Abstract
Context:
Significant increase in vascularity occurs during the transition from normal oral mucosa, through differing degrees of dysplasia, to invasive squamous cell carcinoma (SCC).
Aims:
To evaluate microvessel density (MVD) and vascular endothelial growth factor (VEGF) expression in oral tumorigenesis and correlate it with the clinicopathological characteristics.
Settings and Design:
VEGF expression and MVD were quantified immunohistochemically using anti-VEGF and anti-CD34 antibody.
Materials and Methods:
For this study we used a total of 60 archival specimens, including 10 normal oral mucosa (NOM), 7 mild epithelial dysplasia (Mild ED), 8 moderate epithelial dysplasia (Mod ED), 5 severe epithelial dysplasia (SED), 14 well-differentiated SCC, 11 moderately-differentiated SCC, and 5 poorly-differentiated SCC. VEGF expression was assessed in relation to the localization, intensity, and area of the immunohistochemically stained cells. MVD was evaluated using the Image-Pro® Plus software.
Statistical Analysis:
One-way ANOVA (F test) was carried out for comparing the parameters for multiple groups such as different histopathological grades of dysplasia and carcinoma. Comparison between groups was carried out using the Student's ‘t’ test. Correlations between VEGF score and MVD were estimated using the Karl Pearson coefficient of correlation.
Results:
VEGF and MVD appeared to increase with disease progression and were statistically higher in oral SCC than in epithelial dysplasia and normal buccal mucosa. There was significant correlation between VEGF expression and MVD.
Conclusions:
These findings indicate that VEGF expression is upregulated during head and neck tumorigenesis.
Keywords: Angiogenesis, epithelial dysplasia, image analysis, microvessel density, oral squamous cell carcinoma, vascular endothelial growth factor
INTRODUCTION
Oral squamous cell carcinoma (SCC) is an aggressive epithelial neoplasm. Even with early detection and treatment the overall survival rate is only slightly improved. The role of angiogenesis in neoplasia has been receiving increasing attention in recent times since it can be used as independent prognostic indicator for tumor progression and metastasis and also as a novel second target for anticancer therapy instead of direct tumor cell inhibition.[1]
Angiogenesis is the process of formation of new microvessels from the preexisting vasculature. No solid tumor can probably grow more than 1-2 mm3 in volume, unless it can synthesize its own network of new microvessels. The formation of such a network requires a direct or indirect role of angiogenic factors. Angiogenesis is thought to be initiated by an increase in the level of angiogenic stimuli and a concomitant decrease in the level of angiogenic inhibitors. These factors are produced by tumor cells, stromal cells, and inflammatory cells such as mast cells and macrophages.[2]
As vascular endothelial growth factor (VEGF) is considered as the most potent candidate for the induction of angiogenesis in tumor growth, it has naturally received attention as a potential agent for therapeutic angiogenesis.[3] Therefore, the present study attempts to assess VEGF expression and microvessel density (MVD) in oral tumorigenesis and their correlation with clinicopathologic parameters.
MATERIALS AND METHODS
The present study was carried out on 60 archival specimens, which included 10 normal oral mucosa (NOM), 7 mild epithelial dysplasia (Mild ED), 8 moderate epithelial dysplasia (Mod ED), 5 severe epithelial dysplasia (SED), 14 well-differentiated SCC (WDSCC), 11 moderately-differentiated SCC (MDSCC), and 5 poorly-differentiated SCC (PDSCC). Formalin-fixed paraffin-embedded tissues were sectioned at 3-4 μm thick sections using a semi-automatic microtome. Consecutive sections were placed on slides precoated with egg albumin for routine hematoxylin and eosin staining and on slides coated with poly-L-lysine for immunohistochemical staining for VEGF and CD34.
Determination of VEGF expression
VEGF expression was determined by immunohistochemistry, using a rabbit polyclonal anti-VEGF antibody (BioGenex, USA), to assess localization, intensity, and area of stained cells. Intensity of the stain was scored on the following scale: 0 = no staining, 1 = mild staining, 2 = moderate staining, and 3 = intense staining [Figure 1].
