Abstract
Background:
The etiology of oral lichen planus (OLP) is not fully understood. It is generally considered to be a T-cell mediated chronic inflammatory oral mucosal disease. There is increasing evidence that chronic inflammation is linked to the diseases associated with endothelial dysfunction and is involved in the induction of aberrant angiogenesis.
Aim:
Our aim was to evaluate the role of angiogenesis in the pathogenesis of OLP by immunohistochemistry, using the CD34 antibody.
Materials and Methods:
Forty tissue sections (7 of erosive lichen planus, 18 of reticular oral lichen planus, and 15 of normal oral mucosa), were assessed for microvessel density (MVD) in five selected areas of high inflammatory infiltrate by immunohistochemistry for the expression of CD34 antibody.
Results and Conclusion:
The mean MVD was 44.47 in the control group (normal oral mucosa) and 97.24 in the OLP group, showing that there is increased angiogenesis in the latter. Reticular OLP had mean MVD of 84.61 and erosive OLP had mean MVD of 129.71, showing relatively greater angiogenesis in erosive OLP as compared to reticular OLP. Thus, angiogenesis can be considered to play a role in both the etiopathogenesis and the progression of OLP.
Keywords: Angiogenesis, CD34 antibody, microvessel density
INTRODUCTION
Oral lichen planus (OLP), a common mucocutaneous disease, was first described by Wilson in 1869.[1] Poor described the formation of cavities in lichen planus of the mucosa, corresponding in character to subepithelial bullae and Gougerot described the reticular, annular, plaque, ulcerated, dotted and sclerotic forms of lichen planus.[2]
OLP is considered to be a T-cell mediated autoimmune disease in which cytotoxic CD8+ T-cells trigger the apoptosis in oral epithelial cells. The lichen planus antigen has not been definitely identified and it may be a self-peptide.[1] In most of the studies, OLP is considered to be a T-cell-mediated chronic inflammatory oral mucosal disease.[3] The precise cause of OLP is unknown.[1,3] It is mainly observed among women and symmetrically affects the buccal mucosa, tongue, gingiva, floor of the mouth, lips, and palate.[4] There are essentially two types of OLP: Reticular and erosive.[5]
The role of angiogenesis in the pathogenesis of chronic inflammatory diseases is of considerable interest. A positive feedback loop, in which an inflammatory state promotes angiogenesis and the angiogenesis in turn facilitates chronic inflammation, has been found in some inflammatory diseases.[6] There is increasing evidence that chronic inflammation is tightly linked to diseases associated with endothelial dysfunction, and plays a role in the induction of aberrant angiogenesis.[7] Microvessel density (MVD) evaluation is commonly applied for the estimation of tumor angiogenesis and is widely accepted to play a role in the pathogenesis of some inflammatory conditions also. CD34 monoclonal antibody can be used to highlight the microvessels in inflammatory or neoplastic disorders because of its capability of staining the vascular endothelial cells (membranous staining pattern).[8] The purpose of this study was to evaluate the role of angiogenesis in the pathogenesis of OLP, using CD34 stain to highlight the blood vessels for measuring the MVD. Better understanding of the etiopathological mechanism underlying OLP will help in the development of new treatment strategies, as well as to manage persistent inflammation in patients showing poor response to conventional immunosuppressive drug regimes.
MATERIALS AND METHODS
This study included 40 cases, categorized into two groups: Group I and group II. Group I, the control group, consisted of 15 specimens of normal oral mucosa (buccal mucosa/gingival tissue) from individuals who underwent extractions as part of orthodontic treatment and from the paraffin blocks available with the archives of the Department of Oral and Maxillofacial Pathology and Microbiology, M. M. College of Dental Sciences and Research, Mullana. Group II consisted of 25 diagnosed cases of OLP; these cases were subdivided into two groups: Group IIA and group IIB. Group IIA consisted of 18 diagnosed cases of reticular OLP and group IIB consisted of 7 diagnosed cases of erosive OLP. These cases were obtained either from the blocks of previously diagnosed cases from the archives of the Department of Oral and Maxillofacial Pathology and Microbiology, M. M. College of Dental Sciences and Research, Mullana, or from fresh cases identified at the department of Oral Medicine and Radiology. Case history pertaining to the lesion was obtained and recorded. The samples were fixed in 10% formalin and routinely embedded in paraffin. Sections of 4-μm thickness were cut from the paraffin blocks and were used for immunohistochemical staining.
