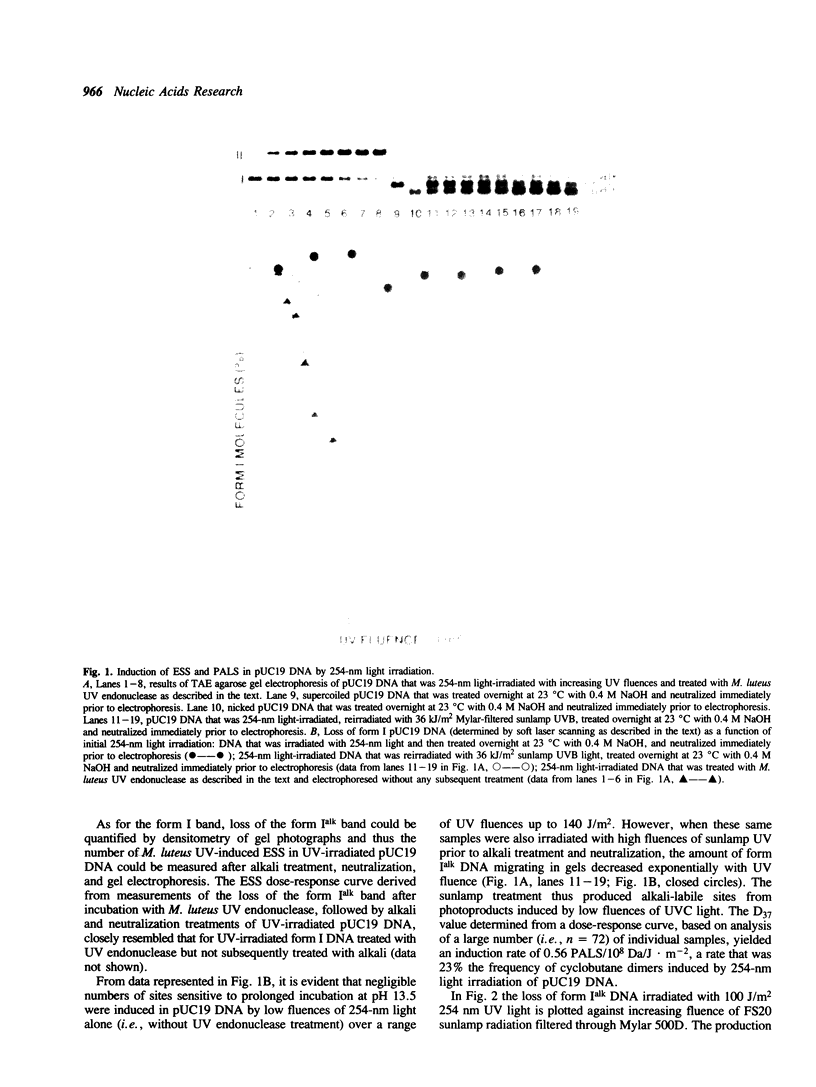
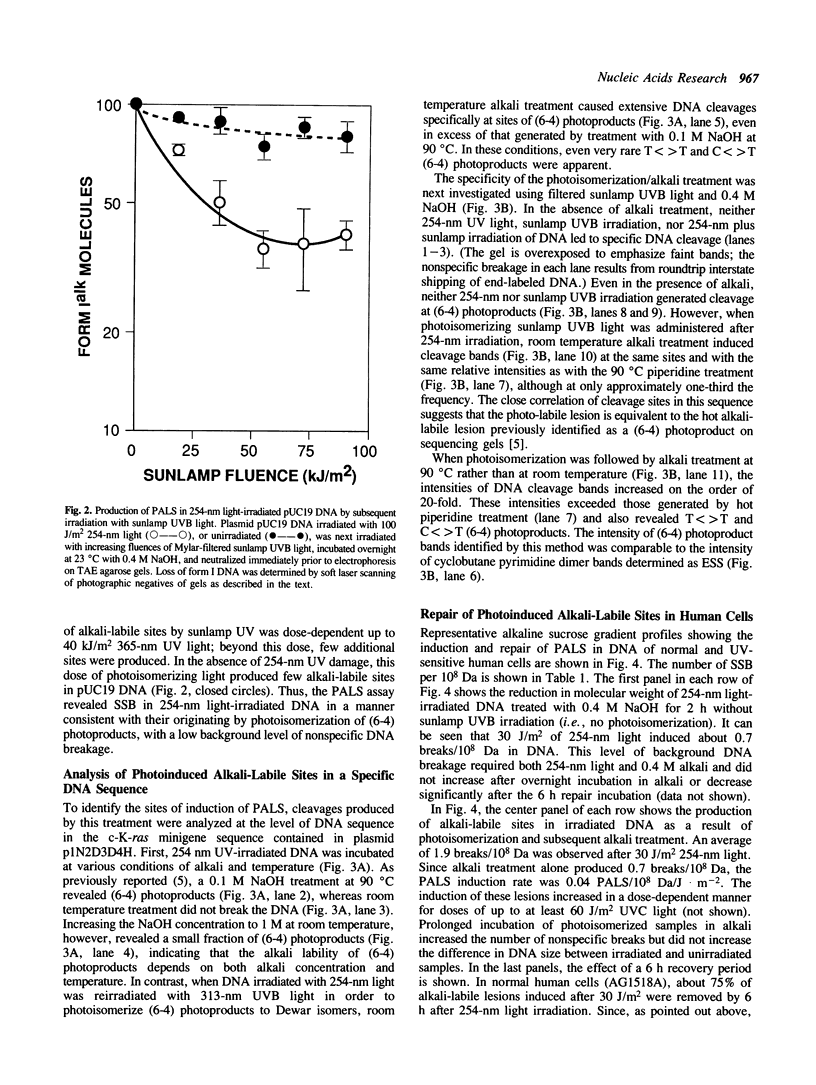
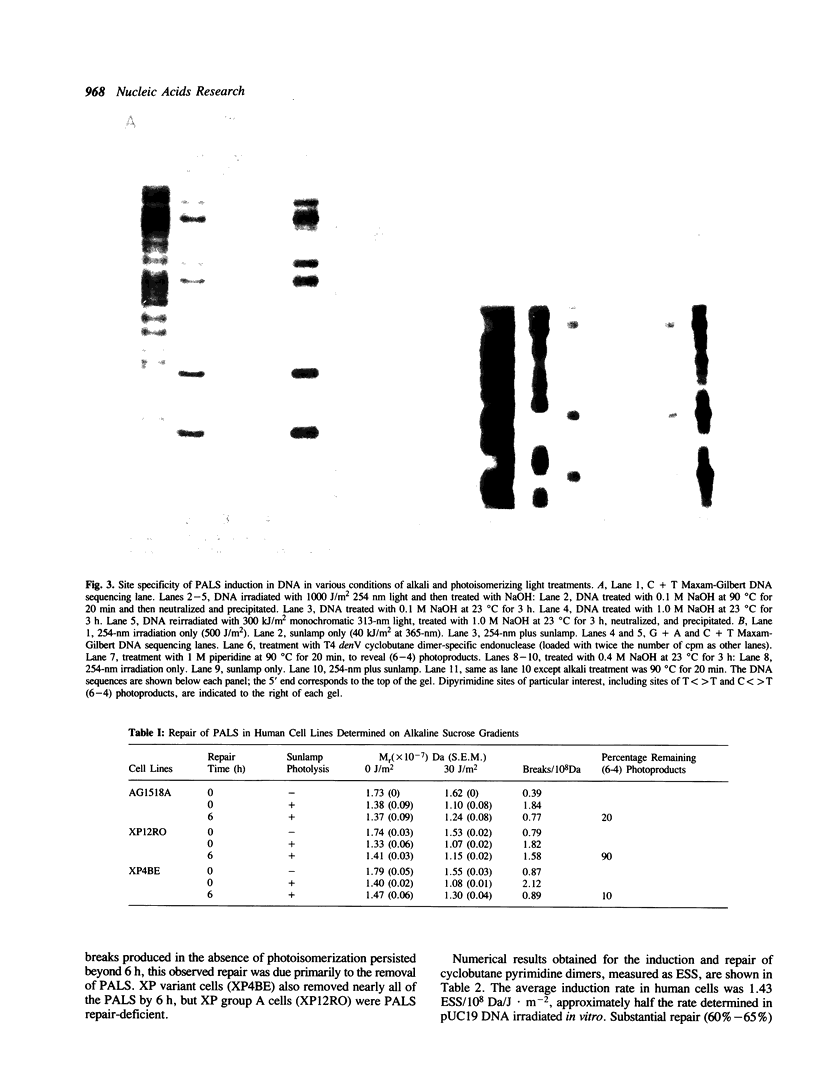
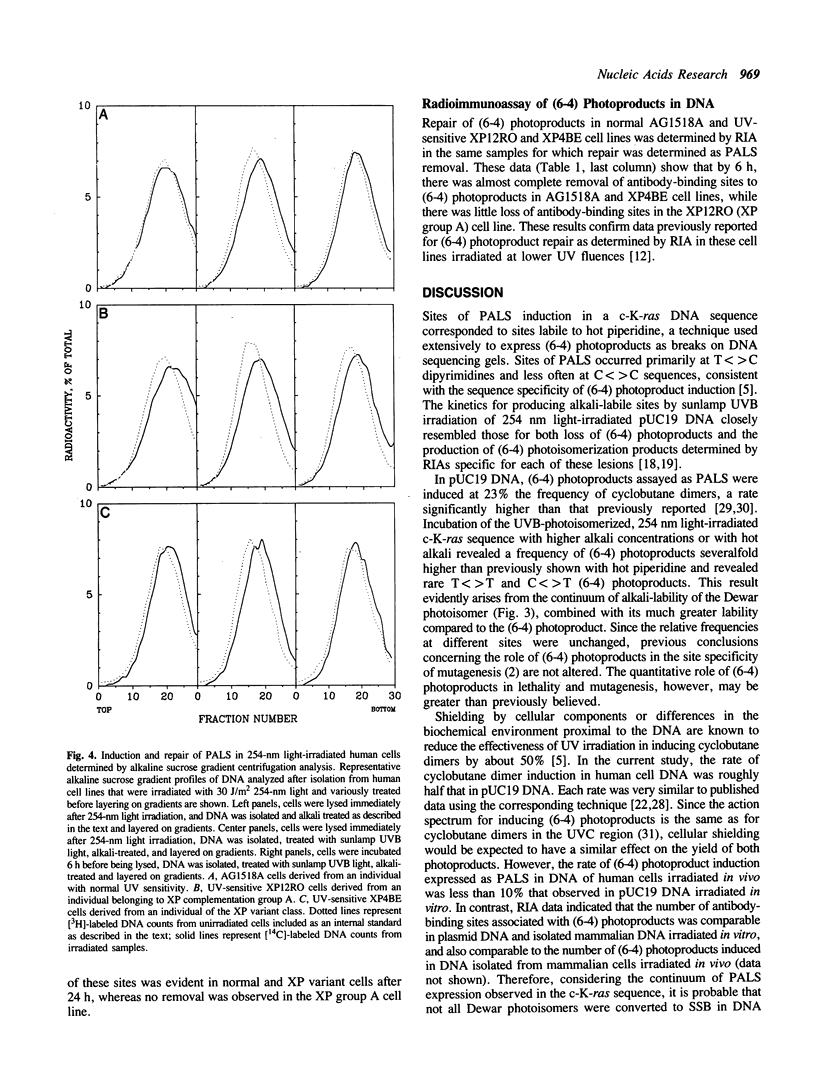
Abstract
UV-induced pyrimidine(6-4)pyrimidone photoproducts in DNA of mammalian cells are apparently repaired much more rapidly than cyclobutane dimers. Since only immunological assays for (6-4) photoproducts have been sensitive enough for repair measurements, it was possible that these apparently rapid repair kinetics reflected a change in physical conformation of antibody-binding sites, resulting in epitope loss rather than excision. To discriminate between these possibilities, we developed a procedure to photochemically convert (6-4) photoproducts to single-strand breaks in UV-irradiated DNA with a background low enough to permit repair measurements. Analysis of a specific DNA sequence indicated that photoinduced alkali-labile sites (PALS) were induced with the same site-specificity as (6-4) photoproducts. Normal human and xeroderma pigmentosum (XP) variant cells rapidly excised (6-4) photoproducts measured as PALS, but little repair was seen in cells from XP complementation group A. These repair kinetics corresponded to those determined in the same samples by radioimmunoassay of (6-4) photoproducts. Thus we conclude that the rapid repair of (6-4) photoproducts observed in UV-irradiated human cells is not the result of a conformational change resulting in epitope loss, but reflects excision of this photoproduct from DNA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brash D. E., Haseltine W. A. UV-induced mutation hotspots occur at DNA damage hotspots. Nature. 1982 Jul 8;298(5870):189–192. doi: 10.1038/298189a0. [DOI] [PubMed] [Google Scholar]