Figure 1.
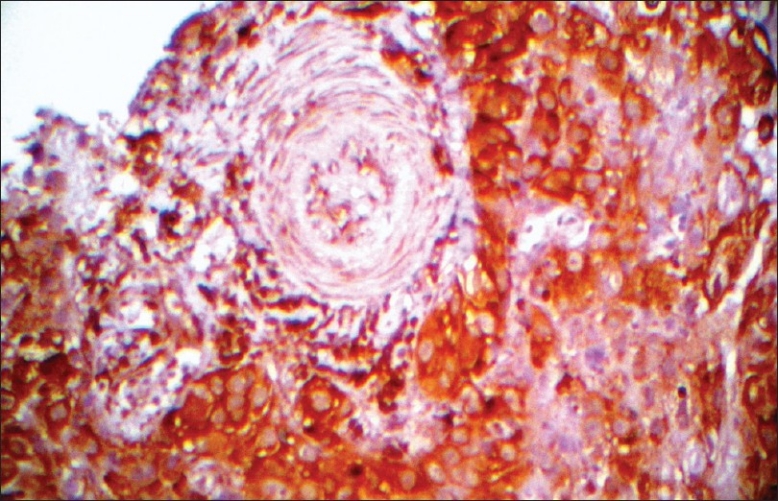
Intense VEGF expression in SED (×400)
The area of staining was scored as follows: 0 = no stained cells in any microscopic field, 1 = <25% of tumor cells stained positively, 2 = 25%-50% of tumor cells stained positively, 3 = 50%-75% of tumor cells stained positively, and 4 = more than 75% of tumor cells stained positively. The sum of the scores for area and intensity of staining were used for statistical analysis.[4]
Determination of microvessel density
To determine MVD, as specified by Weidner et al.,[5] any brown-staining endothelial cell or endothelial cell cluster that was clearly separate from adjacent microvessels, tumor cells, and other connective tissue elements was considered a single, countable microvessel. Vessel lumens, although usually present, were not necessary for a structure to be defined as a microvessel, and red cells were not used to define a vessel lumen. Microvessel counts were determined blindly, the investigator was blinded to this variable. After selecting three microscopic fields of highest neovascularization (vascular hotspots) under low magnification, individual microvessels were manually outlined using freehand draw option in the image analysis software (Pro Plus) and counted under high power. Images of these selected fields along with the marked microvessels were captured [Figure 2]. The mean average scores were tabulated and used for the statistical analysis.
Figure 2.
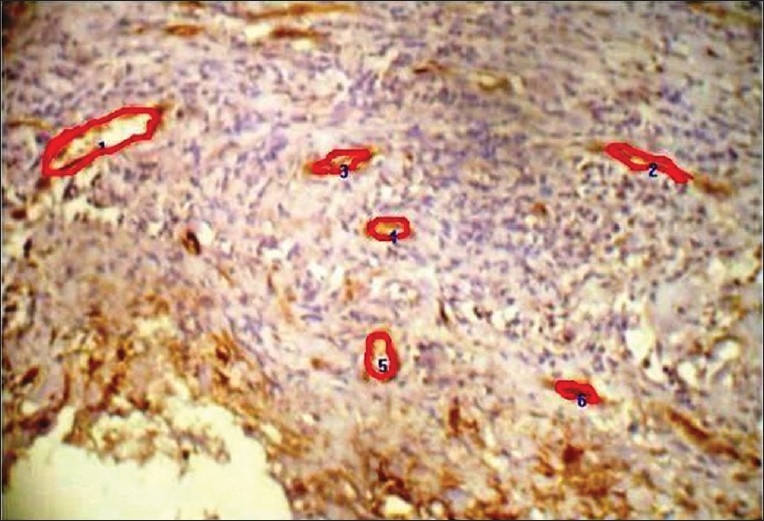
The photomicrograph showing microvessels outlined, traced and numbered using pro plus image analyzer software (×400)
Results were expressed as mean ± standard deviation. One-way ANOVA (F-test) was carried out for comparing the parameters for multiple groups such as different histopathological grades of dysplasia and carcinoma. Comparison between groups was carried out using the Student's ‘t’ test. Correlations between VEGF score and MVD were assessed using the Karl Pearson coefficient of correlation. For all tests a P-value of ≤0.05 was considered to be of statistical significance.
