Abstract
Background:
Squamous cell carcinoma is the most frequent malignancy of the oral cavity. The survival rate of this malignancy has not improved from past two decades. The major factors responsible for this could be due to loco regional and distant metastatic spread. However, the other important prognostic factor is concomitant occurrence and recurrence of multiple primary carcinomas in the head and neck region, which is explained as the concept of field cancerization. The evidence to support the field change in normal mucosa of Oral squamous cell carcinoma (OSCC) through biological markers using immunohistochemistry has always been challenging.
Aim:
Hence, the aim of the present research is to identify changes in the expression of CK 8/18, 19, and MMP-9 to visualize field changes in the clinically normal mucosa adjacent to OSCC and compare with non neoplastic normal oral mucosa.
Materials and Methods:
20 cases of OSCC with radical resection specimens were included in the study. Lesional tissue and adjacent normal looking mucosa were taken during grossing. Ten cases of non-neoplastic normal oral mucosa are also included in the study. Markers such as CK 8/18, CK 19, and MMP-9 are used by the immunohistochemical method in this present study.
Result and Conclusion:
The enhance expression of CK 8/18 (80%), CK 19 (70%), and MMP 9 (90%) in ANM was noted and furthermore in six ANM showing severe dysplasia with enhance expression of CK 8/18, CK 19, and MMP 9 in the apparently normal oral mucosa can suggest a field cancerization.
Keywords: Cytokeratin, field cancerization, matrix metalloproteinase
INTRODUCTION
Oral squamous cell carcinoma (OSCC) represents a major global health problem. It is the sixth most frequently diagnosed malignancy with high incidence of mortality and morbidity rate noted worldwide. Despite improvement in treatment strategies, including novel drug regimes, surgeries, radiotherapy, chemotherapy, the prognosis of OSCC patients remains largely unsatisfactory, due to loco regional recurrences. The 5 year survival rate is less than 50% and the prognosis of advanced cases has not improved much over the past three decades.[1]
The majority of OSCC is preceded by visible changes of the oral mucosa, but at times local or distant occurrence of these malignancies might arise without any obvious macroscopic premalignant lesions being observed. However, several studies support that there is evidence that large areas of mucosa of OSCC patients, whilst appearing normal are genetically altered due to development of the second primary tumor (SPT). Subsequently influences the prognosis even with histopathologically tumor free surgical margins after resection.[2] Depending on both the location of first primary tumor and age of the patient, the incidence of SPT is 10–35%.[3–5]
Different oral field cancerization theories have been proposed like Slaughter et al. in 1953, postulated that the entire mucosa of upper aero digestive tract has a potential for development of premalignant lesions due to multiple genetic abnormalities as result of exposure to several carcinogens and thus oral field cancerization thought to develop independently of each other. They also proposed about the existence of satellites of dysplastic looking epithelium away from the main bulk of the lesion.[6] An alternative theory explains the occurrence of multiple lesions due to migration of transformed cells either by micro metastasis through saliva or by intraepithelial migration of the transformed cells.[7–9]
Recent studies focuses on SPTs in the upper aerodigestive tract have a common clonal origin. Jang et al. in their study regarding clonal relationship to determine genetic relationships among multiple oral cancerous and precancerous lesions have observed that lesion development was synchronous or metachronous. Furthermore, their data stressed on multiple HNSCC could develop from either by field cancerization or mucosal spreading of clonal cells.[10]
Since then several researches have been quoted to support the concept of field change in routine histological specimens like ultra structural changes by exfoliative cytology, image analyzing technique on tissue specimens by research microscope, mirror image biopsy etc.[11,12] The evidence to support the field change in normal mucosa of OSCC through biological markers using immunohistochemistry has always been challenging.
