Abstract
Background:
This study was carried out to assess the innervation patterns in oral cancer using the general neuronal marker protein gene product 9.5 (PGP 9.5) and to find an explanation for why oral cancer tends to be painless in the early stages.
Materials and Methods:
Tumor tissue from 30 unselected patients with oral squamous cell carcinoma was evaluated for this study. PGP 9.5 was used to localize nerve fibers in oral squamous cell carcinoma. An indirect immunofluorescence technique using biotin/FITC streptavidin detection system was used on paraffin wax sections of tumor tissue fixed in neutral buffered formalin.
Results:
There was no PGP9.5 immunoreactivity in the normal tissue adjacent to the tumor in 18 cases out of 30 (60%). In 12/30 of oral squamous cell carcinoma, preexisting nerve fibers were detected in tissue stroma adjacent to the cancer tissue. Labeled nerve fibers between tumor cells were detected only in 2 out of the 30 cases.
Conclusion:
There are no apparent patterns of innervations in the cancer tissues themselves, but there are innervations patterns in the surrounding tissue, which presumably represents preexisting nerves. These results may partly explain why oral cancer tends to be painless in the early stages.
Keywords: Indirect immunofluorescence technique, oral squamous cell carcinoma, tumor innervation
INTRODUCTION
The lesions of oral cancer are always painless in the early stages. There is often severe pain in the later stages, though this represents involvement of the nerves associated with oral cancer itself or is merely the result of an increased stimulation of preexisting nerves or is due to secondary infection of the lesions is unclear.
Perineural invasion and vascular invasion may be adverse prognostic factors in patients with oral squamous cell carcinoma.[1] Perineural invasion is an uncommon spread pattern observed in patients with skin cancer and is associated with a relatively poor prognosis.[2]
Perineural invasion of small nerves is associated with an increased risk of local recurrence and cervical metastasis and is independent of extracapsular spreada predictor of survival for patients with squamous cell carcinoma of the upper aerodigestive tract.[3]
Angiogenesis plays a role in the spread of the cancer, and there is significant correlation between tumor vascular density and lymph node metastasis.[4] Angiogenesis in oral cancer showed a strong correlation with regional recurrence in one study. Although tumor thickness was associated with recurrence, only angiogenesis was a statistically significant predictor of recurrence.[5] Vascular invasion—but not perineural invasion—was significantly associated with death at 5-year follow-up.[1]
The blood vessels inside the tumor are not innervated and the angiogenesis may be regulated by means other than neural.[6] Innervation does not therefore seem to be a prerequisite for tumor growth and metastasis.[7]
Mitchell et al. used the neuronal protein gene product 9.5 (PGP 9.5) to investigate the innervation pattern of benign and malignant breast tumors. An indirect immunofluorescence technique (using streptavidin-biotin complex formation) was used on paraffin sections of tumor tissue fixed in neutral buffered formalin.[6] PGP 9.5-immunoreactive perivascular nerve fibers were observed in the connective tissue stroma around the carcinoma tissue. Connective tissue had large bundles of immunoreactive nerve fibers located at the periphery of the tumor, at the junction between the cancer tissue and normal tissue, although these were possibly immunoreactive nerve fibers unrelated to the tumors. No nerve fibers were observed between the tumor cells. Also, using the neuronal marker protein product (PGP 9.5) to localize the nerves fibers, colorectal carcinoma appeared not to be innervated.[6]
S100 protein (nerve tissue protein) was found in 7 out of 60 rhabdomyosarcoma tumors.[8] S-100 protein, a soluble protein, was reported to be a good marker for Schwann cell structure and/or function.[9]
Neuron-specific enolase (NSE) is an isomer of the glycolytic enzyme enolase, which is found in nerves and neuroendocrine cells. NSE is a neuroendocrine marker that can be detected by immunofluorescence technique in paraffin sections of neuroendocrine neoplasms of the lung.[10] Different levels of NSE have been described in both argyrophilic and nonargyrophilic cancer of the breast.[7]
Neurofilament is a neuronal cytoskeletal element found in central and peripheral neurons.[11] Immunostaining for neurofilament protein was found to be specific for the nervous system.[12]
Monoclonal and polyclonal antibodies to neurofilaments, NSE, glial fibrillary protein, and S100 have been used to demonstrate nerves, ganglion cells, and the supportive glial system of the innervation of various organs. They are therefore possible means for assessing the morphological and functional status of the enteric nervous system.[11,12]
