Abstract
Aims and Objectives:
To know the prevalence of Candidal colonization, and to isolate and know the Candidal species prevalent in the oral cavity from the oral rinse samples collected from the individuals attending to the Voluntary Counseling and Confidential Testing Center (VCCTC) and the antiretro-viral therapy (ART) Center at Government General Hospital, Guntur, Andhra Pradesh, South India.
Materials and Methods:
The study group consisted of 50 HIV negative asymptomatic individuals (Group I); 50 HIV positive individuals (people living with HIV AIDS [PLWHA's]), who are naïve to antiretro-viral therapy (direct walk-in clients of VCCTC) (Group II); and 50 HIV positive individuals with CD4+ count less than 250 and who are started on highly active anti retroviral therapy (HAART) (Group III). Routine mycological tests for the isolation of pure cultures of Candida and also the speciation procedures were done.
Results:
In the study group, 53 (Group I=11; Group II=23; Group III=19) were culture positive. The prevalence of Candida was comparatively high in the age range between 41–50 years in Group II; 51–60 years, in Group III. A male predominance was observed in the Group I (M:F=16:6) and Group III (M:F=20:18), with a slight female predominance in the Group II (F:M=24:22). The overall culture positivity was 35.3%. Candida albicans was the highest prevalent species (47.17% of the isolates). A comparison of the culture positivity with the CD4 counts of the study subjects was statistically highly significant. A pair wise comparison of the culture positivity with that of the colony forming units/mL from the subjects showed a high significance between Group I and Group II, and between Group I and Group III.
Conclusion:
Candidal infections in immuno compromised patients are often severe, rapidly progressive, and difficult to treat and such patients have a definitive risk of developing oral candidiasis wherein, even the members of the normal oral flora may become pathogenic. Candida albicans is the common isolate. Nonalbicans species are also emerging as opportunistic pathogens. In view of this changing pattern, it is strongly recommended that species identification can help in much better treatment strategies, and thus, gain a good control over the disease. The findings of this study would be helpful in any further studies which, if done prospectively on a larger cohort, can be confirmatory.
Keywords: Candida albicans, highly active antiretroviral therapy, human immunodeficiency virus, oral candidiasis
INTRODUCTION
Human immunodeficiency virus (HIV) infection causes the acquired immunodeficiency syndrome (AIDS), which is now recognized as the great pandemic of the second half of the twentieth century.[1]
After Gotileb et al. (1981), first described HIV/AIDS as a disease of global importance, in the members of a gays club, who suffered from Pneumocystis carinii Pneumonia and was thought of as a disease occurring in the high-risk group, the Centers for Disease Control (CDC) reported it as “GRID” (gay-related immune deficiency), stigmatizing the gay community as carriers of this deadly disease.[1]
The researchers at the Pasteur Institute in France isolated a retrovirus in 1983 that was believed to be related to the outbreak of AIDS. Today it has become a generalized epidemic, the world over. HIV/AIDS is a disease with multisystem involvement resulting from the immunodeficiency caused by the T-Lymphotropic Lentivirus, belonging to the Retroviridae family. In 1983, Dr. Luc Montagnier et al., isolated a retro-virus from a West African patient with persistant generalized lymphadenopathy (PGL), which is a manifestation of AIDS, and called it “Lymphadenopathy Associated Virus” (LAV).[1]
In 1986, the international committee on the nomenclature of viruses named it the “Human Immunodeficiency virus” (HIV) and till date two types, HIV – 1 and HIV – 2 are identified. This virus has CD4+ cells as its target and with the help of reverse transcriptase, it gets integrated with the host cell DNA and gets immortalized.
At the end of 2009, there is a global pool of more than 42 millions of people living with HIV/AIDS (PLHAs) (UN AIDS – December 2009). About 4.2 million carriers are present in India, of whom, more than 4 lakhs are in Andhra Pradesh, making it one of the high prevalence states in India with more than 1% carrier rate in Antenatal women. The disease of high risk groups spilled over into the low-risk group through the bridge population and now became a generalized epidemic with more than 0.3% prevalence (NACO - 2009).
Oral candidiasis is one of the most common opportunistic infections in HIV/AIDS, caused by the yeast-like fungus, Candida. There are a number of species of Candida like – Candida albicans; Candida tropicalis; Candida krusei; Candida parakrusei; Candida glabrata; Candida guillermondii; Candida parapsilosis; Candida kefyr/pseudotropicalis; Candida lusitaniae; Candida dubliniensis; Candida viswnathii; Candida stellatoidea etc.