Among the several biological markers cytokeratin (CK) are epithelial specific intermediate filament proteins that are broadly classified on the basis of their molecular weight and isoelectric points into two subfamilies: Type I, acidic with low molecular weight (CK 9–CK 23) and type II, basic with high molecular weight (CK 1–CK 8). There are around 23 CK polypeptides expressed in human epithelia. Each type of epithelium expresses two to four specific pairs based on their differentiation status.[13] These CKs exhibit tissue-specific expressions that have been used as diagnostic markers in cancer and precancer. The expression of CK subtypes such as CK 8/18 and CK 19 in transformed oral lesions has been regarded as an early feature in the premalignant and malignant transformation and invasive potential of OSCC. Furthermore, the altered CKs of CK 7, 8, 13, 16, and 19 was observed at obnormal intraepithelial levels in normal mucosa from HNSCC.[11,12]
Matrix metalloproteinase's (MMP'S) are a family of zinc dependent endopeptidases involved in degradation of ECM components that play relevant role in several steps of tumor progression such as angiogenesis, invasion, and metastasis.[13] OSCC are aggressive tumors with an average unfavorable prognosis, due to loco regional spread and also distant metastasis. One of the important prognostic factors is the early development of occult loco regional micrometastasis.[11] The malignant tumor cells invade into the stroma with no respect to the basement membrane; MMP'S are capable of disintegrating the basement membrane that is a main characteristic of tumor invasion. So far over 20 different members of MMP'S are known. MMP-2, 9, 13, and TIMP-1 seems to play important role in the tumor invasion process for head and neck carcinoma.[13] Furthermore, it has been indicated that a new pattern of transcriptional activation emerges during conversion from benign to malignant lesions. Amongst the several types of MMPs, one gene whose expression is activated by this switch is MMP-9.[13,14]
Hence, the aim of this study is to identify changes in the immunoexpression of CK 8/18, 19, and MMP-9 to visualize field changes in the clinically normal mucosa adjacent to OSCC and compare with non neoplastic normal oral mucosa.
MATERIALS AND METHODS
Case selection
After obtaining the institutional ethical approval for the study, total number of twenty cases of radical resection specimens of OSCC that were received during routine histopathological analysis was included in the study. All these cases had a history of tobacco use with duration of 5 to 15 years. During grossing, tissues were taken from lesion. To check for field change; tissue was taken one centimeter away from apparently normal looking mucosa (ANM). Ten tissue specimens of normal oral mucosa (NOM) from noncancer with no history of tobacco or alcohol habits were included. These tissues were taken either during exposure of impacted tooth or during crown lengthening procedure or from biopsies subsequently reported as normal. All these tissues were routinely fixed in formalin, processed with graded alcohol and paraffin embedded. Two sections of 4 μ thicknesses were obtained. One section of each was stained with routine hematoxylin and eosin to confirm the diagnosis and further to grade dysplasia of ANM as per the modified WHO 2005 classification system. The other section was stained with immunohistochemistry (IHC). For IHC staining, sections were placed on 3-aminopropyl triethoxysilane (APES) (A3648, SIGMA) coated slides and staining protocol was performed by using the Super Sensitive one step PolymerHRP system (QD-600, BIOGENEX). Primary antibodies included CK 19 (AM246-5M), CK 8/18 (AM 131-5M) and MMP-9 (AN 504-5M) from BIOGENEX company.
Immunohistochemistry protocol
The IHC staining protocol was performed as per the steps recommended by the Biogenex. Initially slides were kept overnight in the incubator at 55°C for proper fixation of tissue to the slides, so that there will be limited chances of floating of tissues during antigen retrieval. Subsequently slides were deparaffinized, dehydrated with graded alcohol, and rinsed with distilled water. Antigen retrieval was standardized by using two different buffers by using citrate buffer in EZ-retrieval microwave at 96°C for three cycles. After antigen retrieval sections were thoroughly wiped with tissue paper and subsequently rinsed with phosphate buffer (wash buffer) for 5 min, this step was repeated at every step. The endogenous peroxidase activity was blocked by incubating the slides with 3% H2O2 for 15 min. Power block was used to make a thin casein layer, so that all the epitopes were opened, only after this step wash buffer was not used. Next the slides were incubated with primary antibodies of CK 8/18 and MMP 9 for 1 h whereas CK 19 for 2 h as per the company specifications. At every batch of staining protocol positive and negative controls were taken to determine the false positive/negative expression. Subsequently, slides were further incubated with polymer HRP (horse radish peroxidase) secondary antibody for 30 mins. To visualize the color reaction slides were incubated with freshly prepared DAB chromogen for 10 mins. Then finally slides were counterstained with Harris haematoxylin for 30 s followed by blueing in running tap water. Furthermore, slides were dehydrated, dipped in xyelene and mounted.