PGP 9.5 is the best immunohistological marker for nerves and their processes at all levels of the central and peripheral nervous system and can be clearly visualized in routinely processed tissue using a polyclonal antibody. PGP 9.5 may be a useful marker for tumors that contain both neural and neuroendocrine tissue.[13] The neuronal marker PGP 9.5 has been claimed to be a more sensitive neuronal marker than NSE and neurofibrillary protein. There is a difference in intraneuronal distribution and/or function between NSE and PGP 9.5. PGP 9.5 can be present in all populations and subtypes of nerves and can discriminate the more terminal axonal structures,[14] but NSE and neurofibrillary protein do not stain all populations of nerves and all neuronal regions.[11]
The innervation of oral and dental tissue has been investigated in many studies by using PGP9.5. PGP9.5 is a useful marker for the identification of fine nerve fibers in human teeth. Human radicular dental pulp has been shown to be heavily innervated by PGP9.5 nerve fibers. The PGP9.5-positive nerve fibers penetrate into the predentine and dentine.[15] Garzino et al. used PGP 9.5 to compare the density of oral mucosal innervation in edentulous patients with that in dentate controls and to evaluate changes in the number or type of sensory receptors following placement of implants in the edentulous individuals.[16]
On the other hand, S-100 protein appears to be localized in glial cells only. It has also been found in neuroendocrine cells and nonneural cells.[17]
MATERIALS AND METHODS
Tumor tissues selected randomly from 30 patients (20 males and 10 females) with oral squamous cell carcinoma were evaluated for this study. Tissue samples were selected from 30 surgically resected oral squamous cell carcinomas and fixed in 10% neutral buffered formalin and processed to paraffin wax.
The oral squamous cell carcinomas samples were histologically graded according to the WHO grading system (1971). Seven samples were classified as grade 1, 12 samples as grade 2, and 11 samples as grade 3.
Tissue processing
Standard histology laboratory equipment was used, including hot-wax dispenser, heated water bath, 60°C oven, and microtome.
Tissues were first fixed in 10% neutral buffered formalin solution. Then they were washed in 70% industrial methylated spirits for 30 min, each time. The tissues were trimmed and washed in 95% industrial methylated spirits for 30 min, each time. After that, they were washed in 100% industrial methylated spirits, first for 30 min and then again for 1 hour. Then they were washed in 100% ethanol, first for 30 min and then again for 1 hour. Finally, they washed in clearing agent (xylene), first for 30 min and then again for 1 hour.
Sections were infiltrated with Ralwax at 60°C overnight, and then blocked out. The sections were then dried overnight at 50°C. Finally, cover slips were placed on sections and they were incubated overnight at 60°C.
Immunofluorescent staining method
The apparatus used was standard histology laboratory equipment and included staining troughs for dewaxing and rehydration of sections, a flat-bottomed tray chamber with lid (in which a humid atmosphere can be maintained for antiserum incubations), and conversional fluorescence microscopy.
The solutions used were:
Phosphate-buffered saline (PBS) (pH 7.4)
Antibody diluent: The antibody diluents was made by dissolving 0.5 g sodium azide (bacteriostatic) and 0.5 g bovine serum albumin fraction V in 500 ml of PBS, along with 2.5 ml of Triton® X-100 (detergent agent), and was stored at 4°C.
PBS/Glycerol
Primary antiserum: Polyclonal rabbit anti-PGP9.5 diluted 1:400. The diluted primary antiserum was stored at −20°C
Secondary antiserum: Biotinylated donkey anti-rabbit IgG diluted 1:200. Biotinylated secondary antiserum was stored at 4°C
FITC-streptavidin
Control slides, in which the primary antibody was omitted and substituted with nonimmune rabbit serum, showed no immunoreactivity.
An indirect immunofluorescence method using the biotin/FITC-streptavidin detection system was used.[18] Sections were dewaxed in xylene for 2 min, and each time. Then washed in 100% ethanol for 3 min, each time. Then, they were washed in 95% ethanol for 3 min and then again in 70% ethanol for 3 min. Next, the sections were dipped in distilled water for 10 seconds, and then washed in PBS for at least 5 min. Coverslips supported were arranged on embryo dishes in immunostaining chamber. Wet, folded tissue in the base of the tray ensured the humid atmosphere required to prevent the evaporation of antiserum during prolonged incubations.