C. albicans is the most common clinical isolate, originally with good antifungal susceptibility.[2,3] But with the emergence of other species of Candida as pathogens and a development of change in the susceptibility pattern of the C. albicans, as well as the newer species of Candida, it is necessitating the isolation and identification of the causative species.[4,5]
The advent of highly active anti retroviral therapy (HAART) has changed the scenario of HIV infection and led to a dramatic reduction in the morbidity and mortality. Various foreign reviews have also mentioned that an overall prevalence of the oral manifestations of HIV disease has changed since the advent of HAART. Many studies noted a significant reduction of oral lesions from pre-HAART, to the HAART era.[6] Keeping these things in view, this study was taken up to know the species variations in HIV positive individuals with and without highly active anti retroviral therapy (HAART), attending to the Voluntary Counseling and Confidential Testing Center (VCCTC), Anti Retroviral Therapy (ART) clinic in Government General Hospital, Guntur.
Aims and objectives of the study are
To find out the Candidal carrier state in HIV positive individuals.
To identify the most common species of Candida prevalent in HIV positive individuals with and without HAART, and to compare the results with the control group.
To enumerate the Colony Forming Units (CFU) count, in relation to the immune status of the individuals in the study population.
MATERIALS AND METHODS
This study was carried out in the Department of Microbiology, Guntur Medical College, Guntur and a total of 150 patients attending the Voluntary Counseling and Confidential Testing Centre (VCCTC), and Anti-Retroviral Therapy (ART) centre of Government General Hospital (GGH), Guntur were selected for this study group. All of them were tested for HIV by three tests (COOMB AIDS; TRI DOT; EIA-COOMB), according to National AIDS Control Organization (NACO) guidelines and confirmed by Western Blott. All findings were recorded in a standardized format. The data was coded and entered into the database programme for statistical analysis by SPSS package. The study population was divided into three groups from whom, the samples were collected.
Group I – 50 cases were taken as normal controls [tested negative for HIV by three tests (COOMB AIDS; TRI DOT; EIA-COOMB), according to National AIDS Control Organization (NACO) guidelines and confirmed by Western Blott].
Group II – 50 cases were people living with HIV/AIDS (PLHAs) Naïve to HAART: Walk-in clients in VCCTC, Government General Hospital, Guntur who were positive for HIV by three tests (COOMB AIDS; TRI DOT; EIA-COOMB), according to NACO guidelines and confirmed by Western Blott.
Group III – 50 cases were PLHAs on HAART: Who were having signs and symptoms of oral candidiasis and were registered for HAART and started on three drug regimen.
Informed consent was obtained from all the registrants before collecting the sample. A detailed history was taken with particular reference to name, age, gender, IP/OP number, occupation, address, predisposing factors, onset and duration of complaint, treatment, etc. Ethical details relating to the underlying disease were accounted. A detailed history regarding similar incidence (HIV positivity) in the family members were enquired into.
Oral rinse samples were collected from the subjects of all three groups with the following protocol. Each individual was supplied with 10 ml of sterile phosphate buffer saline solution (PBS 0.1 M, pH 7.2) and was asked to rinse the mouth for 60 s. The oral contents were expelled into a sterile plastic container (Speci-can 30 mL).
The samples were transported immediately to the laboratory and were subjected to various mycological tests. All the samples were collected and processed according to the standard mycological procedures, given by Milne et al.[7] Direct microscopic examination of KOH mount and Gram-stained smear was done to identify the budding yeast cells [Figure 1]. The specimens were inoculated onto Sabouraud's Dextrose Agar (SDA) and incubated at 37°C for 48–72 h. Growth appeared in 1–3 days as creamy white smooth pasty colonies. C. albicans isolates were confirmed by Germ-tube test [Figure 2] and Chlamydospore production on Corn-meal agar by the Dalmau plate technique [Figure 3]. The isolates were then inoculated on Chrome Agar Candida and incubated at 37°C in dark for 48 h and the colonies with pigment were considered for species identification [Figure 4]. Sugar assimilation and fermentation tests were utilized for identification of species. Colony forming unit count was estimated by using the following formula: CFU/mL=1000×number of colonies/4.
Figure 1.
