prevents access of ATP-competitive inhibitors to the ATP binding pocket in Src, thereby protecting pY419 autophosphorylation, conferred resistance of SN12C cells to dasatinib (Fig. 3B) (27, 28). Similarly, expression of v-Src, which naturally expresses the T→I gatekeeper mutation (29), rendered VHL-WT SN12C and ACHN cells resistant to dasatinib (Fig. 3C and fig. S3). Accordingly, SN12C-vSrc xenograft tumors grown in SCID mice were resistant to dasatinib whereas parental SN12C tumors remained dasatinib-sensitive (Fig. 3D).
Figure 3. Src is the relevant target of dasatinib in RCC.
A. SN12C, SN12C-shSrc or SN12C-shSrc cells expressing v-Src (Rescue) were treated with vehicle, 25, 50 or 100 nM dasatinib for 96h and then cell growth was analyzed by cell count. Data are presented as the mean ± S.D. (n = 3). Src expression or knockdown was verified by immunoblot with antibodies against total and pY419 Src. α-tubulin, loading control.
B, C. SN12C cells (Control) or SN12C cells stably expressing (B) dasatinib-resistant Src (Src T338I), or (C) v-Src, were treated with vehicle alone or with 25 or 50 nM dasatinib for 96h and then cell growth was analyzed by cell count. Data are presented as the mean ± S.D. (n = 3). The levels of total Src and pY419 Src were assessed by immunoblot. α-tubulin and β-actin, loading controls.
D. SCID mice bearing SN12C and SN12C v-Src xenografts were treated daily with vehicle or 10 mg/kg of dasatinib by oral gavage. Percent (%) increase in tumor volume is plotted against days following tumor injection. Data are presented as the mean ± S.E.M. (n = 24).
Several controls supported our findings that dasatinib suppressed proliferation in VHL-WT cells by inhibiting Src. First, SN12C cells expressing the BCR-ABL T315I gatekeeper mutant were sensitive to dasatinib, suggesting the dasatinib resistance mediated by Src T388I or v-Src was specific (fig. S4). Secondly, treatment with imatinib, which inhibits ABL, PDGFR, and c-KIT but not Src, had no effect on the proliferation of SN12C or SN12C-shVHL cells (fig. S5a). Finally, saracatinib, a structurally unrelated Src inhibitor (30), repressed proliferation of control SN12C cells but not shVHL cells (Fig. S5b).
Constitutively stabilized HIF confers resistance to dasatinib in VHL-WT cells
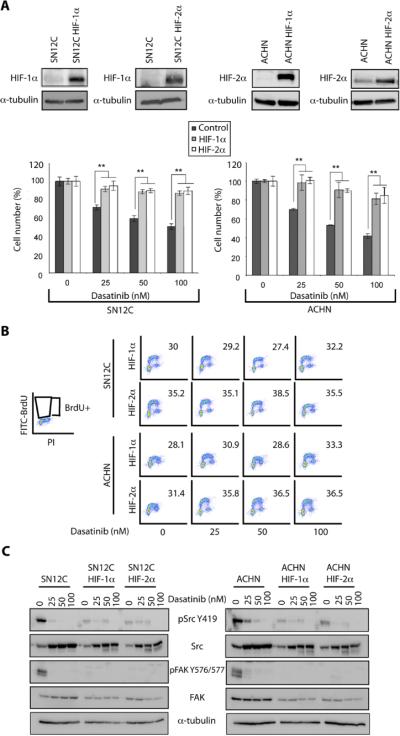
Since the E3 ligase activity of VHL negatively regulates HIF, we asked whether expression of constitutively stable HIF would phenocopy VHL loss by conferring resistance to dasatinib in VHL-WT RCC cells. Indeed, SN12C and ACHN cells expressing constitutively stable HIF-1α-P564A or HIF-2α-P405A,P853A mutants (31) contained reduced levels of Src mRNA and were resistant to the dasatinib-mediated G1 arrest observed in parental SN12C and ACHN cells (Fig. 4A–C, fig. S6 and Table 2). Correspondingly, constitutively stable HIF-1α and HIF-2α inhibited Src signaling output in VHL-WT RCC cells as determined by immunoblot of pY419 Src and phosphorylated Src substrates, including pY576, 577FAK, pY703STAT3 and pY204ERK (Fig. 4D). Conversely, ectopic expression of VHL in VHL-null 786-0 RCC cells resulted in an increase in both total and pY419 Src as well as the phosphorylation and activation of its downstream targets when compared to the parental cells (figs. S7 and S2). Together, these results suggest that HIF represses VHL-mediated Src signaling output.
Figure 4. HIF-α and PTP1B are involved in dasatinib-induced growth inhibition.
A. SN12C and ACHN cells stably expressing mutant HIF-1α (P564A) or HIF-2α (P405A; P853A) were treated with vehicle, 25, 50 or 100 nM dasatinib for 96h and then cell growth was analyzed by cell count. Data are presented as the mean ± S.D. (n = 3, **p<0.01). Overexpression of the mutant forms of HIF-α were validated by immunoblot. α-tubulin, loading control.