- Brash D. E. UV mutagenic photoproducts in Escherichia coli and human cells: a molecular genetics perspective on human skin cancer. Photochem Photobiol. 1988 Jul;48(1):59–66. doi: 10.1111/j.1751-1097.1988.tb02786.x. [DOI] [PubMed] [Google Scholar]
- Clarkson J. M., Hewitt R. R. Significance of dimers to the size of newly synthesized DNA in UV-irradiated Chinese hamster ovary cells. Biophys J. 1976 Oct;16(10):1155–1164. doi: 10.1016/S0006-3495(76)85764-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J. M., Mitchell D. L., Adair G. M. The use of an immunological probe to measure the kinetics of DNA repair in normal and UV-sensitive mammalian cell lines. Mutat Res. 1983 Oct;112(5):287–299. doi: 10.1016/0167-8817(83)90004-4. [DOI] [PubMed] [Google Scholar]
- Eggset G., Volden G., Krokan H. Characterization of antibodies specific for UV-damaged DNA by ELISA. Photochem Photobiol. 1987 Apr;45(4):485–491. doi: 10.1111/j.1751-1097.1987.tb05407.x. [DOI] [PubMed] [Google Scholar]
- Francis A. A., Regan J. D. Detection and repair of a UV-induced photosensitive lesion in the DNA of human cells. Mutat Res. 1986 May;165(3):151–157. doi: 10.1016/0167-8817(86)90049-0. [DOI] [PubMed] [Google Scholar]
- Ikenaga M., Patrick M. H., Jagger J. Photoreactivation of killing in Streptomyces. 3. Action spectra for photolysis of pyrimidine dimers and adducts in S. griseus and S. griseus PHR-1. Photochem Photobiol. 1971 Aug;14(2):175–187. doi: 10.1111/j.1751-1097.1971.tb06161.x. [DOI] [PubMed] [Google Scholar]