Evaluation of immunostaining
The expression of CK 8/18, CK 19, and MMP-9 were determined independently by three oral pathologists. A section was scored according to the staining intensity and staining area. The staining intensity were scored as, no staining (score 0), light yellow (score 1), yellow to brown (score 2), and dark brown (score 3). The staining area were scored as, no staining (score 0), positive staining for less than one-third of tissue section (score 1), positive staining area ranged from one-third to two-third of tissue section (score 2) and positive staining for more than two-third of tissue section (score 3). Sections were considered negative or positive according to the sum of above two scoring systems and a score ≥3 was regarded as positive. Seven high-power fields were randomly selected for observation. Percentages were calculated to determine the expression of these markers.
RESULTS
CK 8 /18: No immunoreactions of CK 8/18 were noted in the oral mucosa of non neoplastic cases. In OSCC only 10% cases showed immunoreactions [Figure 1] where as in ANM tissues group 80% of cases were stained positive for CK 8/18 [Figure 2] and [Table 1].
Figure 1.
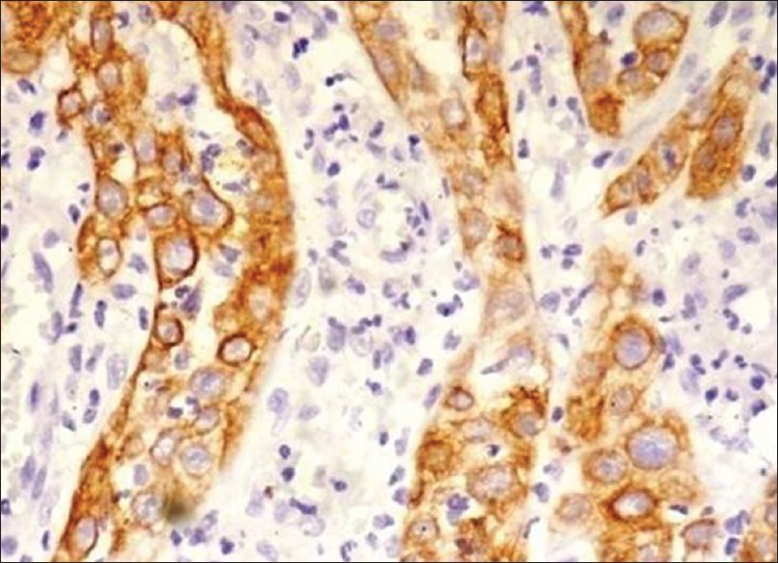
Photomicrograph showing immunohistochemical enhance expression of CK 8/18 in tumor cells of squamous cell carcinoma (×10)
Figure 2.
Photomicrograph showing immunohistochemical enhance expression of CK 8/18 of apparently normal oral mucosa adjacent to squamous cell carcinoma (×10)
Table 1.
Immunoexpression of CK 8/18, CK 19, and MMP-9 in the present study
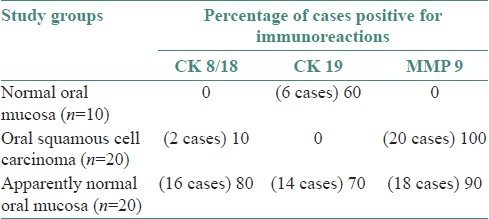
CK 19: In normal oral mucosa 60% of cases showed positive expression throughout the basal cell layer [Figure 3]. Although there was no expression of CK 19 in lesional tissue of OSCC, the normal mucosa adjacent to OSCC (ANM) showed an enhance expression at basal and suprabasal cells in 70% of cases [Figure 4] and [Table 1].
Figure 3.