The sections were incubated in polyclonal rabbit anti-PGP9.5 diluted 1:400 and incubated at 4°C overnight. Biotinylated donkey anti-rabbit IgG diluted 1:200 were applied on sections and they were incubated for 30 min at room temperature. Many molecules of biotin can be conjugated to the Fc portion of one immunoglobulin molecule. Biotin combines tightly with streptavidin.[19] The sections were washed in PBS for 5 min (to remove unbound antibodies) and then incubated in streptavidin-FITC for 30 min in the dark at room temperature. Fluorescein is light sensitive and the sections should remain in the dark, washed in PBS for 5 min in the dark. Finally, they were mounted using PBS/glycerol.
It should be mentioned here that healthy tissues were also studied using immunofluorescence techniques and no evidence of abnormalities was noticed.
RESULTS
In oral squamous cell carcinoma, a characteristic pattern of innervation was observed in the normal tissue adjacent to the tumor. PGP9.5-labeled nerve fibers were detected in the tissue adjacent to the tumor in 12 cases out of 30 (40%). In three tumors, there were quite large bundles of immunoreactive nerve fibers in the supporting tissue around the cancer tissue. A large bundle of PGP9.5-immunoreactive nerve fibers were seen passing through the supporting tissue around the tumor [Figure 1].
Figure 1.
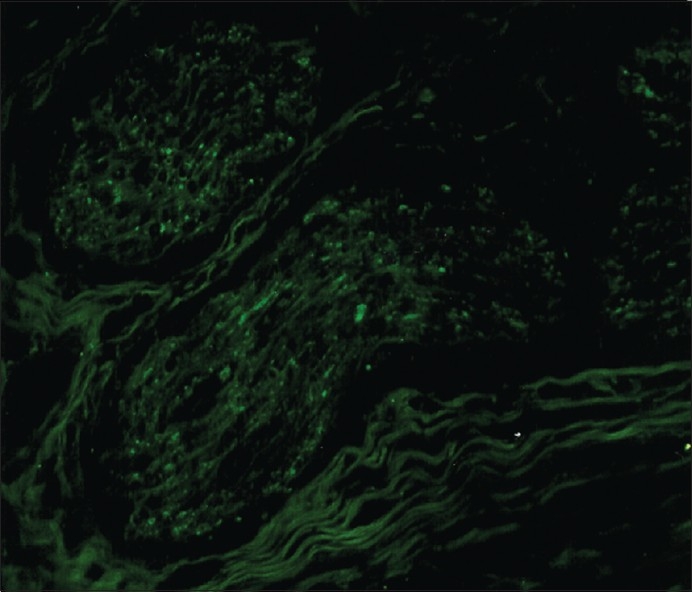
A large bundle of PGP 9.5-immunoreactive nerve fibers passing through supporting tissue around the tumor (out of picture) in a specimen of oral squamous cell carcinoma (original magnification ×240)
There was no evidence of fine nerve fibers passing between the tumor cells unrelated to vasculature (except in two cases). Some blood vessels were immunoreactive for PGP9.5. Normal glandular tissue was also immunoreactive for PGP9.5. PGP9.5-immunoreactive nerve fibers were seen passing through the glandular tissue around the tumor tissue [Figure 2].
Figure 2.
