Abstract
Paget's disease (PD) is a chronic progressive disease of the bone characterized by abnormal bone resorption and deposition affecting either single bone (monostotic) or many bones (polyostotic) with uncertain etiology. We report a case of isolated mandibular involvement in a 65-year-old female patient, clinically asymptomatic with abnormally increased alkaline phosphatase level (1 368.1 U/l). Although prevalence of PD is common in western countries, but rare in Asian chapter, that too isolated mandibular involvement, considering this fact, we report this case of PD for documentation.
Keywords: Bone disease, bone turnover, mandible, osteitis deformans, Paget's disease
INTRODUCTION
Paget's disease (PD) of bone was first described by Sir James Paget under as the term “osteitis deformans” in 1877.[1] It is a chronic progressive disease involving single bone (monostotic) or many bones (polyostotic) of the body, characterized by rapid bone resorption and deposition, resulting in numerous reversal line formation which gives the mosaic pattern to the lamellar bone with profuse local vascularity and fibrous tissue in the marrow.[2] Clinical symptoms include pain, deformity, and may lead to fracture of the affected bone, even though the initial course of the disease may be asymptomatic. The enlarged and deformed bones may compress surrounding nerves and vessels causing neurological symptoms like hearing loss; inexplicably, it is quite unusual in the facial bones. Facial disfigurement may be consequence of enlargement of the maxilla and/or mandible.[3] Radiograph shows characteristic cotton wool appearance and other radiographic finding include well-circumscribed radiolucency, loss of lamina dura, pulpal radio-opacity, root resorption, and hypercementosis.[4–6] The etiology of PD is still obscure, but genetic and environmental factors may play a role. Viruses such as paramyxovirus, canine distember virus, and respiratory syncytial virus are also reported to be the causative agents.[1,3]
Therapeutic agents commonly used include calcitonin, bisphosphonate, and mithramycin.[5] A recent clinical trial suggested that second and third generation bisphosphonate, such as pamidronate and alendronate, were more effective than calcitonin and editronate, the first generation bisphosphonate.[7] Here, we are reporting a very rare case of PD with isolated mandibular involvement.[8]
CASE REPORT
A 67-year-old female patient reported to a private dental clinic in Coimbatore, complaining of swelling in the lower jaw for past few years. History reveals a slow-progressive swelling with no other associated symptoms like pain, discharge, and non-responsive to any medication. Extra oral examination reveals broadening and widening of the lower facial view with thickened lower lip. Intra oral examination showed increased size and altered shape of the mandibular alveolar ridge, pseudo micrognathia of the maxillary arch [Figures 1 and 2], and partially edentulous, thickened cortical plate. Orthopantomograph shows hypercementosis with displacement of involved teeth, cortical thickening of the body of the mandible, and mixed radiolucent and radio opaque areas [Figure 3] on both sides of the mandible. Whole-body X-rays and scans were taken to ascertain the involvement of other bones [Figure 4], confirming that the lesion was restricted to mandible only.
Figure 1.
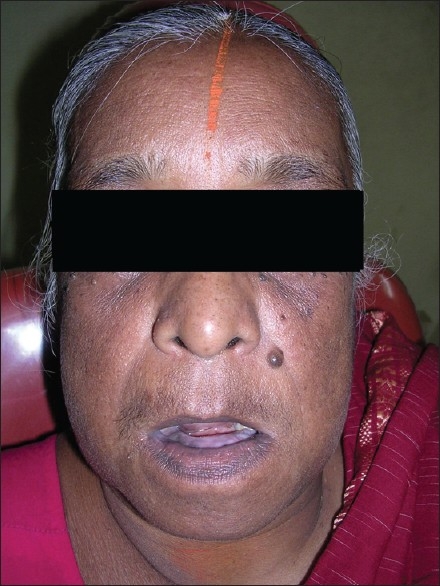
Extra oral view shows large mandible
Figure 2.