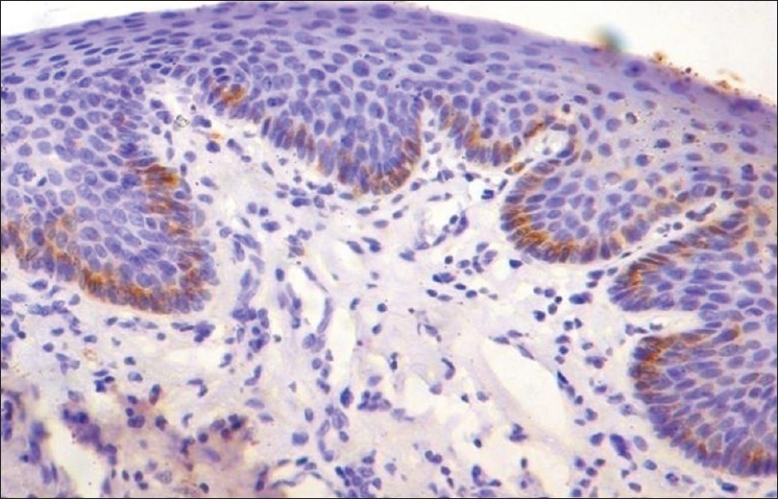
Photomicrograph showing immunohistochemical expression of CK 19 throughout the basal cells of non keratinized normal oral mucosa (×10)
Figure 4.
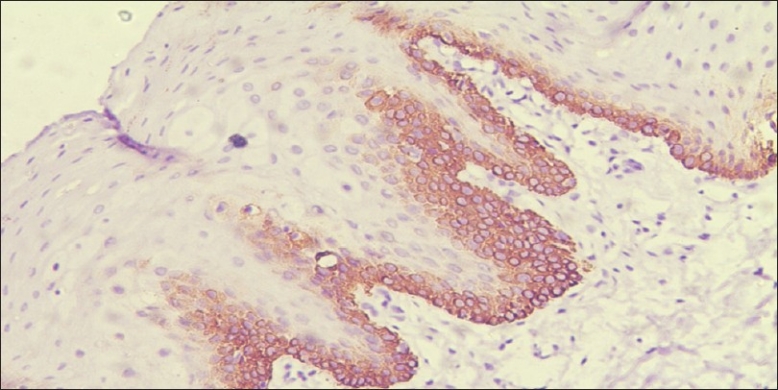
Photomicrograph showing immunohistochemical enhance expression of CK 19 at basal and supra basal cells of apparently normal oral mucosa adjacent to squamous cell carcinoma (×10)
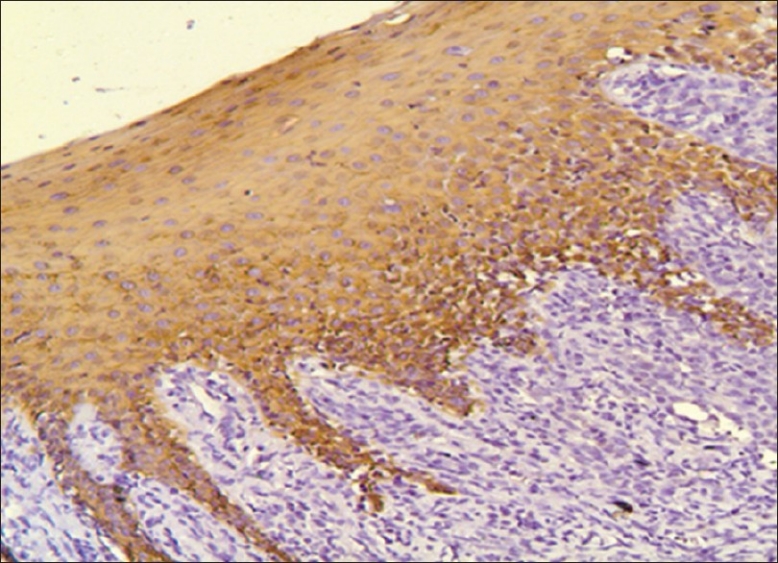
MMP-9: The oral mucosa from the normal non neoplastic group showed no immunostaining in any of the tissues. All cases of OSCC showed immunoreactivity to MMP 9 [Figure 5], subsequently 90% of ANM tissues were found to be immunoreactive to MMP 9 [Figure 6] and [Table 1].