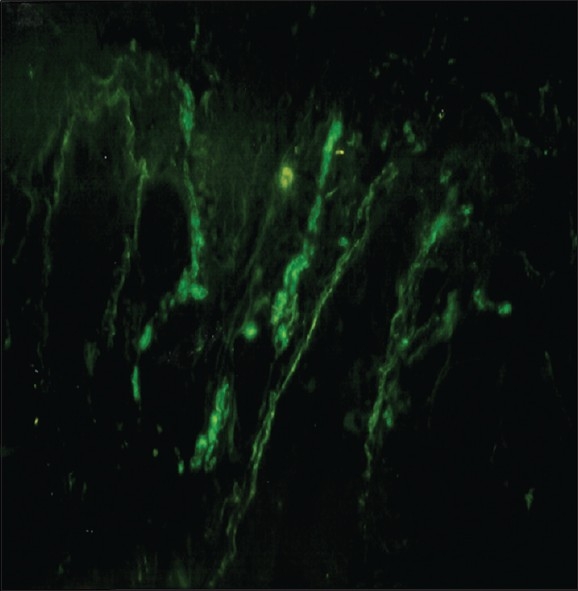
PGP 9.5-immunoreactive nerve fibers passing through the glandular tissue around the tumor tissue (out of picture) in a specimen of oral squamous cell carcinoma (original magnification ×240)
There was no PGP9.5 immunoreactivity in the normal tissue adjacent to the tumor tissue in oral squamous cell carcinoma in 18 cases out of 30 (60%).
The tumor tissue itself, however, was not apparently innervated. PGP9.5-labeled nerve fibers were detected only in 2 cases out of 30 (6.6%) in the tumor tissue itself [Figure 3] One case (stage: TINO, grade 2; site: Floor of the mouth anteriorly) was interesting because it had a fair number of nerve fibers passing between the tumor cells. PGP9.5-immunoreactive fine nerve fibers were seen in the affected area of the tissue samples of this case [Figure 4]. It was the only tumor examined in which it could be concluded that there was an innervation pattern in the tumor tissue itself.
Figure 3.
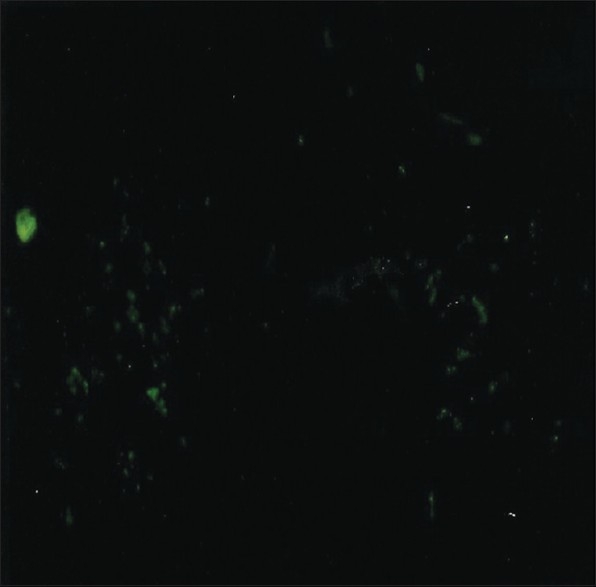
A large number of PGP 9.5-immunoreactive nerve fibers passing through the tumor tissue between the tumor cells in an oral squamous cell carcinoma (original magnification ×240)
Figure 4.
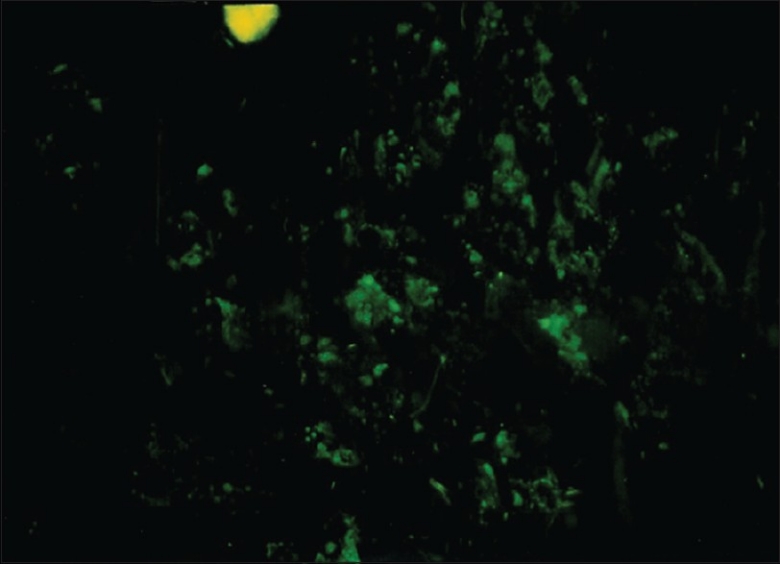
Part of an oral squamous cell carcinoma immunoreacted for PGP 9.5. Labeled nerve fibers can be seen inside the tumor tissue between the tumor cells (original magnification ×480)
In another case (stage: TINO, grade 3; site: Retromolar pad area), a labeled nerve bundle was found within the tumor tissue. This nerve bundle was surrounded by tumor cells. The nerve was small to medium in size and presumably had been engulfed due to tumor expansion [Figure 5].