B. SN12C and ACHN cells stably expressing mutant HIF-1α (P564A) or HIF-2α (P405A; P853A) were treated with vehicle, 25, 50 or 100 nM dasatinib for 48h and then analyzed for BrdU incorporation by flow cytometry as described in Methods.
C. SN12C and ACHN cells stably expressing constitutively stable HIF-1α P564A (SN12C HIF-1α) or HIF-2α P405A; P853A (SN12C HIF-2α) were treated with vehicle alone or with, 25, 50 or 100 nM dasatinib for 18h. Levels of total Src and FAK as well as pSrc Y419 and pFAK Y576/577 were determined by immunoblot. α-tubulin, loading control.
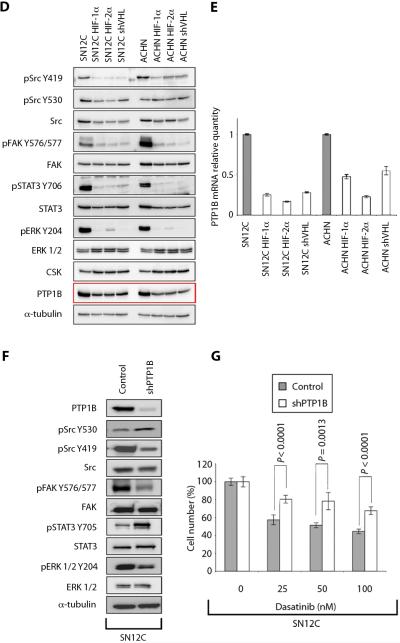
D. Lysates from the SN12C and ACHN mutant HIF-α overexpressing lines, shVHL cells and the parental cell lines were examined for expression of total and/or phospho-specific forms of Src, FAK, ERK1/2, STAT3, CSK and PTP1B by immunoblot. α-tubulin, loading control.
E. The levels of PTP1B mRNA were measured by real-time PCR in SN12C and ACHN HIF-α overexpressing and shVHL cell lines. Levels of PTP1B mRNA in the parental cell lines were normalized to 1. Data are presented as the mean ± S.D. (n = 3).
F. SN12C cells expressing an shRNA targeting PTP1B (shPTP1B) were analyzed by immunoblot for expression levels of total and/or phospho-specific forms of PTP1B, Src, FAK, STAT3, ERK1/2 and α-tubulin.
G. SN12C or shPTP1B cells were treated with vehicle, 25, 50 or 100 nM dasatinib and cell growth was assessed by cell count. Data are presented as the mean ± S.D. (n = 3).
Table 2.
Cell cycle analyses of HIF-α mutant cell lines
| HIF-1α | Dasatinib (nM) | HIF-2α | Dasatinib (nM) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 25 | 50 | 100 | 0 | 25 | 50 | 100 | |||
| SN12C | G1 | 58.3 | 60.1 | 59.2 | 60.4 | G1 | 56.4 | 54.8 | 55.7 | 49.7 |
| S | 26.7 | 25.1 | 27.6 | 26.5 | S | 20.9 | 23.6 | 21.4 | 27.8 | |
| G2/M | 13.3 | 13.3 | 11.4 | 11.1 | G2/M | 21.5 | 20.6 | 22.1 | 21.3 | |
| HIF-1α | Dasatinib (nM) | HIF-2α | Dasatinib (nM) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 25 | 50 | 100 | 0 | 25 | 50 | 100 | |||
| ACHN | G1 | 57.3 | 59.2 | 56.4 | 60.1 | G1 | 59.4 | 53.9 | 51.9 | 55.3 |
| S | 21.6 | 21.6 | 25.1 | 17.6 | S | 20.8 | 27.7 | 27.0 | 24.2 | |
| G2/M | 17.7 | 17.1 | 16.0 | 22.0 | G2/M | 17.5 | 16.1 | 19.3 | 17.7 | |
Cell cycle profiles of SN12C and ACHN cells expressing constitutively stable HIF-1α-P564A (HIF-1α) or HIF-2α-P405A, P853A (HIF-2α) mutants were analyzed by flow cytometry 48h post treatment with dasatinib. Cell population (%) in each cell cycle phase was quantified.
The ability of constitutively stable HIF mutants to promote dasatinib resistance and repress Src signaling output suggested HIF may repress an activator of Src activity. Consistent with this possibility, expression of PTP1B protein and mRNA, which activates Src by dephosphorylation of Y530, was consistently lower in shVHL cells or RCC cells that ectopically express constitutively stable HIF (Figs. 4D and E). Correspondingly, PTP1B protein was decreased in VHL-null 786-0 cells compared to VHL-restored 786-0-VHL cells (fig. S7). Biochemical analysis of Src signaling output suggested PTP1B knockdown phenocopied expression of constitutively stable HIF in VHL-WT cells. SN12C-shPTP1B cells contained reduced pY419 Src and the levels of phosphorylated Src substrates, including pY576, 577FAK and pY204ERK (Fig. 4F) and were relatively resistant to dasatinib-mediated growth inhibition (Fig. 4G). Only pY703STAT3 was unaffected by PTP1B knockdown, which may result from a PTP1B-specific effect on STAT3 and its regulator, JAK (32). As a control, SN12C cells were exposed to hypoxia in vitro or in xenograft tumors. We found that HIF was stabilized but PTP1B was unaffected, suggesting HIF-regulated PTP1B expression may be different under hypoxic conditions (fig. S8).