Figure 5.
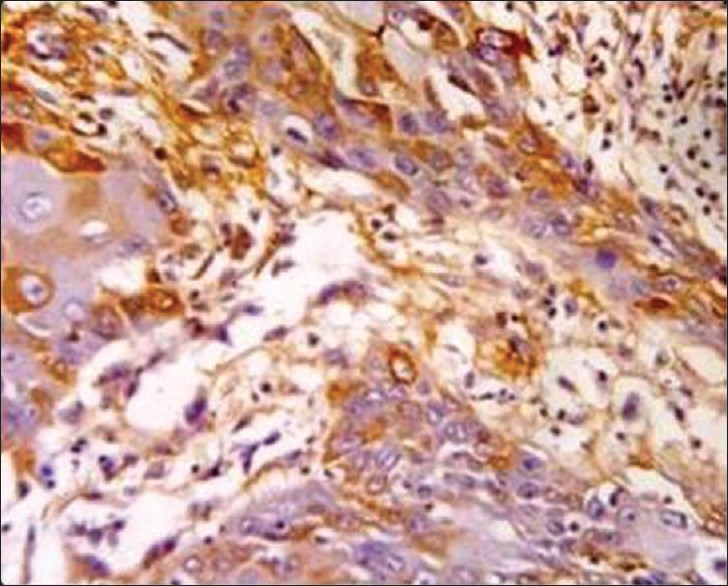
Photomicrograph showing immunohistochemical enhance expression of MMP-9 in the stoma and tumor cells of squamous cell carcinoma (×40)
Figure 6.
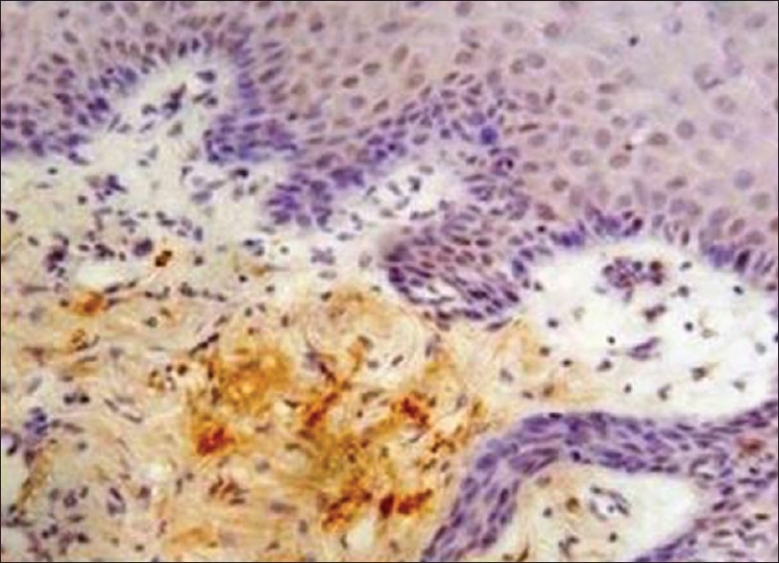
Photomicrograph showing immunohistochemical enhance expression of MMP-9 in the stroma of apparently normal oral mucosa adjacent to squamous cell carcinoma (×10)
Out of 20 cases of ANM on grading dysplasia we noted mild dysplasia (n=4), on immunoexpression very minimal expression of CK 8/18, CK 19, and MMP 9 was observed, moderate dysplasia (n=10) with enhance expression of CK 19 and MMP 9 but minimal expression of CK 8/18. Whereas severe dysplasia (n=6) showed enhance expression of CK 8/18, CK19, and MMP 9. The altered expression of these markers from normal to abnormal pathological tissue can suggest there distinctive role.
