Abstract
Diagnosis of palatal swellings is a challenge. Benign and malignant tumors may be misinterpreted as lesions of inflammatory origin. We present a case of B-cell non-Hodgkin lymphoma on the palate of a 40-year-old male. A number of factors can make the diagnosis of oral lymphoma difficult. Many lymphomas are extranodal, there is usually a prominent superimposed nonspecific inflammatory response and thus they mimic benign lymphoid hyperplasia. It is important for the pathologist to be familiar with features that distinguish benign from malignant lymphoid proliferations.
Keywords: B-cell lymphoma, benign lymphoid hyperplasia, extranodal non-Hodgkin lymphoma, MALT, monocytoid B-cell lymphoma, pseudolymphoma
INTRODUCTION
The diagnosis of palatal lesions is a challenge for any clinician. The palate is a complex area of the mouth, with a variety of native tissue types that give rise to a plethora of pathological conditions, both benign and malignant [Table 1].[1,2] Lymphomas are malignant neoplasms of the lymphocytic cell lines. They mainly involve lymph nodes, spleen, and other nonhematopoietic tissues. They are generally classified as either Hodgkin lymphoma or non-Hodgkin lymphoma (NHL) and may be of either B-lymphocyte or T-lymphocyte origin.[3] Lymphoma is the second most common neoplasm of the head and neck region after squamous cell carcinoma. Nearly 24%–48% of NHL can arise in extranodal locations, and 3%–5% are primarily located in the oral cavity.[4,5]
Table 1.
Differential diagnosis of palatal swellings

Oral lymphomas are relatively rare and are often difficult to diagnose as they may mimic other pathologies such as periodontal diseases, osteomyelitis, or some other malignancy.[6] Lymphoid lesions of the palate can be divided into three categories, the management and prognosis of each category being different:[7]
Primary lymphoma of the palate, with no other lymphomatous lesion detected elsewhere in the body
Lymphoma of the palate occurring as one of the lesions in a case of disseminated lymphoma
Benign lymphoid hyperplasia (BLH) of the palate
In addition, histologically, lymphoid lesions of the palate may be misinterpreted as inflammatory in nature. The purpose of our report is to present a case of B-cell lymphoma on the palate and distinguish it from benign lymphoid hyperplasia (BLH).
CASE REPORT
A 40-year-old man with a swelling in the right palatal region was referred to the department of oral pathology for evaluation and diagnosis. The painless mass was noticed 4 months ago by the patient.
Intraoral examination exhibited a firm, exophytic, oval mass with an intact overlying mucosa in the region of the right hard palate measuring 3 × 4.5 cm in size [Figure 1]. There were no signs of ulceration, bleeding, discharge, or numbness in the area. The patient did not have the habit of chewing tobacco or betel nut. On general examination, he was found to be afebrile, with no palpable lymph nodes in the head and neck region. He did not mention any sudden weight loss in the recent past and the medical history was noncontributory.
Figure 1.
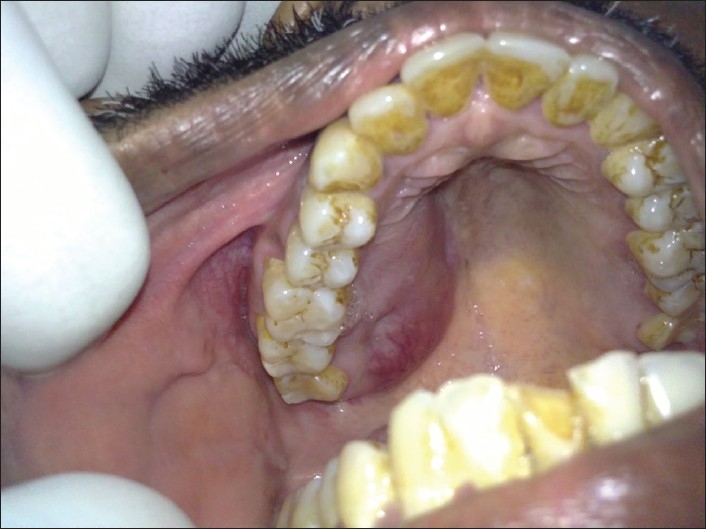
Clinical photograph showing swelling on the palate
CT scan revealed a mass on the right side of the hard palate, with no involvement of the maxillary sinus [Figure 2]. A provisional diagnosis of benign tumor of salivary glands was given.
Figure 2.