Consistent with the reduced activation of Src, PTP1B knockdown cells were less sensitive to dasatinib compared to control cells (Fig. 4G and fig. S9). By contrast, overexpression of the Src regulator Csk in SN12C cells had no effect on dasatinib sensitivity or Src signaling output (fig. S10), which is consistent with work by others that Csk phosphorylation of Src pY530 is complex ((33–37) and see discussion). Together, these results suggest that sensitivity to dasatinib appears to correlate with the capacity to inhibit Src's signaling output.
In addition to repressed levels of PTP1B protein, RCC cells with VHL knockdown or expression of constitutively stable HIF had reduced levels of PTP1B mRNA (Fig. 4E), suggesting HIF may repress PTP1B transcription. In support of this finding, chromatin immunoprecipitation (ChIP) revealed that HIF is enriched at a putative hypoxia response element in the PTP1B promoter in ACHN-cells expressing constitutively stable HIF-1α-P564A but not in parental ACHN cells (fig. S11). This result suggests that HIF-mediated transcriptional regulation of the PTP1B gene contributes to the repression of Src signaling output in VHL-null RCC cells.
Interaction of VHL, HIF, PTP1B and Src in RCC patients
Our identification of a HIF-regulated VHL-PTP1B-Src signaling axis in RCC cell lines provided us with additional markers to rigorously interrogate the presence of this pathway in RCC patients. We constructed a tissue microarray from a second cohort of 131 patients with RCC and performed immunohistochemistry for VHL, HIF-2α, which is the primary driver in VHL-null RCC (38), Src and PTP1B. As controls, we analyzed the HIF transcriptional target, CA-IX, as well as the Src substrate pFAK. Quantification was performed with automated digital image analysis algorithms to rigorously and systematically measure staining intensity (Fig. 5A). An unsupervised hierarchical clustering of the tumors on the basis of the expression of VHL, Src, pFAK and PTP1B was used to generate a heatmap (fig. S12). VHL, Src and PTP1B showed the most similar expression patterns while pFAK and VHL expression were similar, despite pFAK expression being generally lower than the other markers.
Figure 5. Demonstration of inter-relationships between VHL, HIF-α, Src and PTP1B in RCC patients.
A. Quantitative assessment of VHL, PTP1B, Src and HIF-2α expression by immunostaining of RCC TMA. Representative staining images from a patient with strong VHL protein expression (top panel) and from a patient with weak VHL expression (bottom panel) are shown. Corresponding markup images of the color deconvolution algorithm with intensity ranges are shown (Red = strong, orange = moderate, yellow = weak, blue = negative immunoreactivity). For HIF-2α, the nuclear immunostaining algorithm was applied. Scale bar is 50 μm.
B. Spearman Rho correlation coefficients among the biomarkers are listed in the boxes. Red indicates positive correlation and blue indicates negative correlation. P values for these correlations are represented as follows: *p<0.05; **p<0.001; ***p<0.0001 (n = 136).
C. Comparison between Src and VHL protein expression in the RCC tissue microarray
D. Scatter plot of the VHL and Src scores generated from automated image analysis intensity algorithm. The vertical lines represent 5th percentile and median VHL scores, corresponding to thresholds for negative and weak expression, respectively. The horizontal line represents the median for the Src score, where levels below are considered weak expression and levels above are considered strong expression. The upper right (shaded) quadrant depicts the molecular phenotype of tumors with both strong VHL and strong Src expression.
In agreement with the initial RCC clinical dataset (Table S2a), a Spearman rank correlation test of the second RCC clinical dataset again revealed a positive correlation between VHL and Src (r=0.409; p<0.001; Fig 5B). Using these more stringent analyses, only 8% (1/12) of VHL negative tumors had strong Src expression, while 58% (69/119) of VHL strong tumors had strong Src expression, suggesting a highly correlative relationship between VHL and SRC (p=0.0018; Figure 5C and table S3). Conversely, the relationship between VHL and HIF-2α revealed a significant negative correlation (r=−0.132; p=0.036). In agreement with our in vitro findings, PTP1B positively correlated with VHL (r=0.293; p<0.001) but negatively correlated with HIF-2α (r=−0.212; p=0.001), suggesting patient tumors with VHL loss or HIF-2α overexpression may have reduced PTP1B expression. Controls showed positive correlations between HIF-2α and CA-IX and between Src and pFAK as expected (Fig. 5B). A multiple linear regression showed VHL (p <0.0001) and PTP1B (p < 0.0001) to be predictors of Src expression. Additionally, VHL (p < 0.0001) and HIF-2α (p= 0.0021) were independent predictors of PTP1B levels (Table 3). Next, we extracted the datapoints and organized them into a scatter plot representing patient subgroups defined by VHL and Src expression (Fig. 5D). Indeed, 28.6% of the patients were VHL-strong/Src-strong, representing the potential candidates for a prospective phase II clinical trial with dasatinib (table S3).
Table 3.