DISCUSSION
Squamous cell carcinoma of the head and neck is considered to be the most aggressive tumor. The prime requisite prognostic factors for these cancers are with high frequency of loco regional metastatic spread, distant metastasis and early development of occult loco regional micro metastasis. Furthermore, the added important prognostic factor is concomitant occurrence and recurrence of multiple primary carcinomas in head and neck region, which is explained as the concept of field cancerization. This concept explains the occurrence of another tumor at a different site following complete excision and histopathological confirmation of clear margins of primary lesions. However the current surgical practice includes wider excision margin than practiced previously but still OSCC remained with an unfavorable prognosis. Local spread of a solid tumor can be followed at three levels of magnitude: Macroscopic, microscopic, and occult.[11,14] Advance techniques have been identified to determine the relationship of field and emerging tumors, furthermore, to distinguish monoclonal and polyclonal origins. It is thought that initially cell acquires mutation followed by multiplication of these cells to form a patch of altered daughter cells and eventually replacing the surrounding normal tissue without invasive growth.[10]
Identification of distinct biological markers that can help to predict field change is now considered a prime requisite. Recently much attention has been focused on the role of CK in tumor diagnosis and prognosis. These intermediate filaments that are specific to epithelium play an important role in cell migration as well as in intracellular signal transduction pathways. However, it has been noticed that in variety of organs the expression of several CK subtypes varies and also distinctly involved in steps of malignant transformation.[15] Hence, the aim of the present research is to identify the expression of CK'S and MMP-9 in the epithelium of apparently normal oral mucosa adjacent to OSCC to predict field change.
Amongst the several CK subtypes the major difference between the adult and fetal mucosal epithelium the presence of CK 8 and CK 18. Expression of CK 8 and CK 18 is normally observed in fetal buccal mucosa and tongue epithelium until 27 weeks of gestation.[16] The high frequency of expression of these CKs in adult mucosa during malignant transformation can be regarded as return towards embryonic expression pattern.[15] In this study, the expression of CK 8/18 was negative in all NOM, but its expression was enhanced in OSCC (10%) that is progressing toward poorly differentiated than well differentiated [Figure 1]. However, expression of CK 8/18 was enhanced in the epithelium of majority of tissues of ANM (80%) [Figure 2]. Its enhance expression was also noted in all ANM showing severe dysplasia. Previous research also supports that expression of CK 8/18 is enhanced in leukoplakia with dysplasia than compared to without dysplasia and seems to play an important role in progressing to OSCC.[15] The altered cellular morphology and increased cell motility was observed in cell culture studies with a vector of CK 8/18.[17] Furthermore, CK 8/18 can also modulate the transformation process leading to resistance to Fas-induced apoptosis. CK 8/18 is now considered as a marker of altered cells in premalignant stage and early cancer.[18] According to our present research embryonic expression of CK 8/18 in ANM adjacent to OSCC can suggest as an altered mucosa with the field change.