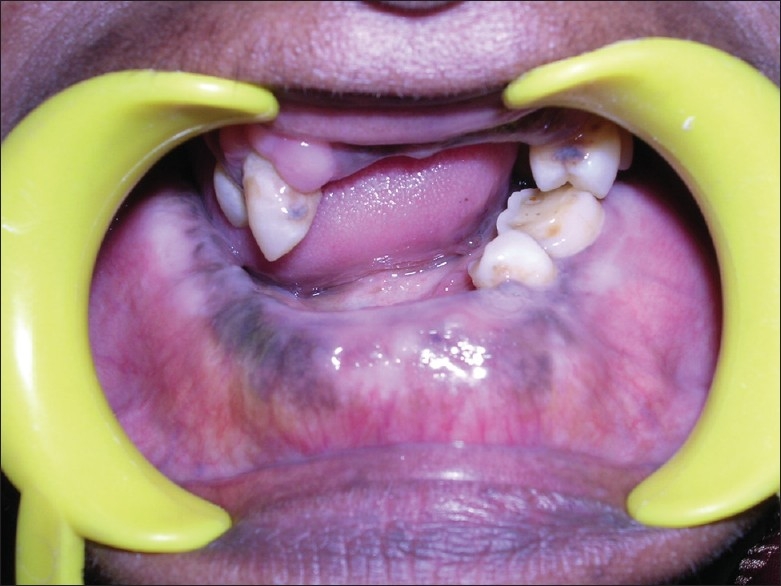
Large size partially edentulous mandible
Figure 3.
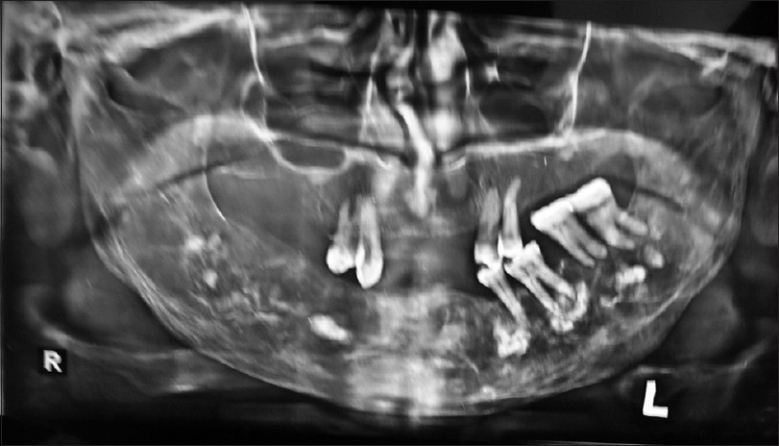
OPG shows hypercementosis of the teeth roots.
Figure 4.
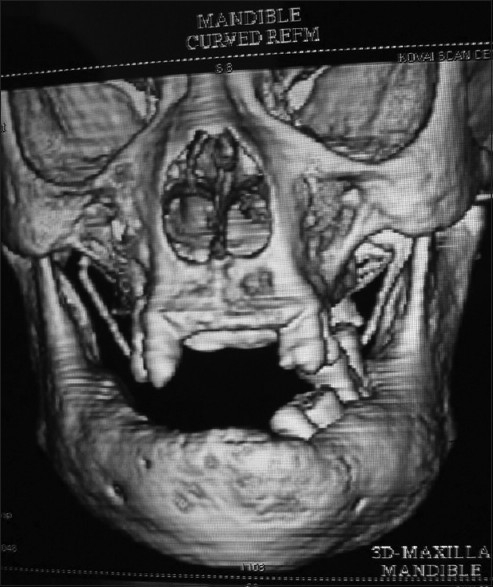
3D scan shows thickened mandibular cortical plate
Incisional biopsy was taken, histopathology of lesion showed increased osteoblastic activity and irregular cortical bones with presence of hematoxyphilic reversal line giving the characteristic mosaic pattern. Marrow spaces were filled with fibro vascular connective tissue, confirming osteitis deformans [Figure 5]. Biochemical analysis showed abnormal increase of alkaline phosphatase (ALP) enzyme level (1368.1 U/l) [Figure 6] while comparing with the normal values of 30-120 U/l (above 17 years) and 74-390 U/l (below 17 years). Serum calcium and phosphorus levels were within the normal limits. Based on the clinical, radiographic, histopathological, and biochemical findings, a diagnosis of mandibular PD was rendered and was explained to the patient.
Figure 5.
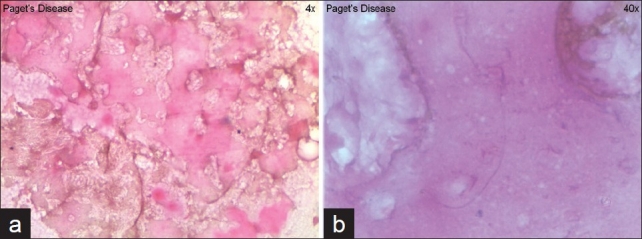
(a) Irregular reversal line, (b) mosaic bone pattern histologically
Figure 6.