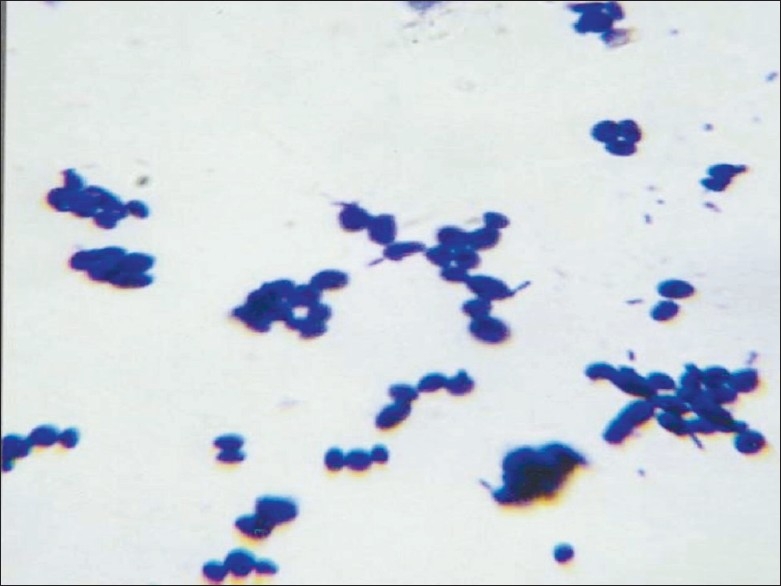
Gram's stained smear showing budding yeast cells and pseudo hyphae – direct smear from centrifuged deposit of the specimen
Figure 2.
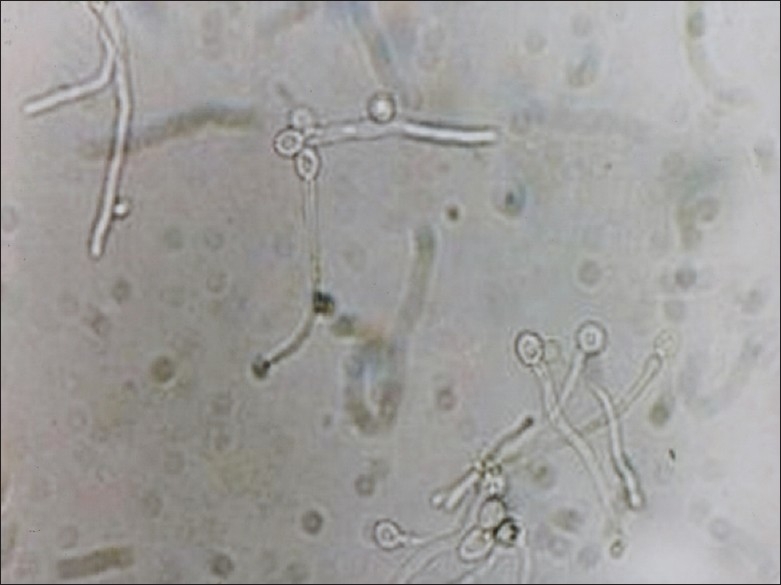
Germ – tube test (Reynolds Braude phenomenon)
Figure 3.
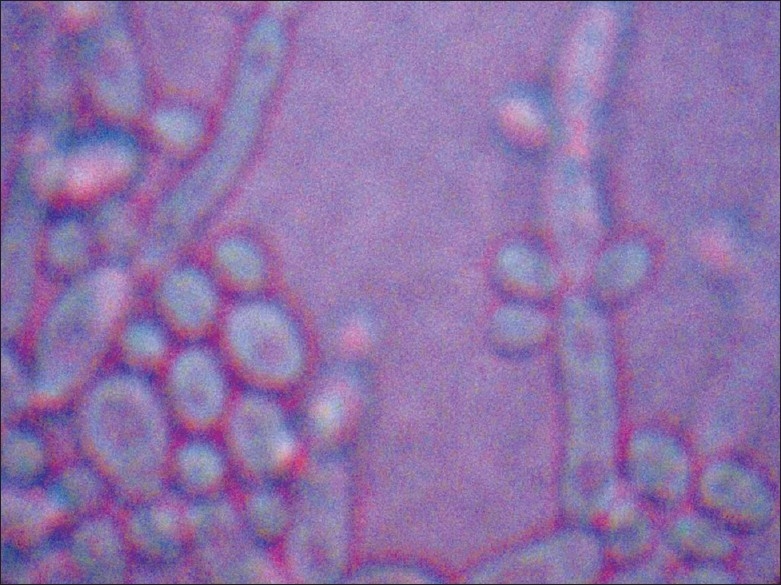
LCB mount from growth of candida albicans on corn-meal agar showing chlamydospores
Figure 4.