The CK expression profile in oral mucosa are always binded with type I high molecular mass keratin peptides with type II low molecular mass. An exception of one specific CK with this is CK 19, which forms filaments in the absence of a type I partner. Much has been learned about the structure and assembly of these filaments by characterization of keratin gene mutations that cause human disease. The smallest keratin, CK 19 was first detected in the OSCC cell lines. The temporal and spatial sequence of expression of CK 19 in epidermal cell development in different stages of human fetus has been reported and it has been suggested that in keratinized normal oral mucosa the down regulation of CK 19 plays an important role in terminal differentiation of superficial squamous cells. Whereas the expression of CK 19 is present throughout the cytoplasm of basal cell layer of nonkeratinized normal oral mucosa. Considering this, it seems that eliminating CK 19 from differentiated keratinocyte is essential for cornified layer formation in the epidermis.[19] In this research we noticed that the expression of CK 19 was present throughout the basal cell layer of 60% of non keratinized normal oral mucosa [Figure 3], whereas its expression was negative in 40% of keratinized mucosa. Basal and supra basal expression was noted in 70% of cases of ANM [Figure 4]. On correlating with dysplasia, we noted its enhance expression in moderate and severe dysplasia of ANM Our result is in correlation with research of Lindberg and Rheinwald, where they suggested that suprabasal CK 19 never occurs in non malignant tissue. They have also emphasized that suprabasal CK 19 expression is not a simple reflection of a hyper proliferative state, but rather a marker of cellular atypia associated with premalignancy.[20] However the expression of CK 19 was not observed in any of the cases of OSCC. Our observation was similar to the previous research of Crowe et al. where they have also observed that in OSCC cell lines expression of CK 19 was consistently down regulated. Furthermore, they have also suggested that whenever there is over expression of CK 19, it decreases invasive potential by diminishing migratory capability.[21] Hence, according to our research the suprabasal expression of CK 19 in 70% of ANM is compatible to suggest an altered epithelial change suggestive of dysplasia with a field change. Furthermore, with its down regulation in OSCC suggesting of invasive potential of the tumor. CK 19 that is interpreted as a marker of dysfunctional epithelial differentiation is true.[16]
Along with the expression of CK 8/18 and CK 19, we noted that over expression of MMP-9 was seen in all cases of OSCC [Figure 5] and also in 90% tissues of ANOM predicting invasive tumor progression[22] [Figure 6] than compared to its expression of NOM. The tissues of ANOM that showed its positive expression of CK 8/18, CK 19 as well as with contemporaneous expression MMP-9 could suggest more confirmatory of field change. However, in ANM showing moderate and severe dysplasia showed enhance expression of MMP 9. Furthermore, all the six ANM showing severe dysplasia with an enhance expression CK 8/18, CK 19, and MMP 9 was observed, suggesting an altered mucosa predicting to be a field change.
CONCLUSION
The enhance expression of CK 8/18, CK 19, and MMP 9 in ANM can predict field cancerization. However, the conclusions in this research are with minimal samples. The journey of a thousand miles must begin with a single step. Further studies should be carried out with proper follow up of radical resection specimens of HNSCC after evaluating these markers in the tumor free surgical margins to predict field change, subsequently to keep these patients under observation.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Tripathi SC, Matta A, Kaur J, Grigull J, Chauhan SS, Thakar A, et al. Nuclear S100A7 is associated with poor prognosis in head and neck cancer. PLoS One. 2010;5:e11939. doi: 10.1371/journal.pone.0011939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parkinson EK. Senescence as a modulator of oral squamous cell carcinoma development. Oral Oncol. 2010;46:840–53. doi: 10.1016/j.oraloncology.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Day GL, Blot WJ, Shore RE, McLaughlin JK, Austin DF, Greenberg RS, et al. Second cancers following oral and pharyngealcancers: Role of tobacco and alcohol. J Natl Cancer Inst. 1994;86:131–7. doi: 10.1093/jnci/86.2.131. [DOI] [PubMed] [Google Scholar]