Multiple Linear Regression
| Outcome and Covariates | Estimated Coefficient | 95% CI | P |
|---|---|---|---|
| Src | |||
| VHL | 0.146 | 0.096–0.196 | 2.55e-08 |
| PTP1B | 0.257 | 0.155–0.359 | 1.20e-06 |
| Intercept | 2.382 | 1.987–2.778 | <2e-16 |
| PTP1B | |||
| VHL | 0.164 | 0.104–0.225 | 2.11e-07 |
| HIF-2α | −0.228 | (−0.373)–(−0.084) | 0.00211 |
| Intercept | 4.298 | 3.796–4.800 | <2e-16 |
Coefficient estimates for two predefined models PTP1B and Src. All variables have been log transformed.
Next, we tested whether this RCC immunohistochemistry profile could be applied to other cancers to predict sensitivity to dasatinib. Using a clinical dataset of transitional cell carcinomas of the bladder, we found the same correlations between VHL, Src, HIF-2α and PTP1B (fig. S13). Taken together, these findings suggest that the immunophenotype of the VHL-PTP1B-Src signaling axis comprises a biomarker signature that not only defines a biologically distinct subgroup of RCC that may benefit from dasatinib or similar Src inhibitors but also points to a wider clinical applicability for these predictive biomarkers in identifying sensitivity to Src inhibitors.
We then explored the cooperating events involved in mediating sensitivity to dasatinib by applying a systems-based approach to map the potential protein-protein interactions, transcriptional information and the signaling networks they impact by using the ROCK-BCFG database (39). We seeded the interaction network searches with targets identified in our experiments and defined a protein interaction network containing 82 nodes that suggest an underlying signaling network involving Src, PTP1B, CA-IX, FAK and VHL together with the transcriptional regulators HIF-1α, HIF2-α and Sp1 (fig. S14).
DISCUSSION
While targeted therapies have been remarkably successful in treating cancers driven by the activation of a single oncogene, these drugs are much less effective in molecularly heterogeneous cancers driven by a multitude of dysregulated signaling networks (16). Successful treatment therefore requires a personalized medicine approach based on robust predictive biomarkers that can stratify patients toward appropriate targeted therapies. Here, we used a quantitative phosphoproteomic screen to identify Src as a potential pharmacologic target in metastatic RCC. Immunohistochemistry of 346 human RCC tumors identified a positive correlation between Src and VHL expression while treatment of VHL-WT xenografts with dasatinib blocked tumor growth in vivo. Conversely, forced expression of HIF, which phenocopied VHL loss, diminished Src's signaling output by downregulation of PTP1B, thereby conferring resistance to dasatinib. HIF binds the PTP1B promoter and reduces PTP1B expression, suggesting HIF controls Src signaling output by regulating PTP1B transcription. Our data suggest that stratifying RCC patients using biomarker profiling for expression of VHL and Src, as well as downstream effector molecules may identify patients likely to respond to Src inhibitors in future RCC clinical trials.
Despite playing a central role in multiple tumorigenic signaling networks, Src itself is rarely mutated in cancers (17). Our data suggest that one mechanism by which tumor cells amplify Src kinase activity is by utilizing gene-autonomous drivers such as PTP1B to dephosphorylate the kinase autoinhibitory domain. The ability of PTP1B knockdown to confer resistance to dasatinib (Fig. 4G and fig. S9) suggests PTP1B may augment Src signaling in RCC cells by channeling inputs from upstream oncogenes, including Ras (40). Unlike PTP1B knockdown, overexpression of Csk did not alter Src pY419 status (fig. S10), suggesting the regulation of Src activation by Csk is complex. Our findings are consistent with reports that Csk phosphorylation of Src Y530 requires interaction of Csk with Csk binding protein (Cbp) in lipid rafts (33–37), suggesting that analysis of Src pY419 or pY530 levels by immunoblot is insufficient to detect minute or compartment-specific changes in Src activation. Our finding that hypoxia-induced stabilization of HIF failed to affect PTP1B expression is in agreement with the HIF-dependent but hypoxia-independent regulation of Ror2 ((fig. S8) and (41)), and is consistent with the model that HIF-mediated inhibition of PTP1B requires that HIF be constitutively stabilized by VHL loss and not by fluctuating O2 levels present in VHL-WT tumors (42). Finally, while our studies cannot exclude the possibility that dasatinib may mediate its effects by inhibiting additional Src family kinases, they nonetheless highlight the therapeutic utility of a pan-SFK inhibitor such as dasatinib to block tumor growth.
Successful implementation of targeted therapies in molecularly heterogeneous cancers requires robust predictive biomarkers. The development of EGFR mutation analysis for stratification of patients with non-small cell lung cancer to EGFR inhibitors supports the feasibility of this approach (43). Therefore, our initial examination of VHL and Src on routinely processed human RCC samples assessed the clinical significance of Src expression. Indeed, RCC patient samples with strong Src expression had a statistically significant reduced overall survival when compared to those with weak expression. This analysis also revealed a positive correlation between VHL and Src using a semi-quantitative scoring protocol that biased toward sensitivity relative to specificity. We then more rigorously interrogated the VHL-Src relationship with enhanced specificity by analyzing VHL, Src, as well as their downstream effector molecules in a second cohort of human RCC tumors. This analysis used unbiased digital image analysis algorithms to objectively quantify staining intensities and to determine correlations between these molecules. Indeed, the VHL-Src relationship was one of the strongest correlations found. In addition, the relationships among VHL, Src, HIF-2α, PTP1B, pFAK and CA-IX were strongly reflected in patient tumors, consistent with our in vitro results. The presence of these associations in clinical samples reveals the strength of the molecular networks identified and supports the testing of these markers in future clinical trials.