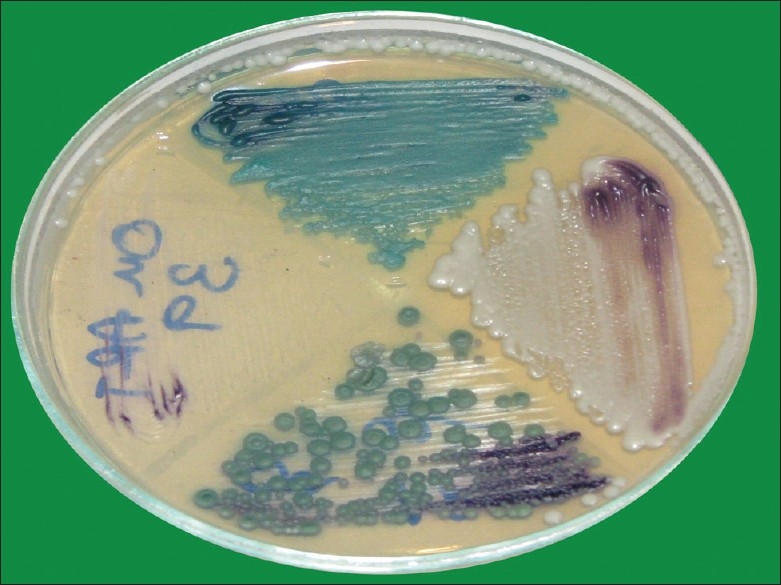
Colored candidal colonies on chrom-agar medium
RESULTS
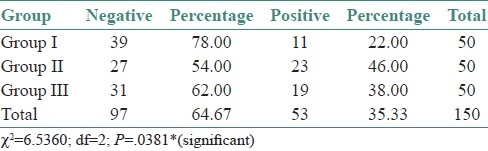
A total of 150 cases were subjected to mycological investigation to confirm the presence of Candida species, of which, 53 were culture positive. In the Group I (n=50), 11 were positives (22%). In the Group II, 23 were positives (46%); and 19 were positive (38%) in the Group III. A correlation between the study groups and culture positivity showed a statistical significance (χ2=6.5360; df=2; P=0.0381*) [Table 1].
Table 1.
Correlation between study groups and culture positivity
Age-wise analysis showed a high positivity for Candidal culture in the age range of 41–50 years among the study Group I. In symptomatic cases, it was 31–40 years. Socioeconomic status showed a significant difference between the high and low income groups, with a statistically significant high positivity in the low socioeconomic group (Paired t-test; t=4.72; P=<0.05).
Overall culture positivity was 35.33%. C. albicans was the most prevalent species (16.67%). This was followed by C. tropicalis (8.00%), C. glabrata (4.67%), C. parapsilosis (1.33%), C. kefyr (0.67%), C. guillermondi (0.67%), C. stellatoidea (0.67%). Two percent of the cases showed a mixture of C. albicans and C. glabrata.
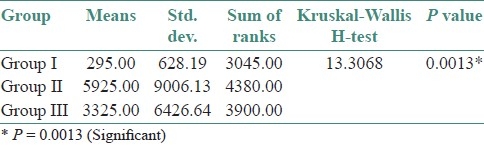
The preponderance of C. albicans over other species was statistically significant (χ2 =15.0801; df=6; P=0.0196*) [Table 2]. Correlation of the culture positivity with CD4 counts showed a high significance with low CD4 count (paired t-test – t=7.75; df=6; P=<0.001) [Table 3]. The three groups were statistically compared, with respect to CFU/mL by Kruskal Wallis ANOVA by RANKS, which showed a high significance (P=0.0013). Pairwise comparisons of all three groups by Mann Whitney U–test showed a significance between Group I and Group II (P=0.0033), and also between Group I and Group III (P=0.0364) [Tables 4–6].
Table 2.
Distribution of study subjects according to study groups andstatus of C. albicans
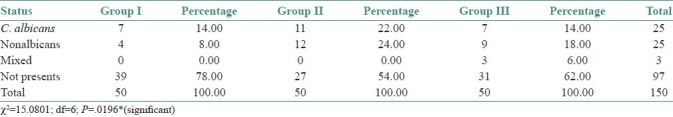
Table 3.
Comparison of culture positivity according to study groups and cd4 counts
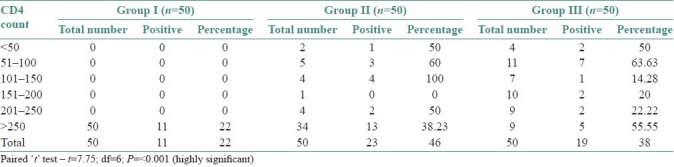
Table 4.
Colony forming units count distribution in cases
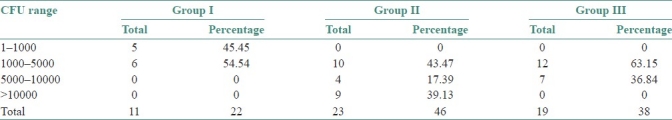
Table 6.
Pairwise comparisons of three groups (i, ii, iii) by Mann Whitney u-test
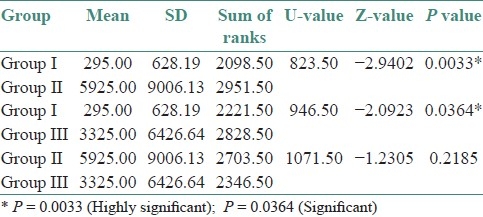
Table 5.