RESULTS
VEGF expression (total score)
The mean and standard deviation of VEGF expression did not uniformly increase with the ascending clinical stages. When the mean scores of VEGF were compared in relation to different histopathologic grades, an increase in VEGF scores was noted from NOM to dysplasia to oral SCC [Graph 1]. Comparison between the groups showed a decrease in the mean VEGF scores from WDSCC to MDSCC to PDSCC [Graph 2].
Graph 1.
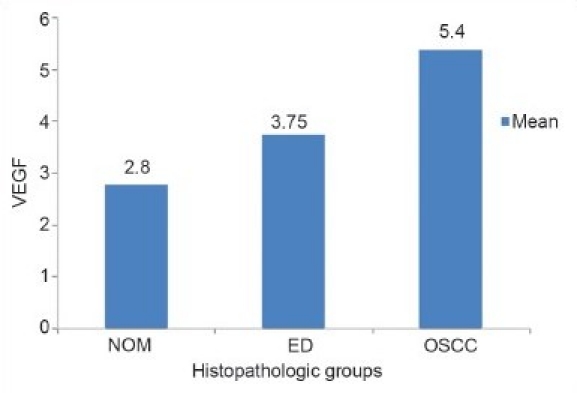
Comparison of VEGF scores between the three histopathologic groups
Graph 2.
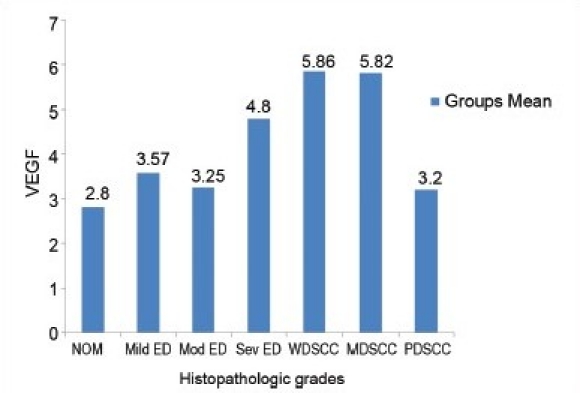
Comparison of VEGF scores between the histopathologic grades
Microvessel density (microvessels/mm2)
The mean and standard deviation of MVD in relation to the clinical stage showed variable results. When the mean scores of MVD were compared in relation to different histopathologic grades, an increase in MVD score from NOM to dysplasia to OSCC was noted [Graph 3]. Comparison between the groups showed a decrease in the mean MVD score from WDSCC to MDSCC to PDSCC [Graph 4].
Graph 3.
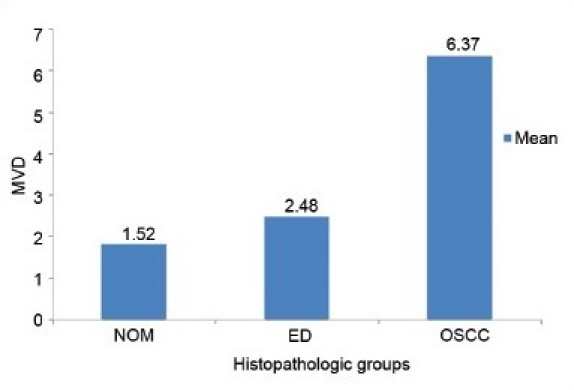
Comparison of MVD scores between the three histopathologic groups
Graph 4.