Figure 5.

A bundle of PGP 9.5-immunorective nerve fibers can be seen in the tumor tissue of oral squamous cell carcinoma, apparently engulfed by tumor tissue (original magnification ×480)
Neither the site of the tumor nor the stage or the grade of tumor had any bearing on the innervation pattern.
Control slides, in which the primary antibody was omitted and substituted with nonimmune rabbit serum, showed no immunoreactivity.
DISCUSSION
The neural marker protein gene product (PGP9.5) that was used in this study is regarded as the best immunohistological marker for nerves and their processes at all levels of the central and peripheral nervous system; it can be clearly visualized in routinely processed tissue using a rabbit polyclonal antibody.[13] PGP9.5 is considered as a more sensitive means of labeling nerve fibers (especially their terminal regions) than NSE and neurofibrillary protein.[14]
Fantini et al. used PGP9.5 to define the innervation patterns of oral mucosa. PGP9.5 displayed denser and more complete staining of neural structures of oral mucosa than the other markers.[20] PGP9.5 is a neuron-specific ubiquitin carboxyl terminal hydrolase estimated to form 1%–2% of total brain protein. It is present at high levels in all differentiated neurons throughout the central and peripheral nervous system at all stages of development.[21]
The indirect staining method that was used in this study is more sensitive than the direct methods. The primary antibody is unlabeled and is identified by a labeled secondary antibody raised to the immunoglobulin of the species providing the primary antibody. Because at least two secondary molecules can bind to each primary antibody molecule, this method is more sensitive than the direct method.[22–24]
Conventional formalin fixation was used in this study and paraffin embedding is often satisfactory for immunofluorescence methods with the sensitive antibodies and detection system that are used. Formalin is preferred over other fixatives in neuropeptide immunocytochemistry as it provides good structural preservation.[22–24]
In the present study, the marker PGP9.5 was used to localize nerve fibers in oral squamous cell carcinoma. PGP9.5 immunoreactivity for nerve fibers was detected in the tissues adjacent to the tumor. This innervation is to be expected in normal tissues adjacent to the tumor. PGP9.5 can evaluate and define the entire normal oral innervation and it is considered as the most reliable general marker for the visualization of oral innervation.[20] In this study, with the exception of two tumors, no innervation was detected in the oral tumor tissues themselves. Innervation detected in the surrounding tissues can be presumed to be preexisting nerves.
It is most likely that the innervated blood vessels in this study were preexisting blood vessels that have come to lie close to the tumor as its size increased.
Perineural invasion and vascular invasion may both be adverse prognostic factors in patients with oral squamous cell carcinoma, but vascular invasion is more significantly associated with death.[1]
The subepithelial and perivascular neural plexuses are clearly and consistently labeled with PGP 9.5 staining in normal oral mucosa.[20]
The tumor tissue itself was not apparently innervated. PGP9.5-labeled nerve fibers were detected in the tumor tissue itself only in 2 cases out of 30. One of these cases had a fair number of nerve fibers passing between the tumor cells. In this case, the PGP 9.5-immunoreactive fine nerve fibers could be seen clearly and it could be concluded that there was an innervation pattern in the tumor tissue itself. In the other case, a medium-sized labeled nerve bundle was found within the tumor tissue. This nerve bundle was surrounded by tumor cells, and had presumably been engulfed by tumor expansion.
Sometimes there were quite large bundles of immunoreactive nerve fibers in the supporting tissue around the cancer tissue; these were presumably preexisting large nerves.
There was no PGP 9.5 immunoreactivity in the normal tissue adjacent to the tumor in 18 cases out of 30 (60%). This observation is not unique. Mitchell et al. also observed that there was no PGP 9.5 immunoreactivity in normal tissue adjacent to breast cancer in 7 out of the 16 cases (44%) in their series.[6]
The cytoplasmic immunoreactivity of the tumor cells in one case of squamous cell carcinoma requires further discussion. PGP 9.5 immunoreactivity of cancer cells has been observed in colorectal carcinoma and breast cancer,[6] but this is the first time it is being reported in oral squamous cell carcinoma. The results of this study offer an explanation for why oral cancer is always painless in the early stages. There was no new nerve supply in oral tumor tissues themselves. The nerve fibers in the surrounding tissues are presumably preexisting nerves.