Comparison of three groups (i, ii, iii) with respect to CFU/mL by Kruskal Wallis anova by ranks
DISCUSSION
Candidiasis is an acute or chronic, superficial or disseminated mycosis which is caused by different species of Candida. The infection can be either primary or secondary. It can be either endogenous or exogenous. Candida is a saprophytic organism under normal circumstances but distinctly pathogenic in a host with depressed defense mechanism. Candida species are members of the normal oral microbiota that can be opportunistic and invade tissue and cause oral Candidiasis.[8]
Candida albicans is the most common species isolated from clinical samples but non-albicans species of Candida are also evolving as pathogens with varying susceptibility to antifungal agents. Hence, accurate identification of Candida species is therefore crucial for successful clinical management.[9]
The present study revealed culture positivity in 53 cases giving an overall prevalence of 35.33%. The positivity rate in the Group I was 22% which revealed Candida carriage even in the normal healthy individuals. The positivity was 46% in Group II and 38% in the Group III. This shows that there is an increased prevalence of Candidal species in the immunocompromized individuals, predisposing them to Oral Candidiasis. Oral carriage of Candida species in healthy as well as in immunocompromized individuals, has been variously reported by different authors.
Cohen et al.,[10] reported the prevalence of Yeast as 35% in oropharynx in healthy volunteers. Campo et al.,[11] reported an oral candidal prevalence of 37.8% in samples of Spanish HIV infected individuals. Hanan et al.,[12] in their study reported an incidence of 30–45% of oral candidiasis in healthy adults, and 95% in HIV positive patients. Zaremba et al.,[13] conducted a study on oral carriage of Candida in healthy individuals and reported a prevalence rate of 63.1%.
Our study revealed a slightly lower Candidal carriage in healthy individuals (22%) than the other reports probably because of other variables which are to be considered, like – Geographical variations, nature, and size of the sample selected and also the method of sample collection.
Estimates of the prevalence of Candida species as human commensal vary considerably according to the size of the sample, type of person sampled and method of sampling adopted.[14]
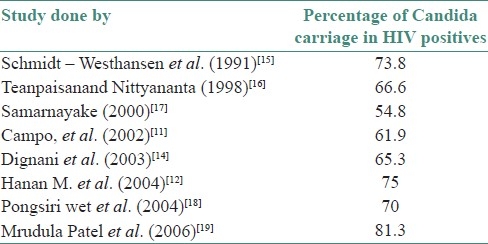
The reports given by various authors on the prevalence of oral Candidal carriage in HIV positives are shown in [Table 7]. The prevalence of Candida in HIV positive individuals in our study (42%) was close to that reported by Samaranayake et al.[17] But in our study on HIV positive individuals undergoing HAART, the carriage was found to be lower (38%), which may probably relate to the changes in immunological status of the individual brought about by HAART.
Table 7.
Literature review of prevalence of oral Candidal carriage in HIV positives
Belazi et al.,[20,21] showed that the oral carriage of Candida species cannot be directly associated with age or gender in relatively healthy people. Certain systemic conditions such as diabetes, defects in immune system, and antibiotic medication may predispose the transformation of benign colonizers such as Candida species into opportunistic pathogens.
In this study, male predominance was seen in the Group I (16%) and Group III (20%) cases. The positivity in females was comparatively less (Group I – 6% and Group III – 24%). Group II showed a little higher incidence in females with 24% positivity, compared to males (22%). Our findings were concurrent with that reported by Ranganathan et al. , from Chennai, South India.[22]
This study revealed that a total of 53 isolates were culture positive for Candida species. Candida albicans was the most commonly isolated species in all the study groups with an overall positivity of 16.67%, nonalbicans (includes all the other species and also mixed cultures) being 18.66%.
Recent epidemiological trend is the emergence of less pathogenic species of non-albicans Candida species as significant opportunistic pathogens.[23] Data from various multicentric surveys as reported by Pfaller in 1996 establish the pathogenic role of non-albicans species which were isolated from cases of systemic Candidiasis.[24] Oral candidiasis caused by non-albicans Candida was well established and reported by many authors.[25,26]
In the present study, among the non-albicans species, C. tropicalis was the most commonly isolated species, accounting for 8.00% of the isolates, which was also similar to that reported by Ranganathan et al.[25]
Similarly, other authors also reported 56% of C. albicans and 44% of non-albicans species in hospitalized patients, which almost correlates to the present study.[9,25,27] Zaremba et al., reported Candida albicans as the most frequently isolated species with 53.5% and non-albicans species with 22.5%.[14] Mrudula Patel et al., reported 78.6% of C. albicans as the most common species isolated and 21.4% of nonalbicans species.[19] In the present study, an attempt is made to know the prevalence of Candida in HIV positive individuals, as immune-suppression is always associated with the disease. CD4 count is a marker of immunosuppression and also helps in prognosticating the case. When the positivity rate was compared to the CD4 count, present study revealed a higher positivity with low CD4 count, that is, <150/mm3.