Inactivation of the VHL tumor suppressor gene is the most prevalent driver mutation in RCC, accounting for ~60% of all tumors (5, 6). Thus, despite the fact that ~40% of RCC patients have VHL-positive cancer, they are treated as VHL-negative cancers. Unfortunately, the absence of biomarker-driven treatment protocols in RCC together with the fact that VHL-positive patients are excluded from many registration trials precludes meaningful understanding of the mechanisms of response or resistance. Thus, the singular approach currently used to treat RCC underscores the need for rational treatment strategies for VHL-positive RCC. Our findings suggest that Src inhibition may represent a rational treatment option in renal cancers that have retained VHL protein expression. Additionally, analyzing functional readouts of VHL and Src activity by means of HIF, CA-IX and pFAK expression would enhance specificity since functional VHL would confer low HIF and CA-IX expression while elevated Src signaling output would correlate with increased pFAK levels. While the ideal treatment subgroup would include those tumors that are VHL-strong, Src-strong, pFAK-strong, HIF-weak, and CA-IX-weak, the most effective biomarker combination can only be determined from future clinical studies in which outcomes following Src inhibitor treatment are known. Indeed, since Src inhibitors such as dasatinib and saracatinib have been clinically tested, our data suggest that these biomarker analyses may be rapidly translated to a phase II clinical trial in patients with metastatic RCC.
Collectively, our results suggest a fundamental change in RCC treatment is warranted. Specifically, patients should be selected upfront based on the presence of a molecular phenotype. The simplicity of our approach lies in two elements: using immunohistochemical-based assays on routinely processed clinical samples and the targeting of a well-characterized oncogene for which there already exists clinically active drugs. The key challenges ahead are assessing intratumor heterogeneity and standardization of methods across diagnostic laboratories. In summary, we conclude that stratifying RCC patients based on the identification of a VHL-PTP1B-Src signaling axis will identify a subgroup of patients likely to respond to Src inhibitors in future RCC clinical trials.
MATERIAL AND METHODS
Sample Preparation, Peptide Immunoprecipitation and Mass Spectrometry Analysis
SN12C and SN12C-shVHL cells were maintained in DMEM supplemented with 10% FBS. Cells (40–50% confluence per 10 cm plate) were seeded for 24h, washed twice with PBS and then incubated for 24h in serum-free media. Cells were stimulated with 10% serum for 10 min and harvested in 900 μl/dish 8M Urea. Unstimulated cells were used as controls. Cells were lysed in 8M urea, subjected to reduction, alkylation and trypsin digestion as previously described (15). Peptides were desalted on a C18 Sep-Pak Plus cartridge (Waters), eluted with 25% acetonitrile and lyophilized to dryness. Lyophilized peptides were subjected to labeling with the iTRAQ 8-plex reagent (Applied Biosystems). Peptide immunoprecipitation was performed as described (15). Briefly, 30 μg of protein G Plus-agarose beads (Sigma) were incubated with 12 μg of each of the antiphosphotyrosine antibodies (pTyr100 (Cell Signaling Technology), PT66 (Perkin Elmer) and 4G10 (Millipore) in 200 μl of immunoprecipitation buffer (100 mM Tris, 100 mM NaCl, 1% Nonidet P-40, pH 7.4) for 8h at 4°C. Beads were washed with rinse buffer (100 mM Tris, 100 mM NaCl, pH 7.4) and retained peptides were eluted from antibody with 70 μL of elution buffer (100 mM glycine, pH 2.5) for 1h at room temperature. Immobilized metal affinity chromatography was performed to enrich for phosphorylated peptides, and peptides retained on the column were eluted with 250mM sodium phosphate (pH 8.0) and analyzed by electrospray ionization liquid chromatography tandem MS on a QqTof (QSTAR Elite, Applied Biosystems) as described (15).
Phosphopeptide Sequencing, Quantification and Analysis
MS/MS spectra were extracted, searched, and quantified by using Protein Pilot (Applied Biosystems). Phosphorylation sites and peptide sequence assignments were validated by manual confirmation of raw MS/MS data. Peak areas of iTRAQ marker ions (m/z 113, 114, 115, 116, 117, 118, 119, and 121) were normalized with values from the iTRAQ marker ion peak areas of nonphosphorylated peptides in supernatant of the immunoprecipitation. Each condition was normalized against the 113 channel to obtain fold changes across all eight conditions. Table S1 represents the mean and standard deviation of two biological replicate experiments.
Tissue microarrays
Two separate RCC patient clinical databases were used to construct the TMAs described in our experiment. The first TMA comprised of 215 clear cell RCCs collected from nephrectomies performed at the University Hospital of Zurich. All RCC samples were histologically reviewed by one pathologist (H.M.). This study was approved by the local commission of ethics. Tumor specific survival data were obtained by reviewing the hospital records and by the cancer registry of the Canton of Zurich. The second RCC TMA consisted of 131 nephrectomies performed for kidney cancer at the Royal Marsden Hospital, London. This study protocol was approved by the hospital ethics review board. All tumors arrayed from this second dataset were histologically reviewed by one pathologist (G.V.T.).