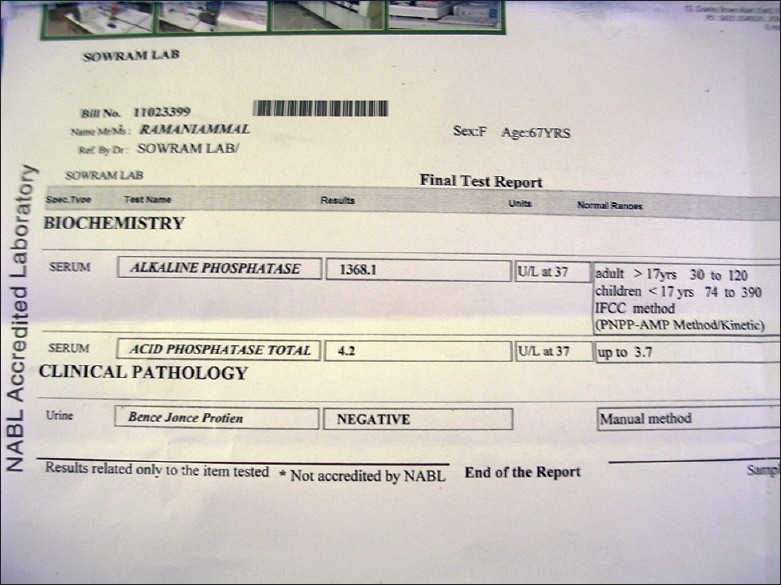
Biochemical report
DISCUSSION
Although PD is a relatively common disease in western countries like England, United States, New Zealand, Canada, South Africa, and France,[1,3] incidence in Asian population that too among Indians are very rare. Britain has the highest recorded prevalence. Within Britain and America, it is not uniformly distributed among all the states. There is localized area of high prevalence in Lancashire and Yorkshire in North America. Prevalence is markedly higher in New York than in Atlanta, although the disease rare in Africa. Incidence among American blacks and whites are equal.
PD is a geriatric disease reported above 5th to 6th decades of life, occurs both in men and women, with male predominance of approximately 3:2 ratio.[9] Shashank et al. have described juvenile form of PD different from the adult form. Etiology of PD is unknown, but there is a hypothesis that vitamin D deficiency in children may express later as PD. Wide variations in PD prevalence are not understood. Studies have suggested an association with viral infection.[10] Inclusion bodies resembling paramyxovirus nucleocapsid particles have been observed in pagetic osteoclasts by electron microscopy[11,12] and canine distemper virus has been localized by in-situ hybridization in the bone cells of patients with disorder.[13] There is familial tendency of occurrence of PD and it is associated with polymorphisms in DNA coding in centrosome structure. Genetics could therefore also play a major role in determining prevalence of PD.[14] But, it is evident from the decline in disease prevalence in Britain that undifferentiated environmental factors is also a major contributor.[15]
The diagnosis of PD of the jaw is generally made only on clinical grounds. The specimen is received commonly after prosthetic repair. Isolated involvement of one or both the jaws, the clinical and radiographic appearances may be interpreted as those of chronic sclerosing osteomyelitis in such condition, histological examination of bone or teeth may be suggestive if not conclusive performance of biochemical test and adequate follow-up of the patient. If the disease affects isolated areas of bone such as maxilla or mandible, the biochemical values will be within the normal limits, whereas our case shows an increase of ALP level to many folds (1368.1 U/l) despite isolate involvement of mandible.[16]
As the stresses of mastication causes very active turnover of bone, the jaw bones contain numerous irregular reversal lines than does bone from other parts.[17] Healing infectious periostitis with excessive bone formation as a possible cause of numerous cement lines produces chaotic (mosaic) bone architecture. But, when mosaics appear in conditions other than PD, their extent is limited and more regular, this fact coincide with our case, irregular mosaic pattern with numerous reversal line in our histopathological study.
Since the major defect in PD is exaggerated bone remodeling, treatment is based on powerful antiresorptive agent. Bisphosphonates form the main stay of medical management of PD. These agents suppress or reduce bone resorption by osteoclasts. Five bisphosphonates are approved by the US food and drug administration for the treatment of PD. These include pamidronate, which is given intravenously, etidronate, tiludronate, alendronate, and risedronate, all of which are taken orally. The case series from Vellore and Mumbai suggests that lower doses of alendronate might work well in Indian patients.[18,19] Calcitonin therapy is conventional but now infrequently used therapy. Pain management and surgery are also used when indicated.