Oral candidiasis is perhaps the earliest prognosticator of HIV infection and it has been described even in the first documented case of HIV disease.[28] The degree of immunosuppression influences the risk, severity and anatomic location of the disease. Vaginal candidiasis tends to occur when CD4+ T-lymphocytes count is normal or near normal, oral candidiasis when count is less than 300/mm3 and oesophagitis when count is <100/mm3.[29,30]
The introduction of HAART has reduced the incidence of oral candidiasis, as reported by many authors. Highly Active Anti Retroviral Therapy has reduced the incidence of oropharyngeal Candidiasis.[31] This is postulated to be due to the increased immune responsiveness, as well as the action of Protease Inhibitors (PI) in HAART, on the Secretory Aspartyl Proteinases (SAP), which is an important virulence factor of C. albicans.[32–34]
The introduction of Highly Active Antiretroviral Therapy (HAART) has reduced the incidence of Oral candidiasis as reported by many authors. Patton et al., reported significant decrease in the prevalence of Oral candidiasis from 47.6% to 37.5%, probably attributable to HAART therapy,[34] which is closer to our study.
Candida being a member of the normal flora of the oral cavity, in the present study an attempt is made to enumerate Colony Forming Units (CFU) of Candida present in the oral rinse samples collected from different groups, to know the significance of the count.
CFU was <5000/mL in the Group I. Majority of patients in the Group II showed CFU count in the range of 1000–5000 (43.47%), a few cases in the range of 5000–10000 (17.39%), and a considerable number of cases showed a CFU count of >10000 (39.13%). In the Group III, most of the cases showed <5000 CFU count (63.15%), and a few cases were in the range of 5000–10000 (36.84%).
A comparative analysis of the three groups by Kruskal Wallis Anova by Ranks has shown significance between the Group I and the other two groups. But there was no statistical significance between the Groups II and III. A similar report was given by Mrudula Patel et al.,[19] Similar observations with variable percentage of positivity were also reported from Thailand (adults 66%; children 70%); Hongkong (54.8%), Italy (64%) and India (65.3%) by Teanpaisan and Nittyanantha;[16] Samarnayake et al.;[17] Campo et al.;[11] and Dignani et al.,[14] respectively.
A low CFU count in PLHA's on HAART, compared to PLHA's who are not receiving HAART indicates a good response to HAART in successfully keeping the opportunistic infection under control.
CONCLUSION
Candidal infections in immunocompromized patients are often severe, rapidly progressive, and difficult to treat and such patients have a definitive risk of developing oral Candidiasis, which is an important oral manifestation in PLHA's. Age and sex has no significance on Candidal carriage. Immune status has a definite impact on Candidal carriage. Candida albicans was the predominant species in our study population, though nonalbicans Candida was also isolated. Immunological status of the individual has a definite impact on the severity of the disease as reflected by the association of high CFU with low CD4 counts. In view of the changing pattern of the species of Candida, their isolation and identification is important and can help in much better treatment strategies, and thus, gain a good control over the disease. The findings of this study would be helpful in any further studies which, if done prospectively on a larger cohort, can be confirmatory.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Willey JM, Sherwood LM, Woolverton CJ. Prescott, Harley and Klein's Microbiology. 7th ed. New York: McGraw Hill; 2008. pp. 925–30. [Google Scholar]
- 2.Sobel JD, Ohmit SE, Schuman P, Klein RS, Mayer K, Duerr A, et al. The evolution of Candida species and fluconazole susceptibility among oral and vaginal isolates recovered from human immunodeficiency virus (HIV)-seropositive and at-risk HIV-seronegative women. J Infect Dis. 2001;183:286–93. [PubMed] [Google Scholar]
- 3.Xu J, Mitchell TG. Geographical differences in human oral yeast flora. Clin Infect Dis. 2003;36:221–4. doi: 10.1086/345672. [DOI] [PubMed] [Google Scholar]
- 4.McQuillen DP, Zingman BS, Meunier F, Levitz SM. Invasive infections due to Candida krusei: Report of 10 cases of fungemia that include three cases of endophthalmitis. Clin Infect Dis. 1992;14:472–8. doi: 10.1093/clinids/14.2.472. [DOI] [PubMed] [Google Scholar]
- 5.Fidel PL, Jr, Vazquez JA, Sobel JD. Candid aglabrata: Review of epidemiology, pathogenesis, and clinicaldiseasewithcomparisonto C. albicans. Clin Microbiol Rev. 1999;12:80–96. doi: 10.1128/cmr.12.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reznik DA, Daniels CO. RN-BC, HIV Dent – Oral Manifestations. [Last accessed 2007]. Available from: http://www.hivdent.org/_oralmanifestations .