Immunohistochemistry
Immunohistochemical staining was performed for CD34 antibody (Dako). Briefly, endogenous peroxidase activity was blocked with 0.6% H2O2 and sections were incubated at room temperature with 100 μl of prediluted primary CD34 antibody (Dako). After washing in phosphate-buffered saline (PBS), secondary antibody was applied on the section and then washed with PBS. Diaminobenzidine Hyrochloride was applied till a brown color appeared. After counterstaining with hematoxylin, the sections were mounted with dibutyl phthalate xylol (DPX) and the slides were observed under a Nikon® research microscope. Negative and positive controls were used simultaneously to assess the specificity and reliability of the staining process. PBS was substituted for the primary antibodies of CD34 as a negative control, and placental tissue sections with known CD34 positivity were used as the positive control.
Quantification of microvessel density
Microvessels were highlighted on the sections of OLP and normal oral mucosal tissue by immunostaining with anti-CD34 monoclonal antibody [Figures 1–3]. Any single brown-stained cell or cluster of endothelial cells that were clearly separated from adjacent microvessels, histiocytes, and other connective tissue elements were considered as a single vessel.
Figure 1.
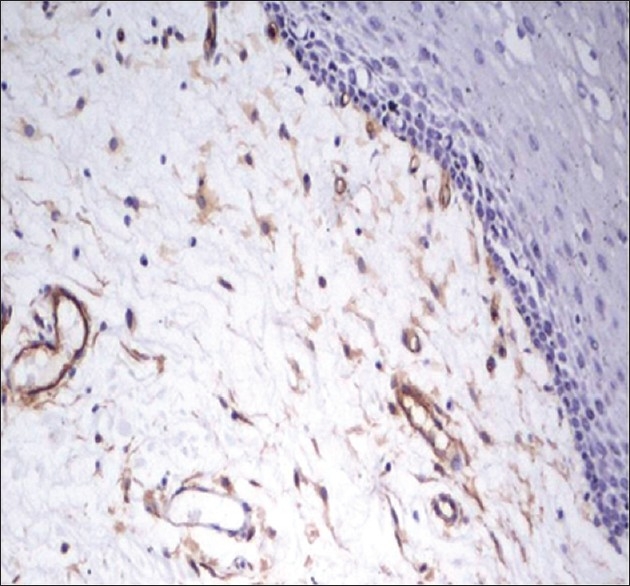
CD34-stained brown-colored blood vessels in normal oral mucosal tissue
Figure 3.
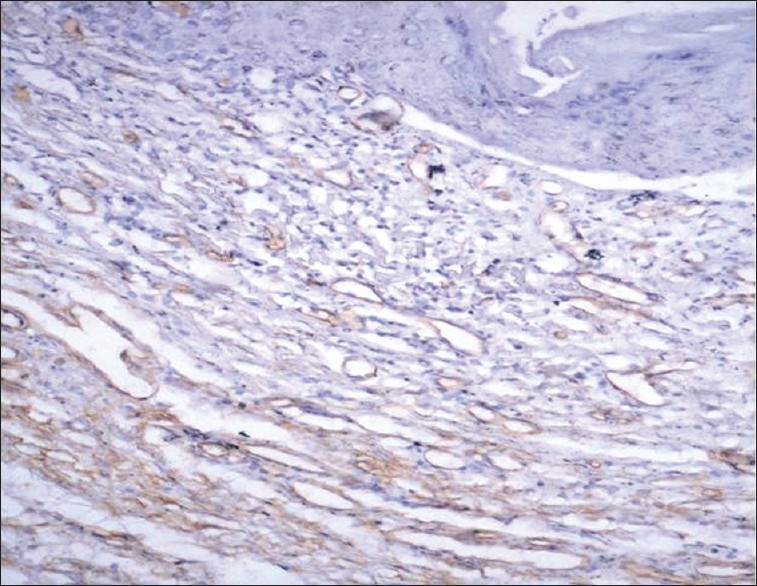
CD34-stained brown-colored blood vessels in erosive oral lichen planus
CD34-stained sections were scanned at low magnification to identify the most vascular areas (hot spot areas), which were almost exclusively localized within the inflammatory infiltrate. A maximum of five fields were selected and the images were captured on the computer under the ×20 objective of a Nikon research microscope (Y-THR-L; Japan; 0132508) for counting of the number of blood vessels in hot spot areas.