- Lippke J. A., Gordon L. K., Brash D. E., Haseltine W. A. Distribution of UV light-induced damage in a defined sequence of human DNA: detection of alkaline-sensitive lesions at pyrimidine nucleoside-cytidine sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3388–3392. doi: 10.1073/pnas.78.6.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D. L., Clarkson J. M. Induction of photoproducts in synthetic polynucleotides by far and near ultraviolet radiation. Photochem Photobiol. 1984 Dec;40(6):735–741. doi: 10.1111/j.1751-1097.1984.tb04645.x. [DOI] [PubMed] [Google Scholar]
- Mitchell D. L., Clarkson J. M. The development of a radioimmunoassay for the detection of photoproducts in mammalian cell DNA. Biochim Biophys Acta. 1981 Aug 27;655(1):54–60. doi: 10.1016/0005-2787(81)90066-6. [DOI] [PubMed] [Google Scholar]
- Mitchell D. L., Haipek C. A., Clarkson J. M. (6-4)Photoproducts are removed from the DNA of UV-irradiated mammalian cells more efficiently than cyclobutane pyrimidine dimers. Mutat Res. 1985 Jul;143(3):109–112. doi: 10.1016/s0165-7992(85)80018-x. [DOI] [PubMed] [Google Scholar]
- Mitchell D. L., Haipek C. A., Clarkson J. M. Xeroderma pigmentosum variant cells are not defective in the repair of (6-4) photoproducts. Int J Radiat Biol Relat Stud Phys Chem Med. 1987 Aug;52(2):201–205. doi: 10.1080/09553008714551661. [DOI] [PubMed] [Google Scholar]
- Mitchell D. L., Humphrey R. M., Adair G. M., Thompson L. H., Clarkson J. M. Repair of (6-4)photoproducts correlates with split-dose recovery in UV-irradiated normal and hypersensitive rodent cells. Mutat Res. 1988 Jan;193(1):53–63. doi: 10.1016/0167-8817(88)90007-7. [DOI] [PubMed] [Google Scholar]