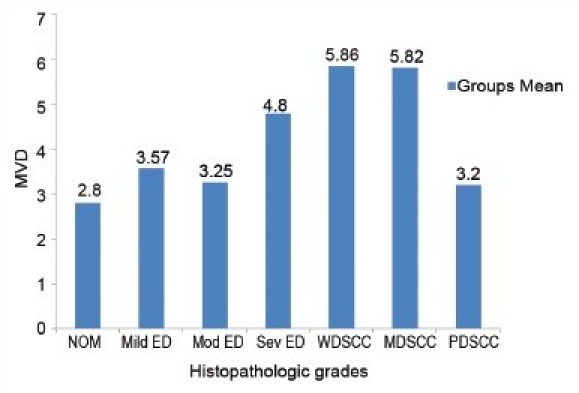
Comparison of MVD scores between the histopathologic grades
To assess the correlation between VEGF and MVD, the Karl Pearson correlation coefficient was calculated, and the results showed a positive correlation, with high statistical significance (r=0.61, P<.00).
DISCUSSION
In the present study, both VEGF expression and MVD did not show an uniform increase with the ascending clinical stages of oral SCC. Whereas, in relation to the histopathologic grades, an increase from NOM to dysplasia to oral SCC was noted. A comparison between the groups showed a decrease in the mean scores of VEGF as well as MVD from WDSCC to MDSCC to PDSCC, which is similar to the finding in the studies by Shintani et al.[6] But overall, an increase in mean scores from NOM to dysplasia to OSCC was observed histopathologically, which is similar to the findings of Gasparini et al.,[7] Williams et al.,[8] Pazouki et al.,[9] Denhart et al.,[10] Sauter et al.,[11] Iamaroon et al.,[12] and Shang et al.[13] However, studies by several other authors have showed contrasting results, e.g., Leedy et al.,[14] Gleich et al.,[15] Moriyama et al.,[16] Tae et al.,[17] and Carlile et al.[3]
A probable explanation could be that nutrition is necessary for the initial establishment and growth of tumor mass but, eventually, the differentiating tumor houses a number of various modulating factors such as VEGF isoforms (VEGF-C and VEGF-D),[18] iNOS,[4,13] endothelins,[19,20] CXCL-5, CXCL-8,[18,21] and COX-2,[4,22] with various oncogenes like ras[23] and tumor suppressor genes like p53[7] acting upon it. As stated by several authors,[21,24,25] activated oncogenes and/or inactivated tumor suppressor genes bring about increased VEGF expression, firstly, through the ras-raf-MAPK pathway and, secondly, through progressive tumor growth, which eventually induces hypoxia.
The differences between the results of various studies[26,27] could be due to the different antibodies (CD-31, CD-34, factor VIII, etc.) used by several authors to define endothelium and the different methodologies used in the assessment of parameters, besides the interobserver variation. The nonavailability of direct methods to measure angiogenic activity could be one of the causes; none of the existing methods can differentiate or mark a resting or active angiogenic vessel. Differences between immunohistochemical protocols, for instance, selection of the paraffin block, level of section within the tissue block (superficial or deep), and the issue of hot spot selection may have also contributed to variations in the results. The lack of reproducibility of measurements due to inadequate standardization and different methodology used also adds to it. Additionally, quantifying the MVD involves selecting the most angiogenic areas, and this may not always be representative of the tumor. Expression of the various VEGF isoforms within the tissues, together with cross-reactivity of antibodies, could also contribute to the bias.
Finally, a positive correlation was seen between VEGF and MVD (r = 0.61, P<.00); the strong statistical significance confirming that there is close correlation between them and that VEGF plays a role in early neovascularization of OSCC as described by Sauter et al.[11] and Wulfing et al.[19] However, the majority of research studies have shown contrasting results.[3,15–17]
These findings suggest that angiogenesis increases with disease progression. VEGF may indeed be referred to as an important mitogen responsible for the neovascularization of these tumors, and MVD can be used as an indicator for disease progression. These findings also indicate that suppression of the angiogenic functions of VEGF can be the basis of a new treatment for oral squamous cell carcinoma.