Pain and tenderness usually develop when a malignant lesion becomes secondarily infected or if the lesion invades sensory nerves.
CONCLUSIONS
There is no apparent innervation of the cancer tissues themselves.
There is an innervation pattern in the surroundings tissue, presumably preexisting nerves.
Normal glandular tissue associated with the oral cancer is innervated.
Innervated blood vessels were seen, probably preexisting blood vessels that had come close to the tumor as the size of the tumor increased.
The results of this study explain why oral cancer is always painless in the early stages.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Kurtz KA, Hoffman HT, Zimmerman MB, Robinson RA. Perineural and vascular invasion in oral cavity squamous carcinoma: Increased incidence on re-review of slides and by using immunohistochemical enhancement. Arch Pathol Lab Med. 2005;129:354–9. doi: 10.5858/2005-129-354-PAVIIO. [DOI] [PubMed] [Google Scholar]
- 2.Mendenhall WM, Amdur RJ, Williams LS, Mancuso AA, Stringer SP, Price Mendenhall N, et al. Carcinoma of the skin of the head and neck with perineural invasion. Head Neck. 2002;24:78–83. doi: 10.1002/hed.10025. [DOI] [PubMed] [Google Scholar]
- 3.Fagan JJ, Collins B, Barnes L, D’Amico F, Myers EN, Johnson JT. Perineural invasion in squamous cell carcinoma of the head and neck. Arch Otolarngeal Head Neck Surg. 1998;124:637–40. doi: 10.1001/archotol.124.6.637. [DOI] [PubMed] [Google Scholar]
- 4.Penfold CN, Partridge M, Rojas R, Langdon JD. The role of angiogenesis in the spread of oral squamous cell carcinoma. Br J Oral Maxillofac Surg. 1996;34:37–41. doi: 10.1016/s0266-4356(96)90133-3. [DOI] [PubMed] [Google Scholar]
- 5.Williams JK, Carlson GW, Cohen C, Derose PB, Hunter S, Jurkiewicz MJ. Tumour angiogenesis as a prognostic factor in oral cavity tumours. Am J Surg. 1994;168:73–380. doi: 10.1016/s0002-9610(05)80079-0. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell BS, Schumacher U, Kaiserling E. Are tumours innervated? Immunohistological investigation using antibodies against the neuronal marker protein gene product 9.5 (PGP9.5) in benign, malignant and experimental tumours. Tumour Biol. 1994;15:269–74. doi: 10.1159/000217901. [DOI] [PubMed] [Google Scholar]
- 7.Schumacher U, Adam E, Flavell DA, Boehm D, Loizidou M, Brooks S, et al. A model system for metastasing human colon cancer in SCID mice. Verh Anat Ges. 1993;88:185. [Google Scholar]
- 8.Coindre JM, de Mascarel A, Trojani M, de Mascarel I, Pages A. Immunohistochemical study of rhabdomyosarcoma. Unexpected staining with S100 protein and cytokeratin. J Pathol. 1988;155:127–32. doi: 10.1002/path.1711550209. [DOI] [PubMed] [Google Scholar]
- 9.Alm P, Lundberg LM, Wharton J, Polak JM. Ontogenic development of the guinea pig uterine innervation. An immunohistochemical study of different neuronal markers, neuropeptides and S-100 protein. Histochemistry. 1988;90:19–24. doi: 10.1007/BF00495701. [DOI] [PubMed] [Google Scholar]
- 10.Springall DR, Lackie P, Levene MM, Marangos PJ, Polak JM. Immunostaining of neurone specific enolase is a valuable aid to the cytological diagnosis of neuroendocrine tumours of the lung. J Pathol. 1984;143:259–65. doi: 10.1002/path.1711430405. [DOI] [PubMed] [Google Scholar]
- 11.Bishop AE, Polak JM, Facer P, Ferri GL, Marangos PJ, Pearse AG. Neurone specific enolase, a common marker for endocrine cells and innervation of the gut and pancreas. Gastroenterology. 1982;83:902–12. [PubMed] [Google Scholar]