Statistical analysis
Data entry and analysis was performed using SPSS statistical Software. The unpaired t test was used for statistical analysis. Statistical significance was defined as P<.05.
RESULTS
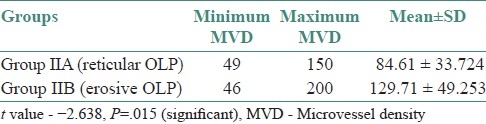
In the present study, of the difference between mean MVD in group I (44.47) and that in group II (97.24) was statistically highly significant (P=.001) [Table 1]. A comparison of the mean MVD in group IIA (84.61) with that in group IIB (129.71) also showed a statistically significant difference (P=.015) [Table 2].
Table 1.
Comparison of microvessel density between group I and group II
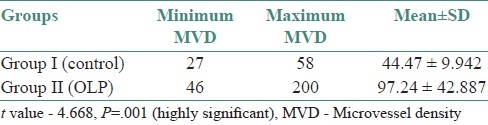
Table 2.
Comparison of MVD between group IIA and group IIB
DISCUSSION
The etiopathogenesis of OLP has always been controversial. At different times, various concepts have been quoted by different authors but the differences have not yet been sorted.
As an autoimmune disease with inflammatory origin and chronic progression, OLP satisfies all the prerequisites of hypoxia which is essential for angiogenesis.[9] Macrophages and the other cells of the immune system produce vascular endothelial growth factor (VEGF), which stimulates the degradation, proliferation, and migration of endothelial cells. VEGF regulates vascular permeability, which is very important for the initiation of angiogenesis.[9] TNF-alpha, IL-1 alpha, interleukin-6, and interleukin-8 also upregulate the expression of VEGF, and these factors have been found in the oral fluids of patients with OLP. Also, OLP shows significantly increased VEGF as compared to normal oral mucosa.[6] Proangiogenic and angiogenic factors such as histamine, heparin, chymase, bFGF, VEGF, and TGF-beta are produced by mast cells,[10] and an increase in the number of mast cells has been observed in OLP.[11] A significant correlation between mast cell density and MVD has been found during the evolution of oral squamous cell carcinoma from normal oral tissue through premalignant lesion with various degrees of dysplasia to carcinoma.[10]
The malignant potential of OLP remains controversial, and different research groups have proposed different approaches and interpretations. It appears advisable to carry out a meticulous follow-up of patients with OLP for early detection of the malignant transformation of suspected lesions.[12] Malignant transformation of OLP may be related to, or dependent on, a series of molecular stimuli originating in the inflammatory infiltrate.[13] Some molecules and radicals generated by inflammatory cells can act as mutagenic agents for epithelial cells or influence important cell cycle regulation mechanisms, e.g., apoptosis, cell cycle arrest, and cell proliferation, among others.[12] A source of possible mutation in OLP derives from the action of cyclooxygenase-2 (COX-2) that is produced by inflammatory infiltrating cells. Apart from its other actions, COX-2 also intervenes in the metabolism of arachidonic acid, generating the carcinogenic metabolite malondialdehyde, which can damage DNA.[13]