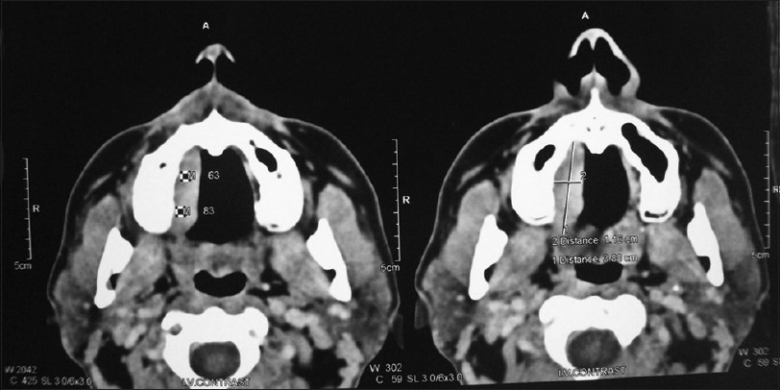
Computed tomography scan of patient
An excisional biopsy [Figure 3] was performed under local anesthesia. and a bony crater-like defect was seen on the palatal bone after soft tissue removal. Histopathological examination of sections of the resected specimen revealed an intact stratified squamous epithelium with underlying vaguely follicular and diffuse proliferation of lymphoid cells [Figure 4a and b]. The follicle-like structures were composed of central large cells (giving a ‘washed-out’ appearance) surrounded by a thin rim of small, round lymphocytes. The central large cells had abundant pinkish cytoplasm and convoluted nuclei with inconspicuous nucleoli [Figure 5]. Epimyoepithelial islands were also observed. On microscopic examination of hematoxylin and eosin (H and E)–stained sections, there was a conflict of opinion over the distinction between benign (reactive) lymphoid hyperplasia (pseudolymphoma) and non-Hodgkin lymphoma. Immunohistochemical investigations were performed to resolve the issue. The large lymphoid cells showed immunoreactivity for CD20 and the rim of small lymphocytes were positive for CD5 [Figure 6]. The large lymphoid cells were negative for CD5, Bcl-2, and CD10 [Figures 7–9]. The Ki67 proliferative index was 1% [Figure 10].
Figure 3.
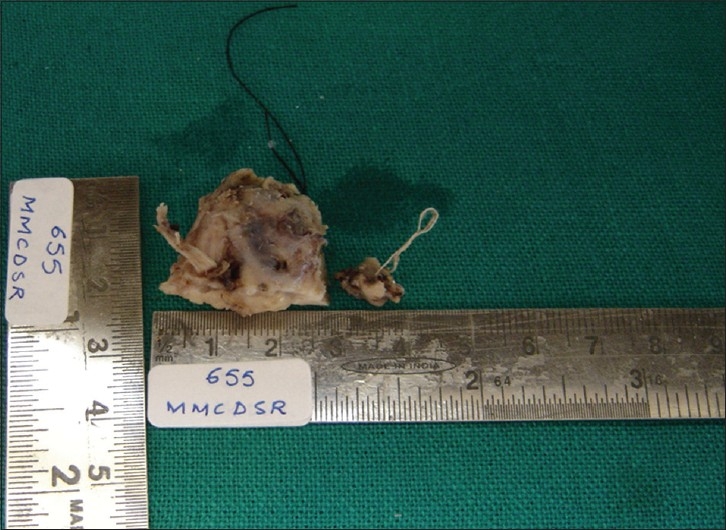
Photograph of gross excisional tissue
Figure 4.
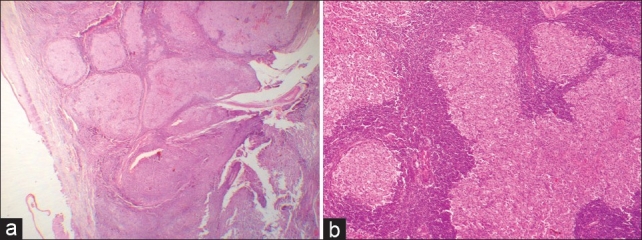
(a) The overlying epithelium is seen separated from follicles. The histological picture in this view gives the impression of reactive follicular hyperplasia of the lymphoid tissue (H and E, stain; original magnification, ×2.5). (b) High-power view of the follicular pattern gives the impression of a reactive lesion (H and E, stain; original magnification, ×10)
Figure 5.