- Mitchell D. L., Nairn R. S., Alvillar J. A., Clarkson J. M. Loss of thymine dimers from mammalian cell DNA. The kinetics for antibody-binding sites are not the same as that for T4 endonuclease V sites. Biochim Biophys Acta. 1982 Jun 30;697(3):270–277. doi: 10.1016/0167-4781(82)90089-6. [DOI] [PubMed] [Google Scholar]
- Mitchell D. L., Nairn R. S. The (6-4) photoproduct and human skin cancer. Photodermatol. 1988 Apr;5(2):61–64. [PubMed] [Google Scholar]
- Mitchell D. L., Nairn R. S. The biology of the (6-4) photoproduct. Photochem Photobiol. 1989 Jun;49(6):805–819. doi: 10.1111/j.1751-1097.1989.tb05578.x. [DOI] [PubMed] [Google Scholar]
- Mitchell D. L., Rosenstein B. S. The use of specific radioimmunoassay to determine action spectra for the photolysis of (6-4) photoproducts. Photochem Photobiol. 1987 Jun;45(6):781–786. doi: 10.1111/j.1751-1097.1987.tb07882.x. [DOI] [PubMed] [Google Scholar]
- Mitchell D. L. The induction and repair of lesions produced by the photolysis of (6-4) photoproducts in normal and UV-hypersensitive human cells. Mutat Res. 1988 Nov;194(3):227–237. doi: 10.1016/0167-8817(88)90024-7. [DOI] [PubMed] [Google Scholar]
- Mitchell D. L. The relative cytotoxicity of (6-4) photoproducts and cyclobutane dimers in mammalian cells. Photochem Photobiol. 1988 Jul;48(1):51–57. doi: 10.1111/j.1751-1097.1988.tb02785.x. [DOI] [PubMed] [Google Scholar]
- Mori T., Matsunaga T., Hirose T., Nikaido O. Establishment of a monoclonal antibody recognizing ultraviolet light-induced (6-4) photoproducts. Mutat Res. 1988 Nov;194(3):263–270. doi: 10.1016/0167-8817(88)90028-4. [DOI] [PubMed] [Google Scholar]