Immunohistochemistry
The first TMA was stained using the ultraView Universal DAB Detection Kit (Ventana, Tucson, Arizona, USA). A clear cell RCC tumor with strong membranous Src positivity was used as positive control. Negative controls were identical array sections stained in the absence of the primary antibody. Immunohistochemistry can yield false positivity at the margin or edges of tissue (i.e. edge effect) and this needs to be considered when scoring tissue microarray (TMA) cores. Therefore, to minimize false positivity, we used a conservative 5% cutoff, i.e. any tumors with <5% cytoplasmic and/or membranous staining was considered negative and any tumors with >5% cytoplasmic and/or membranous staining was considered positive. Next, positive Src expression was analyzed subjectively based on antibody staining intensity as either having weak or strong cytoplasmic and/or membranous immunoreactivity by an experienced pathologist (H.M.) VHL immunostaining was similarly scored (44).
The second TMA was processed using EnVision Kits (DAKO), SuperSensitive IHC Detection Systems (BioGenex) or VECTASTAIN ABC Kit (Vector Labs) according to manufacturer's instruction. Negative controls slides were used in every run (incubated in DAKO Universal Negative Control Mouse/Rabbit). Diaminobenzidine tetrahydrochloride (DAB) was used as the enzyme substrate for visualization and counterstained with hematoxylin.
Image acquisition, management and automated analysis
The Aperio ScanScope CS slide Scanner (Aperio Technologies) was used to capture whole slide digital images with a 20× objective. Slides were de-arrayed to visualize individual cores, using the TMA Lab (Aperio). A color deconvolution algorithm (Aperio) was used to develop a quantitative scoring model for measuring cytoplasmic immunoreactivity in TMAs consecutively stained with VHL, Src, CA-IX, PTP1B and pFAK. A nuclear algorithm was used to quantify HIF-2α and Ki-67 nuclear positivity. The algorithm was calibrated to individual staining patterns (range of hues and saturation) and three intensity ranges were generated, weak: yellow; moderate: orange; strong: red and immunonegative: blue. For pixels that satisfy the color specification, the algorithm counted the number and intensity-sum in each intensity range, along with three additional quantities: average intensity, ratio of strong/total number, and average intensity of weak positive pixels. The algorithm was calibrated for both cytoplasmic and nuclear expression by constructing receiver operator curves for Hue, Hue width and color saturation. A pseudo-color “mark-up” image was generated from the algorithm and verified to ensure that specified inputs were measuring the desired color and intensity ranges. All “mark up” images were inspected by a pathologist (G.V.T) to confirm the accuracy of the algorithm. The final automated score was assessed for each core as the product of corrected average intensity and corrected positive pixel percentage.
Supplementary Material
Acknowledgements
We thank C.W.Ryan, W.Y.Kim, K.Ellwood-Yen, M.Ashcroft, S.Mittnacth, I.K.Mellinghoff, J.T.Erler and R.Dresbeck for helpful discussions; J.G.Braun for academic support; L.Iwai, N.Martin, C.Garcia and J.Dukes for technical expertise; M.C.Costello for artwork; W.Kaelin (HHMI) for the HIF-1α and -2α prolyl hydroxylase mutants; J.Massagué (HHMI) for the Src shRNA, chicken Src and Src T388I mutant; W.Ohh for the 786-0 VHL WT plasmid; M. Okada and S. Nada for the CSK plasmids; T.Mori, S.McWeeney, S.Mongue-Tchokote from the Biostatistics Shared Resource of the Knight Cancer Institute (NCI P30 CA 069533). M.E.G. thanks the RMH foundation.
Funding: K.M.A. is supported by NRSA T32 GM71338 and award RMS1112 from the Radiological Society of North America. This study was supported by NIH grants DK37274, CA151564 (G.T.), 1KL2 RR024141 01 through OCTRI and UL1 RR024140 (J.J.A.), R01CA149253-01 (D.Z.Q), P30 CA069533 13S5 through OHSU-Knight Cancer Institute and the PNW Prostate SPORE (G.V.T.), Knight Cancer Institute award (G.T.), VHL Family Alliance, STOP Cancer Foundation, Experimental Cancer Medicine Centre network and the Institute of Cancer Research (G.V.T.).
Footnotes
Author contributions: N.S, E.V., K.S., H.G. and D.Z.Q. performed the cellular and biochemical experiments; M.G. conducted the immunohistochemical assays; P.S and H.M. performed and analyzed the survival correlations; B.A.L., D.B. and A.C. performed the statistical analysis on the tissue microarray and network interaction map; P.H. conducted the proteomics experiments; P.H., B.A.L and P.N. analyzed the proteomics data; K.M.A. and G.T. performed the in vitro kinase assays; L.G. and J.J.A. performed ChIP experiments; M.E.G., J.L., S.B.K., J.D.B. and T.M.B. provided clinical samples for analysis and clinical insight; N.S, E.V., K.M.A., G.T., C.L.S. and P.W. provided critical input into the overall research direction; G.V.T. directed the research and wrote the manuscript with input from all the co-authors.