ACKNOWLEDGEMENT
We thank all the staff members in the department of oral and maxillofacial pathology for their help.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Braunwald, Fassci, Kasper . Harrison's Principles of Internal Medicine. 5th ed. Vol. 1. New York: Mc. Graw Hill Companies; 2003. pp. 517–29. [Google Scholar]
- 2.Polverini PJ. The pathophysiology of angiogenesis. Crit Rev Oral Biol Med. 1995;6:23047. doi: 10.1177/10454411950060030501. [DOI] [PubMed] [Google Scholar]
- 3.Carlile J, Harada K, Baillie R, Macluskey M, Chisholm DM, Ogden GR, et al. Vascular endothelial growth factor (VEGF) expression in oral tissues: Possible relevance to angiogenesis, tumor progression and field cancerization. J Oral Pathol Med. 2001;30:449–57. doi: 10.1034/j.1600-0714.2001.030008449.x. [DOI] [PubMed] [Google Scholar]
- 4.Sappayatosok K. Expression of pro-inflammatory protein iNOS, VEGF and COX-2 in OSCC, relationship with angiogenesis and their clinico-pathologic correlation. Med Oral Patol Oral Cir Bucal. 2009;14:E319–24. [PubMed] [Google Scholar]
- 5.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis- correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 6.Shintani S, Li C, Ishikaw T, Maihara M, Nakashiro K, Hamakawa H. Expression of vascular endothelial growth factor A, B, C and D in oral squamous cell carcinoma. Oral Oncol. 2004;40:13–20. doi: 10.1016/s1368-8375(03)00127-1. [DOI] [PubMed] [Google Scholar]
- 7.Gasparini G, Weidner N, Maluta S, Pozza F, Boracchi P, Mezzetti M, et al. Intratumoral microvessel density and p53 protein: Correlation with metastasis in head- and- neck squamous carcinoma. Int J Cancer. 1993;55:739–44. doi: 10.1002/ijc.2910550507. [DOI] [PubMed] [Google Scholar]
- 8.Williams JK, Carlson GW, Cohen C, Derose PB, Hunter S, Jurkiewicz MJ. Tumor angiogenesis as a prognostic factor in oral cavity tumors. Am J Surg. 1994;168:37880. doi: 10.1016/s0002-9610(05)80079-0. [DOI] [PubMed] [Google Scholar]
- 9.Pazouki S, Chisholm DM, Adi MM, Carmichael G, Farquharson M, Ogden GR, et al. The association between tumor progression and vascularity in the oral mucosa. J Pathol. 1997;183:39–43. doi: 10.1002/(SICI)1096-9896(199709)183:1<39::AID-PATH1088>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 10.Denhart BC, Guidi AJ, Tognazzi K, Dvorak HF, Brown LF. Vascular permeability factor/vascular endothelial growth factor and its receptors in oral and laryngeal squamous cell carcinoma and dysplasia. Lab Invest. 1997;77:659–64. [PubMed] [Google Scholar]
- 11.Sauter ER, Nesbit M, Watson JC, Klein-Szanto A, Litwin S, Herlyn M. Vascular endothelial growth factor is a marker of tumor invasion and metastasis in squamous cell carcinomas of head and neck. Clin Cancer Res. 1999;5:775–82. [PubMed] [Google Scholar]
- 12.Iamaroon A, Pongsiriwet S, Jittidecharaks S, Pattanaporn K, Prapayasatok S, Wanachantararak S. Increase of mast cells and tumor angiogenesis in oral squamous cell carcinoma. J Oral Pathol Med. 2003;32:195–9. doi: 10.1034/j.1600-0714.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- 13.Shang ZJ, Li ZB, Li JR. VEGF is up-regulated by hypoxic stimulation and related to tumor angiogenesis and severity of disease in oral squamous cell carcinoma: In vitro and in vivo studies. Int J Oral Maxillofac Surg. 2006;35:533–8. doi: 10.1016/j.ijom.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Leedy DA, Trune DR, Kronz JD, Weidner N, Cohen JI. Tumor angiogenesis, the p53 antigen and cervical metastasis squamous cell carcinoma of tongue. Otolaryngol Head Neck Surg. 1994;111:417–22. doi: 10.1177/019459989411100405. [DOI] [PubMed] [Google Scholar]