- 4.Cianfriglia F, Di Gregorio DA, Manieri A. Multiple primary tumoursin patients with oral squamous cell carcinoma. Oral Oncol. 1999;35:157–63. doi: 10.1016/s1368-8375(98)00105-5. [DOI] [PubMed] [Google Scholar]
- 5.Dhooge IJ, De Vos M, Van Cauwenberge PB. Multiple primarymalignant tumors in patients with head and neck cancer: Results of a prospectivestudy and future perspectives. Laryngoscope. 1998;108:250–6. doi: 10.1097/00005537-199802000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963–8. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 7.Califano J, van der Riet P, Westra W, Nawroz H, Clayman G, Piantadosi S, et al. Genetic progression model for head and neck cancer: Implications for field cancerization. Cancer Res. 1996;56:2488–92. [PubMed] [Google Scholar]
- 8.Bedi GC, Westra WH, Gabrielson E, Koch W, Sidransky D. Multiple head and neck tumors: Evidence for a common clonal origin. Cancer Res. 1996;56:2484–7. [PubMed] [Google Scholar]
- 9.Partridge M, Emilion G, Pateromichelakis S, Phillips E, Langdon J. Field Cancerisation of the oral cavity: Comparison of the spectrum of molecular alterations in cases presenting with both dysplastic and malignant lesions. Oral Oncol. 1997;33:332–7. doi: 10.1016/s1368-8375(97)00035-3. [DOI] [PubMed] [Google Scholar]
- 10.Jang SJ, Chiba I, Hirai A, Hong WK, Mao L. Multiple oral squamous epithelial lesions: Are they genetically related? Oncogene. 2001;20:2235–42. doi: 10.1038/sj.onc.1204311. [DOI] [PubMed] [Google Scholar]
- 11.Ogden GR, Lane EB, Hopwood DV, Chisholm DM. Evidence for field change in oral cancer based on Cytokeratin expression. Br J Cancer. 1993;67:1324–30. doi: 10.1038/bjc.1993.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bosch FX, Ouhayoun JP, Bader BL, Collin C, Grund C, Lee I, et al. Extensive changes in cytokeratins expression patterns inpathologically affected human gingiva. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;58:59–77. doi: 10.1007/BF02890059. [DOI] [PubMed] [Google Scholar]
- 13.Ranganathan K, Kavitha R, Sawant SS, Vaidya MM. Cytokeratin expression in oral submucous fibrosis-an immunohistochemical study. J Oral Pathol Med. 2006;35:25–32. doi: 10.1111/j.1600-0714.2005.00366.x. [DOI] [PubMed] [Google Scholar]
- 14.Kuropkat C, Plehn S, Herz U, Dünne AA, Renz H, Werner JA. Tumour marker potential of serum matrix metalloproteinase in patients with head and neck cancer. Anticancer Res. 2002;22:2221–8. [PubMed] [Google Scholar]
- 15.Fillies T, Jogschies M, Kleinheinz J, Brandt B, Joos U, Buerger H. Cytokeratin alteration in oral leukoplakia and oral squamous cell carcinoma. Oncol Rep. 2007;18:639–43. [PubMed] [Google Scholar]
- 16.Pelisser A, Ouhayoun JP, Sawaf MH, Forest N. Changes in Cytokeratin expression during the development of the human oral mucosa. J Periodontal Res. 1992;27:588–98. doi: 10.1111/j.1600-0765.1992.tb01741.x. [DOI] [PubMed] [Google Scholar]
- 17.Rahul U, Sawant S, Dange P, Kalraiya R, Ingle A, Vidya M. Implications of cytokeratins of CK 8/18 filament formation in stratified epithelial cells: Induction of transformed phenotype. Int J Cancer. 2004;20:662–8. doi: 10.1002/ijc.20349. [DOI] [PubMed] [Google Scholar]
- 18.Gilbert S, Loranger A, Daigel N, Marceau N. Simple epithelium keratins 8 and 18 provide resistance to Fas mediated apoptosis. The protection through a receptor-targeting modulation. J Cell Biol. 2006;154:763–73. doi: 10.1083/jcb.200102130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu MH, Yang PC, Chang LT, Chao CF. Temporal and spatial sequence expression of cytokeratin K19 in cultured human keratinocyte. Proc Natl Sci Counc Repub China B. 2000;24:169–77. [PubMed] [Google Scholar]
- 20.Lindberg K, Rheinwald JG. Suprabasal 40 Kd Keratin (K19) expression as an immunohistologic marker of premalignancy in oral epithelium. Am J Pathol. 1989;134:89–98. [PMC free article] [PubMed] [Google Scholar]
- 21.Crowe DL, Milo GE, Shuler CF. Keratin 19 down regulation by oral squamous cell carcinoma lines increases invasive potential. J Dent Res. 1999;78:1256–63. doi: 10.1177/00220345990780061001. [DOI] [PubMed] [Google Scholar]
- 22.Kupferman ME, Fini ME, Muller WJ, Weber R, Cheng Yi, Muschel RJ. Matrix metalloproteinase 9 promoter activity is induced coincident with invasion during tumour progression. Am J Pathol. 2000;157:1777–83. doi: 10.1016/S0002-9440(10)64815-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