- 7.Milne LJ. Fungi, Mackie and McCartney Practical Medical Microbiology. In: Collee JG, editor. Chapter 41. 14th ed. Vol. 2. Edinburgh: Churchill Livingstone; 1996. pp. 695–717. [Google Scholar]
- 8.Rippon JW. Medical Mycology. 3rd ed. Philadelphia: W.B. Saunders; 1998. Candidiasis and the Pathogenic Yeasts; pp. 536–81. [Google Scholar]
- 9.Shaheen MA, Taha M. Species Identification of Candida isolates obtained from oral lesions of Hospitalized and Non-hospitalized patients with Oral Candidiasis. Egypt Dermatol J. 2006;2:14. [Google Scholar]
- 10.Cohen R, Roth FJ, Delgado E, Ahearn DG, Kalser MH. Fungal flora of the normal human small and large intestine. N Engl J Med. 1969;280:638–41. doi: 10.1056/NEJM196903202801204. [DOI] [PubMed] [Google Scholar]
- 11.Campo J, Del Romero J, Castilla J, García S, Rodríguez C. Bascones AOral candidiasis as a clinical marker related to viral load, CD4 lymphocyte count and CD4 lymphocyte percentage in HIV-infected patients. J Oral Pathol Med. 2002;31:5–10. doi: 10.1034/j.1600-0714.2002.310102.x. [DOI] [PubMed] [Google Scholar]
- 12.Al-Abeid HM, Abu-Elteen KH, Elkarmi AZ, Hamad MA. Isolation and characterization of Candida spp. in Jordanian cancer patients: Prevalence, pathogenic determinants, and antifungal sensitivity. Jpn J Infect Dis. 2004;57:279–84. [PubMed] [Google Scholar]
- 13.Zaremba ML, Daniluk T, Rozkiewicz D, Cylwik-Rokicka D, Kierklo A, Tokajuk G, et al. Incidence rate of Candida species in the oral cavity of middle-aged and elderly subjects. Adv Med Sci. 2006;51(Suppl 1):233–6. [PubMed] [Google Scholar]
- 14.Dignani MC, Joseph Solomkin, Anaisse EJ. Textbook of Clinical Mycology. In: Anaisse, McGinnis, Pfaller, editors. Chapter 8. 1st ed. Philadelphia: Churchill Livingstone; 2003. pp. 195–239. [Google Scholar]
- 15.Schmidt-Westhausen AM, Priepke F, Bergmann FJ, Reichart PA. Decline in the rate of oral opportunistic infections following introduction of highly active antiretroviral therapy. J Oral Pathol Med. 2000;29:336–41. doi: 10.1034/j.1600-0714.2000.290708.x. [DOI] [PubMed] [Google Scholar]
- 16.Teanpaisan R, Nittyananta W. Prevalence of Candida species in AIDS patients and HIV-free subjects in Thailand. J Oral Pathol Med. 1998;27:4–7. doi: 10.1111/j.1600-0714.1998.tb02082.x. [DOI] [PubMed] [Google Scholar]
- 17.Tsang CS, Samaranayake LP. Oral yeasts and coliforms in HIV-infected individuals in Hong Kong. Mycoses. 2000;43:303–8. doi: 10.1046/j.1439-0507.2000.00584.x. [DOI] [PubMed] [Google Scholar]
- 18.Kerdpon D, Pongsiriwet S, Pangsomboon K, Iamaroon A, Kampoo K, Sretrirutchai S, et al. Oral manifestations of HIV infection in relation to clinical and CD4 immunological status in northern and southern Thai patients. Oral Dis. 2004;10:138–44. doi: 10.1046/j.1601-0825.2003.00990.x. [DOI] [PubMed] [Google Scholar]
- 19.Patel M, Shackleton JT, Coogan MM. Effect of antifungal treatment on the prevalence of yeasts in HIV-infected subjects. J Med Microbiol. 2006;55:127–84. doi: 10.1099/jmm.0.46588-0. [DOI] [PubMed] [Google Scholar]
- 20.Belazi M, Velegraki A, Fleva A, Gidarakou I, Papanaum L, Baka D, et al. Candidal overgrowth in diabetic patients: Potential predisposing factors. Mycoses. 2005;48:192–6. doi: 10.1111/j.1439-0507.2005.01124.x. [DOI] [PubMed] [Google Scholar]
- 21.Ikebe K, Morii K, Matsuda K, Hata K, Nokubi T. Association of candidal activity with denture use and salivary flow in symptom-free adults over 60 years. J Oral Rehabil. 2006;33:36–42. doi: 10.1111/j.1365-2842.2006.01527.x. [DOI] [PubMed] [Google Scholar]