Competing Interest: The authors have no competing interests
REFERENCES AND NOTES
- 1.Gupta K, Miller JD, Li JZ, Russell MW, Charbonneau C. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev. 2008 May;34:193. doi: 10.1016/j.ctrv.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Janzen NK, Kim HL, Figlin RA, Belldegrun AS. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am. 2003 Nov;30:843. doi: 10.1016/s0094-0143(03)00056-9. [DOI] [PubMed] [Google Scholar]
- 3.Lam JS, Leppert JT, Figlin RA, Belldegrun AS. Surveillance following radical or partial nephrectomy for renal cell carcinoma. Curr Urol Rep. 2005 Feb;6:7. doi: 10.1007/s11934-005-0062-x. [DOI] [PubMed] [Google Scholar]
- 4.Bonsib SM. Renal cystic diseases and renal neoplasms: a mini-review. Clin J Am Soc Nephrol. 2009 Dec;4:1998. doi: 10.2215/CJN.02020309. [DOI] [PubMed] [Google Scholar]
- 5.Dalgliesh GL, et al. Systematic sequencing of renal carcinoma reveals inactivation of histone modifying genes. Nature. 2010 Jan 21;463:360. doi: 10.1038/nature08672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim WY, Kaelin WG. Role of VHL gene mutation in human cancer. J Clin Oncol. 2004 Dec 15;22:4991. doi: 10.1200/JCO.2004.05.061. [DOI] [PubMed] [Google Scholar]
- 7.Thomas GV, et al. Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nat Med. 2006 Jan;12:122. doi: 10.1038/nm1337. [DOI] [PubMed] [Google Scholar]
- 8.Rini BI, Atkins MB. Resistance to targeted therapy in renal-cell carcinoma. Lancet Oncol. 2009 Oct;10:992. doi: 10.1016/S1470-2045(09)70240-2. [DOI] [PubMed] [Google Scholar]
- 9.Rini BI, et al. A phase I study of sunitinib plus bevacizumab in advanced solid tumors. Clin Cancer Res. 2009 Oct 1;15:6277. doi: 10.1158/1078-0432.CCR-09-0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Motzer RJ, et al. Treatment outcome and survival associated with metastatic renal cell carcinoma of non-clear-cell histology. J Clin Oncol. 2002 May 1;20:2376. doi: 10.1200/JCO.2002.11.123. [DOI] [PubMed] [Google Scholar]
- 11.Ronnen EA, et al. Treatment outcome for metastatic papillary renal cell carcinoma patients. Cancer. 2006 Dec 1;107:2617. doi: 10.1002/cncr.22340. [DOI] [PubMed] [Google Scholar]
- 12.Demetri GD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002 Aug 15;347:472. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 13.Druker BJ, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001 Apr 5;344:1038. doi: 10.1056/NEJM200104053441402. [DOI] [PubMed] [Google Scholar]
- 14.Flaherty KT, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010 Aug 26;363:809. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang PH, et al. Quantitative analysis of EGFRvIII cellular signaling networks reveals a combinatorial therapeutic strategy for glioblastoma. Proc Natl Acad Sci U S A. 2007 Jul 31;104:12867. doi: 10.1073/pnas.0705158104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stommel JM, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007 Oct 12;318:287. doi: 10.1126/science.1142946. [DOI] [PubMed] [Google Scholar]
- 17.Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004 Jun;4:470. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- 18.Pan J, et al. Stromal derived factor-1 (SDF-1/CXCL12) and CXCR4 in renal cell carcinoma metastasis. Mol Cancer. 2006;5:56. doi: 10.1186/1476-4598-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pantuck AJ, An J, Liu H, Rettig MB. NF-kappaB-dependent plasticity of the epithelial to mesenchymal transition induced by Von Hippel-Lindau inactivation in renal cell carcinomas. Cancer Res. Jan 15;70:752. doi: 10.1158/0008-5472.CAN-09-2211. [DOI] [PubMed] [Google Scholar]
- 20.Turcotte S, et al. A molecule targeting VHL-deficient renal cell carcinoma that induces autophagy. Cancer Cell. 2008 Jul 8;14:90. doi: 10.1016/j.ccr.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishizawar R, Parsons SJ. c-Src and cooperating partners in human cancer. Cancer Cell. 2004 Sep;6:209. doi: 10.1016/j.ccr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Wheeler DL, Iida M, Dunn EF. The role of Src in solid tumors. Oncologist. 2009 Jul;14:667. doi: 10.1634/theoncologist.2009-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buettner R, Mesa T, Vultur A, Lee F, Jove R. Inhibition of Src family kinases with dasatinib blocks migration and invasion of human melanoma cells. Mol Cancer Res. 2008 Nov;6:1766. doi: 10.1158/1541-7786.MCR-08-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gwanmesia PM, et al. The effect of the dual Src/Abl kinase inhibitor AZD0530 on Philadelphia positive leukaemia cell lines. BMC Cancer. 2009;9:53. doi: 10.1186/1471-2407-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schweppe RE, et al. Inhibition of Src with AZD0530 reveals the Src-Focal Adhesion kinase complex as a novel therapeutic target in papillary and anaplastic thyroid cancer. J Clin Endocrinol Metab. 2009 Jun;94:2199. doi: 10.1210/jc.2008-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frame MC. Src in cancer: deregulation and consequences for cell behaviour. Biochim Biophys Acta. 2002 Jun 21;1602:114. doi: 10.1016/s0304-419x(02)00040-9. [DOI] [PubMed] [Google Scholar]