- 15.Gleich LL, Biddinger PW, Pavelic ZP, Gluckman JL. Tumor angiogenesis in T1 oral cavity squamous cell carcinoma: Role in predicting tumor aggressiveness. Head Neck. 1996;18:343–6. doi: 10.1002/(SICI)1097-0347(199607/08)18:4<343::AID-HED5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 16.Moriyama M, Kumagai S, Kawashiri S, Kojima K, Kakihara K, Yamamoto E. Immunohistochemical study of tumor angiogenesis in oral squamous cell carcinoma. Oral Oncol. 1997;33:369–74. doi: 10.1016/s1368-8375(97)00025-0. [DOI] [PubMed] [Google Scholar]
- 17.Tae K, El-Naggar AK, Yoo E, Feng L, Lee JJ, Hong WK, et al. Expression of vascular endothelial growth factor and microvessel density in head and neck tumorigenesis. Clin Cancer Res. 2000;6:2821–8. [PubMed] [Google Scholar]
- 18.Benke EM, Ji Y, Patel V, Wang H, Miyazaki H, Yeudall WA. VEGF-C contributes to head and neck squamous cell carcinoma growth and motility. Oral Oncol. 2010;46:E19–24. doi: 10.1016/j.oraloncology.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Wülfing P, Kersting C, Tio J, Fischer RJ, Wülfing C, Poremba C, et al. Endothelin-1-, endothelin-A-, and endothelin-B-receptor expression is correlated with vascular endothelial growth factor expression and angiogenesis in breast cancer. Clin Cancer Res. 2004;10:2393–440. doi: 10.1158/1078-0432.ccr-03-0115. [DOI] [PubMed] [Google Scholar]
- 20.Hebert C, Siavash H, Norris K, Nikitakis NG, Sauk JJ. Endostatin inhibits nitric oxide and diminishes VEGF and collagen XVIII in squamous carcinoma cells. Int J Cancer. 2005;114:195–201. doi: 10.1002/ijc.20692. [DOI] [PubMed] [Google Scholar]
- 21.Gupta MK, Qin RY. Mechanism and its regulation to tumor-induced angiogenesis. World J Gastroenterol. 2003;9:1144–55. doi: 10.3748/wjg.v9.i6.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallo O, Franchi A, Magnelli L, Sardi I, Vannacci A, Boddi V, et al. Cyclooxygenase-2 pathway correlates with VEGF expression in head and neck cancer. Implications for tumor angiogenesis and metastasis. Neoplasia. 2001;3:53–61. doi: 10.1038/sj.neo.7900127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rak J, Mitsuhashi Y, Bayko L, Filmus J, Shirasawa S, Sasazuki T, et al. Mutant ras oncogenes upregulate VEGF/VPF expression: Implications for induction and inhibition of tumor angiogenesis. Cancer Res. 1995;55:4575–80. [PubMed] [Google Scholar]
- 24.Maeda T, Matsumura S, Hiranuma H, Jikko A, Furukawa S, Ishida T, et al. Expression of vascular endothelial growth factor in human oral squamous cell carcinoma: Its association with tumor progression and p53 gene status. J Clin Pathol. 1998;51:771–5. doi: 10.1136/jcp.51.10.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Högmo A, Kuylenstierna R, Lindholm J, Munck-Wikland E. Predictive value of malignancy grading systems, DNA content, p53, and angiogenesis for stage I tongue carcinomas. J Clin Pathol. 1999;52:35–40. doi: 10.1136/jcp.52.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hannen EJ, Riediger D. The quantification of angiogenesis in relation to metastasis in oral cancer: A review. Int J Oral Maxillofac Surg. 2004;33:2–7. doi: 10.1054/ijom.2003.0433. [DOI] [PubMed] [Google Scholar]
- 27.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis–correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]