- Protić-Sabljić M., Kraemer K. H. One pyrimidine dimer inactivates expression of a transfected gene in xeroderma pigmentosum cells. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6622–6626. doi: 10.1073/pnas.82.19.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstein B. S., Mitchell D. L. Action spectra for the induction of pyrimidine(6-4)pyrimidone photoproducts and cyclobutane pyrimidine dimers in normal human skin fibroblasts. Photochem Photobiol. 1987 Jun;45(6):775–780. doi: 10.1111/j.1751-1097.1987.tb07881.x. [DOI] [PubMed] [Google Scholar]
- Rosenstein B. S. Photoreactivation of ICR 2A frog cells exposed to solar UV wavelengths. Photochem Photobiol. 1984 Aug;40(2):207–213. doi: 10.1111/j.1751-1097.1984.tb04577.x. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Sibghatullah, Husain I., Carlton W., Sancar A. Human nucleotide excision repair in vitro: repair of pyrimidine dimers, psoralen and cisplatin adducts by HeLa cell-free extract. Nucleic Acids Res. 1989 Jun 26;17(12):4471–4484. doi: 10.1093/nar/17.12.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. H., Mitchell D. L., Regan J. D., Bouffler S. D., Stewart S. A., Carrier W. L., Nairn R. S., Johnson R. T. CHO mutant UV61 removes (6-4) photoproducts but not cyclobutane dimers. Mutagenesis. 1989 Mar;4(2):140–146. doi: 10.1093/mutage/4.2.140. [DOI] [PubMed] [Google Scholar]
- Wood R. D. Repair of pyrimidine dimer ultraviolet light photoproducts by human cell extracts. Biochemistry. 1989 Oct 17;28(21):8287–8292. doi: 10.1021/bi00447a005. [DOI] [PubMed] [Google Scholar]
- Yamamoto F., Perucho M. Activation of a human c-K-ras oncogene. Nucleic Acids Res. 1984 Dec 11;12(23):8873–8885. doi: 10.1093/nar/12.23.8873. [DOI] [PMC free article] [PubMed] [Google Scholar]