Recruitment and retention of lymphocytes is a requisite event for lichen planus. Attraction of lymphocytes to a particular site would require cytokine-mediated upregulation of adhesion molecules on endothelial cells and concomitant expression of receptor molecules by circulating lymphocytes. In OLP, there is in fact increased expression of several vascular adhesion molecules (known by the acronyms ELAM-1, ICAM-1, and VCAM-1) and infiltrating lymphocytes that express reciprocal receptors (known as L-selectin, LFA-1, and VLA4), supporting the hypothesis that there is activation of a lymphocyte homing mechanism in lichen planus.[14]
Thus, if angiogenesis is increased, it will lead to more recruitment and retention of lymphocytes or inflammatory infiltrate, or progression of disease or recurrence of the lesions; the inflammatory infiltrate can further lead to carcinogenesis. The link between complement factors and etiopathogenesis of oral lichen planus is not clear. Complements can be just extravasated proteins from the leaking vessels in oral lichen planus.[15]
CD34, a myeloid progenitor cell antigen present in endothelial cells, is detectable in all types of endothelium. The monoclonal antibody against CD34 reacts with the endothelium of arteries and venules and has been found to stain capillary endothelium most intensely, because it is highly sensitive for endothelium and produces lowest background staining.[16] Therefore, in the present study, MVD was estimated by CD34 immunostaining. In the present study, comparison of mean MVD between group I (44.47) and group II (97.24) showed a statistically highly significant result. Our results are in agreement with the studies done by Jin et al.,[17] Tao et al.,[6] and Scardina et al.[9]
There is a great need to understand the etiopathogenesis and progression of OLP.[18] Our study suggests that angiogenesis is significantly increased in OLP as compared to normal oral mucosa [Figure 1], also in erosive OLP [Figure 3] as compared to reticular OLP [Figure 2]; this suggests that angiogenesis is one of the main contributing factors in the progression of OLP. Malignant transformation in OLP is said to occur in 0.3%–3% of cases and is more common in the erosive form.[1] Thus, the increased MVD in erosive OLP as compared to reticular OLP in our study could be suggested as one of the factors contributing to this higher malignant potential.
Figure 2.
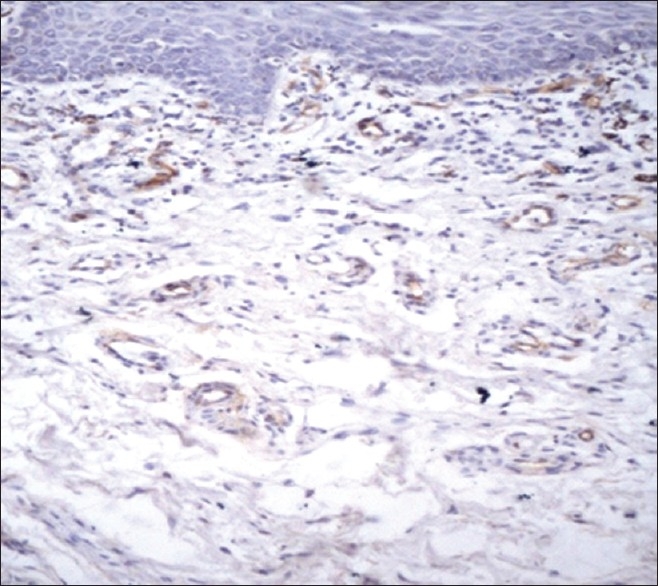
CD34-stained brown-colored blood vessels in reticular oral lichen planus
Angiogenesis has long been known to be closely linked to chronic inflammation, and it is a component of various chronic inflammatory diseases. However, the exact etiopathological mechanism of OLP is still not clear. Most of the studies have not been able to demonstrate a direct relation between angiogenesis and OLP. Antiangiogenic drugs are not commonly used in OLP patients. If antiangiogenic drugs can be demonstrated to be beneficial in OLP patients, it would reduce the dependency on corticosteroid drugs.
Considering the role of angiogenesis in the inflammatory lesions of OLP, and the fact that some OLP patients respond poorly to conventional immunosuppressive drugs, angiogenesis could be a possible therapeutic target to reverse the persistent and stubborn inflammation. Thus, antiangiogenic drugs can be used in future for the treatment of OLP.