- 22.Ranganathan K, Devi MU, Saraswathi TR, Kumarasamy N, Solomon S, Johnson N. Oral Lesions and Conditions Associated with Human Immunodeficiency Virus Infection in 1000 South Indian Patients. Ann Acad Med Singapore. 2004;33(Suppl):37s–42s. [PubMed] [Google Scholar]
- 23.Fleming RV, Walsh TJ, Anaissie EJ. Emerging and less common fungal pathogens. Infect Dis Clinic North Am. 2002;16:915–33. doi: 10.1016/s0891-5520(02)00041-7. [DOI] [PubMed] [Google Scholar]
- 24.Pfaller MA, Houston A, Coffmann S. Application of CHROMagar Candida for rapid screening of clinical specimens for Candida albicans, Candida tropicalis, Candida krusei, and Candida (Torulopsis) glabrata. J ClinMicrobiol. 1996;34:58–61. doi: 10.1128/jcm.34.1.58-61.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ranganathan K, Reddy BV, Kumarasamy N, Solomon S, Viswanathan R, Johnson NW. Oral lesions and conditions associated with Human immunodeficiency virus infection in 300 south Indian patients. Oral Dis. 2000;6:152–7. doi: 10.1111/j.1601-0825.2000.tb00326.x. [DOI] [PubMed] [Google Scholar]
- 26.Qi QG, Hu T, Zhou XD. Frequency of species and Molecular Characterization of Oral Candida and Distribution in Host of different ages in China. J Oral Pathol Med. 2005;34:350–6. doi: 10.1111/j.1600-0714.2005.00326.x. [DOI] [PubMed] [Google Scholar]
- 27.Dorko E, Pilipcinec E, Tkacikova L. Incidence of Candida tropicalis in Clinical Samples over 5 year period. Pak J BiolSci. 2000;3:606–9. [Google Scholar]
- 28.Imam N, Carpenter CC, Mayer KH, Fisher A, Stein M, Danforth SB. Hierarchical pattern of mucosal candida infections in HIV-seropositive women. Am J Med. 1990;89:142–6. doi: 10.1016/0002-9343(90)90291-k. [DOI] [PubMed] [Google Scholar]
- 29.Fichtenbaum CJ, Powderley WG. Refractory mucosal candidiasis in patients with human immunodeficiency virus infection. Clin Infect Dis. 1998;26:556–65. doi: 10.1086/514571. [DOI] [PubMed] [Google Scholar]
- 30.Pantaleo G, Cohen OJ, Schacker T, Vaccarezza M, Graziosi C, Rizzardi GP, et al. Evolution pattern of HIV replication and distribution in lymph nodes following primary infection: Implications for anti-viral therapy. Nat Med. 1998;4:341–5. doi: 10.1038/nm0398-341. [DOI] [PubMed] [Google Scholar]
- 31.Cassone A, De Bernardis F, Torosantucci A, Tacconelli E, Tumbarello M, Cauda R. In vitro and in vivo anticandidal activity of human immunodeficiency virus protease inhibitors. J Infect Dis. 1999;180:448–53. doi: 10.1086/314871. [DOI] [PubMed] [Google Scholar]
- 32.Gruber A, Speth C, Lukasser-Vogl E, Zangerle R, Borg-von Zepelin M, Dierich MP, et al. Human immunodeficiency virus type 1 protease inhibitor attenuates Candida albicans virulence properties in vitro. Immunopharmacology. 1999;41:227–34. doi: 10.1016/s0162-3109(99)00035-1. [DOI] [PubMed] [Google Scholar]
- 33.Calderone RA, Fonzi WA. Virulence factors of Candida albicans. Trends Microbiol. 2001;9:327–35. doi: 10.1016/s0966-842x(01)02094-7. [DOI] [PubMed] [Google Scholar]
- 34.Patton LL, McKaig R, Strauss R, Rogers D, Eron JJ., Jr Changing prevalence of oral manifestations of human immuno-deficiency virus in the era of protease inhibitor therapy. Oral Surg Oral Med Oral Pathol Oral RadiolEndod. 2000;89:299–304. doi: 10.1016/s1079-2104(00)70092-8. [DOI] [PubMed] [Google Scholar]