- 27.Zhang XH, et al. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell. 2009 Jul 7;16:67. doi: 10.1016/j.ccr.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lombardo LJ, et al. Discovery of N-(2-chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4- ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004 Dec 30;47:6658. doi: 10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, et al. Structural basis for selective inhibition of Src family kinases by PP1. Chem Biol. 1999 Sep;6:671. doi: 10.1016/s1074-5521(99)80118-5. [DOI] [PubMed] [Google Scholar]
- 30.Ple PA, et al. Discovery of a new class of anilinoquinazoline inhibitors with high affinity and specificity for the tyrosine kinase domain of c-Src. J Med Chem. 2004 Feb 12;47:871. doi: 10.1021/jm030317k. [DOI] [PubMed] [Google Scholar]
- 31.Kondo K, Klco J, Nakamura E, Lechpammer M, Kaelin WG., Jr. Inhibition of HIF is necessary for tumor suppression by the von Hippel-Lindau protein. Cancer Cell. 2002 Apr;1:237. doi: 10.1016/s1535-6108(02)00043-0. [DOI] [PubMed] [Google Scholar]
- 32.Lund IK, Hansen JA, Andersen HS, Moller NP, Billestrup N. Mechanism of protein tyrosine phosphatase 1B-mediated inhibition of leptin signalling. J Mol Endocrinol. 2005 Apr;34:339. doi: 10.1677/jme.1.01694. [DOI] [PubMed] [Google Scholar]
- 33.Boerner RJ, et al. Correlation of the phosphorylation states of pp60c-src with tyrosine kinase activity: the intramolecular pY530-SH2 complex retains significant activity if Y419 is phosphorylated. Biochemistry. 1996 Jul 23;35:9519. doi: 10.1021/bi960248u. [DOI] [PubMed] [Google Scholar]
- 34.Moarefi I, et al. Activation of the Src-family tyrosine kinase Hck by SH3 domain displacement. Nature. 1997 Feb 13;385:650. doi: 10.1038/385650a0. [DOI] [PubMed] [Google Scholar]
- 35.Sun G, Sharma AK, Budde RJ. Autophosphorylation of Src and Yes blocks their inactivation by Csk phosphorylation. Oncogene. 1998 Sep 24;17:1587. doi: 10.1038/sj.onc.1202076. [DOI] [PubMed] [Google Scholar]
- 36.Matsubara T, et al. Cbp recruitment of Csk into lipid rafts is critical to c-Src kinase activity and bone resorption in osteoclasts. J Bone Miner Res. 2010 May;25:1068. doi: 10.1359/jbmr.091039. [DOI] [PubMed] [Google Scholar]
- 37.Veracini L, et al. The Csk-binding protein PAG regulates PDGF-induced Src mitogenic signaling via GM1. J Cell Biol. 2008 Aug 11;182:603. doi: 10.1083/jcb.200705102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaelin WG., Jr. Kidney cancer: now available in a new flavor. Cancer Cell. 2008 Dec 9;14:423. doi: 10.1016/j.ccr.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 39.Richardson CJ, et al. MoKCa database--mutations of kinases in cancer. Nucleic Acids Res. 2009 Jan;37:D824. doi: 10.1093/nar/gkn832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dube N, Cheng A, Tremblay ML. The role of protein tyrosine phosphatase 1B in Ras signaling. Proc Natl Acad Sci U S A. 2004 Feb 17;101:1834. doi: 10.1073/pnas.0304242101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wright TM, Rathmell WK. Identification of Ror2 as a hypoxia-inducible factor target in von Hippel-Lindau-associated renal cell carcinoma. J Biol Chem. Apr 23;285:12916. doi: 10.1074/jbc.M109.073924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan DA, Sutphin PD, Denko NC, Giaccia AJ. Role of prolyl hydroxylation in oncogenically stabilized hypoxia-inducible factor-1alpha. J Biol Chem. 2002 Oct 18;277:40112. doi: 10.1074/jbc.M206922200. [DOI] [PubMed] [Google Scholar]
- 43.Gridelli C, et al. Gefitinib as first-line treatment for patients with advanced non-small-cell lung cancer with activating Epidermal Growth Factor Receptor mutation: Implications for clinical practice and open issues. Lung Cancer. 2011 Apr;72:3. doi: 10.1016/j.lungcan.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 44.Dahinden C, et al. Mining tissue microarray data to uncover combinations of biomarker expression patterns that improve intermediate staging and grading of clear cell renal cell cancer. Clin Cancer Res. Jan 1;16:88. doi: 10.1158/1078-0432.CCR-09-0260. [DOI] [PubMed] [Google Scholar]
- 45.Cluster3.0. http://bonsai.ims.utokyo.ac.jp/~mdehoon/software/cluster/software.htm.
- 46.TreeView. http://rana.lbl.gov/EisenSoftware.htm.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.