CONCLUSION
From this study, we conclude that angiogenesis can be considered to play a role in both the etiopathogenesis and progression of OLP. Our findings could be helpful for formulating new treatment strategies for OLP utilizing antiangiogenic medications.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Rajendran R, Sivapathasundharam B. Shafer's Textbook of Oral Pathology. 6th ed. Amsterdam: Elsevier; 2009. [Google Scholar]
- 2.McCarthy PL, Shklar G. Diseases of the Oral Mucosa. 2nd ed. Philadelphia: Lea and Febige; 1980. [Google Scholar]
- 3.Sugerman PB, Savage NW, Walsh LJ, Zhao ZZ, Zhou XJ, Khan A, et al. The pathogenesis of oral lichen planus. Crit Rev Oral Biol Med. 2002;13:350–64. doi: 10.1177/154411130201300405. [DOI] [PubMed] [Google Scholar]
- 4.Sahebjamee M, Kalati FA. Management of Oral lichen planus. Arch Iran Med. 2005;8:252–6. [Google Scholar]
- 5.Brant JM, Vasconcelos AC, Rodrigues LV. Role of apoptosis in erosive and reticular oral lichen planus exhibiting variable epithelial thickness. Braz Dent J. 2008;19:179–85. doi: 10.1590/s0103-64402008000300001. [DOI] [PubMed] [Google Scholar]
- 6.Tao X, Huang Y, Li R, Quing R, Ma L, Rhodus NL, et al. Assessment of local angiogenesis and vascular endothelial growth factors in the patients with atrophic-erosive and reticular oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:661–9. doi: 10.1016/j.tripleo.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 7.Rajashekhar G, Willuweit A, Patterson CE, Sun P, Hilbig A, Breier G, et al. Continuous endothelial cell activation increases angiogenesis: Evidence for the direct role of endothelium linking angiogenesis and inflammation. J Vasc Res. 2006;43:193–204. doi: 10.1159/000090949. [DOI] [PubMed] [Google Scholar]
- 8.Hussein MR. Evaluation of angiogenesis in normal and lichen planus skin by CD34 protein immunohistochemistry: Preliminary findings. Cell Biol Int. 2007;31:1292–5. doi: 10.1016/j.cellbi.2007.03.035. [DOI] [PubMed] [Google Scholar]
- 9.Scardina GA, Ruggieri A, Messina P, Maresi E. Angiogenesis of oral lichen planus: A possible pathogenetic mechanism. Med Oral Patol Oral Cir Bucal. 2009;14:e558–62. doi: 10.4317/medoral.14.e558. [DOI] [PubMed] [Google Scholar]
- 10.Michailidou EZ, Markopoulous AK, Antoniades DZ. Mast cells and angiogenesis in oral malignant and premalignant lesions. Open Dent J. 2008;2:126–32. doi: 10.2174/1874210600802010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao ZZ, Savage NW, Walsh LJ. Association between mast cells and laminin in oral lichen planus. J Oral Pathol Med. 1998;27:163–7. doi: 10.1111/j.1600-0714.1998.tb01934.x. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez-Moles MA, Scully C, Gil-Montoya JA. Oral lichen planus: Controversies surrounding malignant transformation. Oral Dis. 2008;14:229–43. doi: 10.1111/j.1601-0825.2008.01441.x. [DOI] [PubMed] [Google Scholar]
- 13.Mignogna MD, Fedele S, Lo Russo L, Lo Muzio L, Bucci E. Immune activation and chronic inflammation as the cause of malignancy in oral lichen planus: Is there any evidence? Oral Oncol. 2004;40:120–30. doi: 10.1016/j.oraloncology.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Regezi JA, Sciubba JJ, Jordan RCK. Oral Pathology; Clinical Pathological Correlations. 4th ed. St. Louis: Saunders; 2003. [Google Scholar]
- 15.Laskaris G, Sklavounou A, Angelopoulos A. Direct immunoflorescence in oral lichen planus. Oral Surg Oral Med Oral Pathol. 1982;53:483–7. doi: 10.1016/0030-4220(82)90461-3. [DOI] [PubMed] [Google Scholar]
- 16.Mazur G, Wróbel T, Dziegiel P, Jeleń M, Kuliczkowski K, Zabel M. Angiogenesis measured by expression of CD 34 antigen in lymph nodes of patients with non – Hodgkin's lymphoma. Folia Histochem Cytobiol. 2004;42:241–3. [PubMed] [Google Scholar]
- 17.Jin Y, Tipoe GL, White FH, Yang L. A quantitative investigation of immunocytochemically stained blood vessels in normal, benign, premalignant and malignant human oral cheeq epithelium. Virchows Arch. 1995;427:145–51. doi: 10.1007/BF00196519. [DOI] [PubMed] [Google Scholar]
- 18.Shetty S, Thomas P, Chatra L, Shenai P, Rao P, Babu S. An association between serum cortisol levels in erosive and nonerosive oral lichen planus patients. Webmedcentral. 2011;1:1–15. [Google Scholar]