- 12.Hacker GW, Polak JM, Springall DR, Ballesta J, Cadieux A, Gu J, et al. Antibodies to neurofilament protein and other brain protein reveal the innervation of peripheral organs. Histochemistry. 1985;82:581–93. doi: 10.1007/BF00489980. [DOI] [PubMed] [Google Scholar]
- 13.Wilson PO, Barber PC, Hamid QA, Power BF, Dhillon AP, Rode J, et al. The immunolocalization of protein gene product 9.5 using rabbit polyclonal and mouse monoclonal antibodies. Br J Exp Path. 1988;69:91–104. [PMC free article] [PubMed] [Google Scholar]
- 14.Lundberg LM, Alm P, Wharton J, Polak JM. Protein gene product 9.5 (PGP 9.5). A new neuronal marker visualizing the whole uterine innervation and pregnancy induced and developmental changes in the guinea pig. Histochemistry. 1988;90:9–17. doi: 10.1007/BF00495700. [DOI] [PubMed] [Google Scholar]
- 15.Maeda T, Honma S, Takano Y. Dense innervation of human radicular dental pulp as revealed by immunocytochemistry for protein gene-product 9.5. Arch Oral Biol. 1994;39:563–8. doi: 10.1016/0003-9969(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 16.Garzino M, Ramieri G, Panzica G, Preti G. Changes in the density of protein gene product 9.5 immunoreactivity nerve fibers in human oral mucosa under implant retained overdentures. Arch Oral Biol. 1996;41:1073–9. doi: 10.1016/s0003-9969(96)00038-6. [DOI] [PubMed] [Google Scholar]
- 17.Trojanowski JQ, Lee VM. Monoclonal and polyclonal antibodies against neural antigens. Diagnostic application for studies of central and peripheralneuron system tumors. Human pathol. 1982;14:281–5. doi: 10.1016/s0046-8177(83)80111-7. [DOI] [PubMed] [Google Scholar]
- 18.Coggi G, Dell Orto P, Vial G. Avidin-biotin methods. In: Polak JM, Van Noorden S, editors. Immonocytochemistry, modern methods and applications. Wright, Bristol: 1986. pp. 54–70. [Google Scholar]
- 19.Bancroft JD, Cook HC. Immunocytochemistry. In: Bancroft JD, Cook HC, editors. Manual of histological techniques and their diagnostic application. Edinburgh: Churchill Livingstone; 1994. pp. 263–87. [Google Scholar]
- 20.Fantini F, Giannetti A, Benassi L, Cattaneo V, Magnoni C, Pincelli C. Nerve growth factor receptor and neurochemical markers in human oral mucosa: An immunohistochemical study. Dermatology. 1995;190:186–91. doi: 10.1159/000246682. [DOI] [PubMed] [Google Scholar]
- 21.Schofield JN, Day IN, Thompson RJ, Edwards YH. PGP 9.5, a Ubiquitin C-terminal hydrolase; pattern of mRNA and protein expression during neural development in the mouse. Brain Res. 1995;85:229–38. doi: 10.1016/0165-3806(94)00217-n. [DOI] [PubMed] [Google Scholar]
- 22.Schumacher U, Mitchell BS, Kaiserling E. The neuronal marker protein gene protein 9.5 (PGP9.5) is phenotypically expressed in human breast epithelium, milk and in benign and malignant tumours. DNA Cell Biol. 1994;13:839–43. doi: 10.1089/dna.1994.13.839. [DOI] [PubMed] [Google Scholar]
- 23.Van Noorden S. Tissue preparation and immunostaining techniques for light microscopy. In: Polak JM, Van Noorden S, editors. In: Immunocytochemistry, modern methods and applications. Wright, Bristol: 1986. pp. 26–53. [Google Scholar]
- 24.Pogue KM, Johnston CF. Sample immunocytochemical methods for regulatory proteins. In: Irvine GB, Williams C, editors. In: Methods in molecular biology, neuropeptide protocols. Totowa NJ: Humana Press Inc; 1997. pp. 283–91. [DOI] [PubMed] [Google Scholar]