CONCLUSION
We report a rare case of isolated mandibular PD in a 65-year-old Indian female patient with abnormal increase of ALP level (1368.1 U/l) considered to be unique in our case.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Wang WC, Cheng YS, Chen CH, Lin YJ, Chen YK, Lin LM. Paget's disease of bone in a Chinese patient: A case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:727–33. doi: 10.1016/j.tripleo.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Kanis JA. Editor Pathophysiology and treatment of Paget's diseases of bone. 2nd ed. London: Martin Dunitz; 1998. [Google Scholar]
- 3.Fredrick R, Singer CH. Bone program. Santa Monica, California: A Publication of the Paget Foundation for Paget's Disease of Bone and Related Disorders; 2008. Paget's disease of bone. [Google Scholar]
- 4.Neville BW, Damm DD, Allen CM, Bouquot JE. Bone pathology. 2nd ed. Philadelphia: WB Saunders; 2002. Paget's disease of bone. Oral and Maxillofacial Pathology. Chapter 14. [Google Scholar]
- 5.Bender IB. Paget's disease. J Endod. 2003;29:720–3. doi: 10.1097/00004770-200311000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Smith NH. Monostotic Paget's disease of the mandible presenting with progressive resorption of the teeth. Oral Surg Oral Med Oral Pathol. 1978;46:246–55. doi: 10.1016/0030-4220(78)90199-8. [DOI] [PubMed] [Google Scholar]
- 7.Walsh JP, Ward LC, Stewart GO, Will RK, Criddle RA, Prince RL, et al. A randomized clinical trial comparing oral alendronate and intravenous pamidronate for the treatment of Paget's disease of bone. Bone. 2004;34:747–54. doi: 10.1016/j.bone.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Shawaff Authmanand. Paget's disease of mandible. J Oral Surg Oral Med Oral Pathol. 1969;l28:6. doi: 10.1016/0030-4220(69)90341-7. [DOI] [PubMed] [Google Scholar]
- 9.Joshi SR, Ambhore S, Butala N, Patwardhan M, Kulkarni M, Pai B, et al. Paget's disease from western India. J Assoc Physicians India. 2006;54:535–8. [PubMed] [Google Scholar]
- 10.Baslé MF, Fournier JG, Rozenblatt S, Rebel A, Bouteille M. Measles virus RNA detected in paget's disease bone tissue by in situ hybridization. J Gen Virol. 1986;67:907–13. doi: 10.1099/0022-1317-67-5-907. [DOI] [PubMed] [Google Scholar]
- 11.Mills BG, Singer FR, Weiner LP. Evidence for both respiratory syncitial virus and measles virus antigen in the osteoclasts of patients with paget's disease of bone. Clin Orthop. 1984;183:303–11. [PubMed] [Google Scholar]
- 12.Reddy SV, Singer FR, Rodoman GD. Bone marrow mononuclear cells from patients with Paget's disease contain measles virus nucleocapsid messenger ribonucleic acid that has mutations in a specific region of the sequence. J Clin Endocrinol Metab. 1995;80:2108–11. doi: 10.1210/jcem.80.7.7608263. [DOI] [PubMed] [Google Scholar]
- 13.Gordon MT, Anderson DC, Sharpe PT, Mee P. Consistent expression of canine distemper virus in Paget's disease. Bone. 1997;20:565. [Google Scholar]
- 14.Darroszewaka A, Stuart H. Ralston genetics of paget's disease of bone. Clin Sci. 2005;109:257–63. doi: 10.1042/CS20050053. [DOI] [PubMed] [Google Scholar]
- 15.Siris ES, Rodoman GD. Paget's disease of bone. In: Favus MJ, editor. Primer on metabolic bone diseases and disorders of mineral metabolism. 5th ed. Washington: American society for bone and mineral research; 2003. pp. 495–506. [Google Scholar]
- 16.Cahn L. Bone pathology as it relates to some phases of oral surgery. Oral Surg Oral Med Oral Pathol. 1948;1:917–33. doi: 10.1016/0030-4220(48)90116-9. [DOI] [PubMed] [Google Scholar]
- 17.Jaffe HL. Arch path. Vol. 15. Chicago: Dover Publications; 1933. Paget's disease of bone; pp. 83–131. [Google Scholar]
- 18.Anjali, Thomas N, Rajaratnam S, Shanthly N, Oommen R, Seshadri MS. Paget's disease of bone: Experience from a centre on southern India. J Assoc Physicians India. 2006;54:525–9. [PubMed] [Google Scholar]
- 19.Joshi SR, Ambhore S, Butala N, Patwardhan M, Kulkarni M, Pai B, et al. Paget's disease from western India. J Assoc Physicians India. 2006;54:535–8. [PubMed] [Google Scholar]