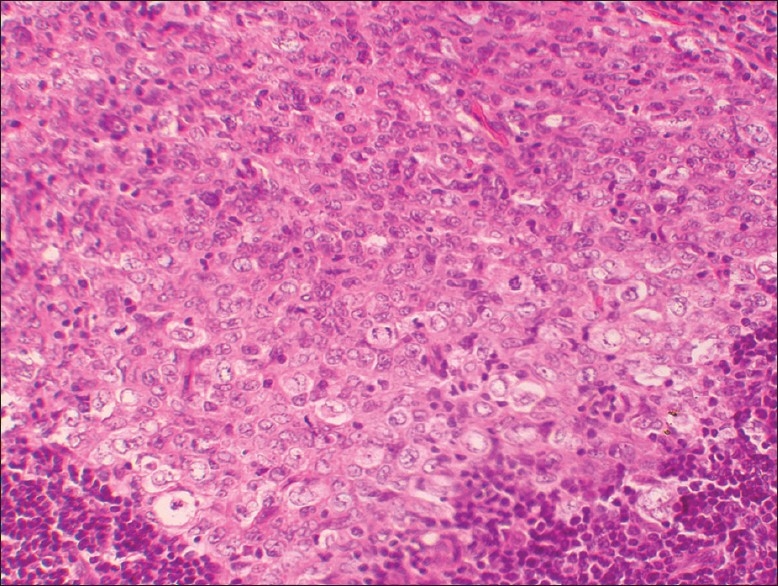
Magnified view showing central larger cells with mitotic figures, convoluted nuclei, and inconspicuous nucleoli. Peripherally, small round lymphocytes are seen (H and E, stain; original magnification, ×20)
Figure 6.
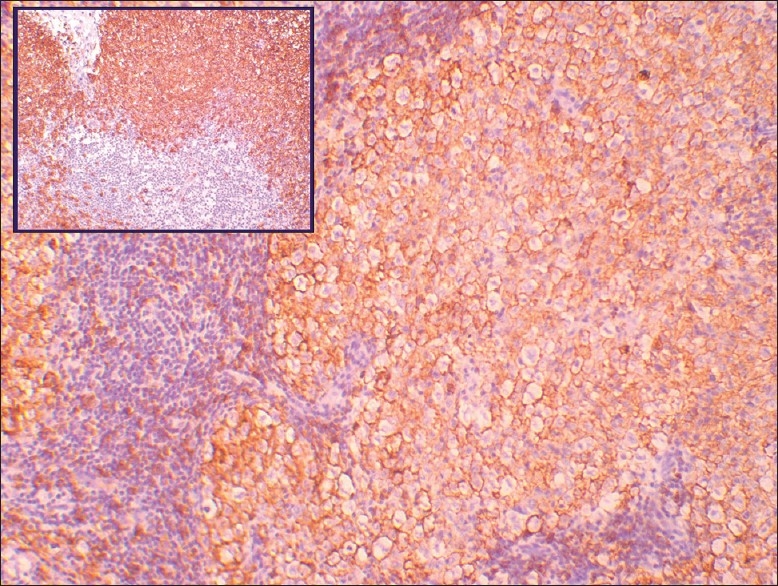
The large lymphoid cells show positive immunoreactivity for CD20, whereas the peripheral small lymphocytes are negative. Inset shows control stain (original magnification, ×10)
Figure 7.
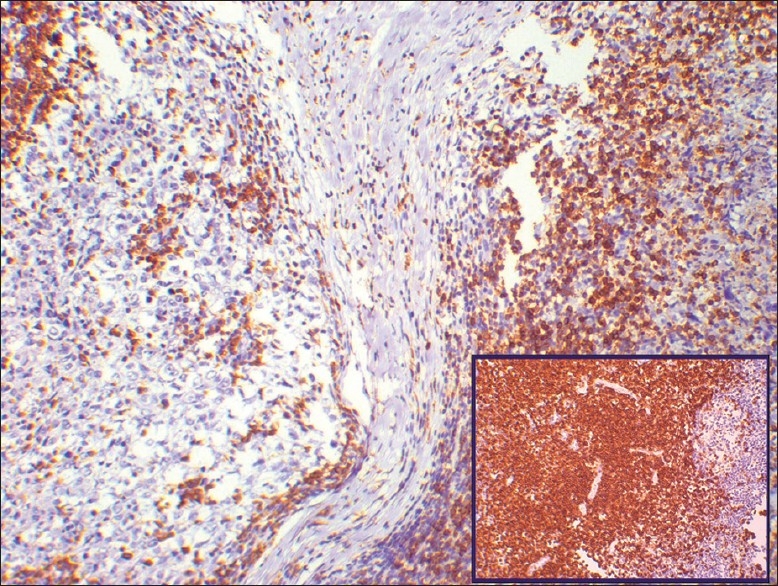
The peripheral small lymphocytes show positive immunoreactivity for CD5, whereas the central large cells are negative. Inset shows control stain (original magnification, ×10)
Figure 9.
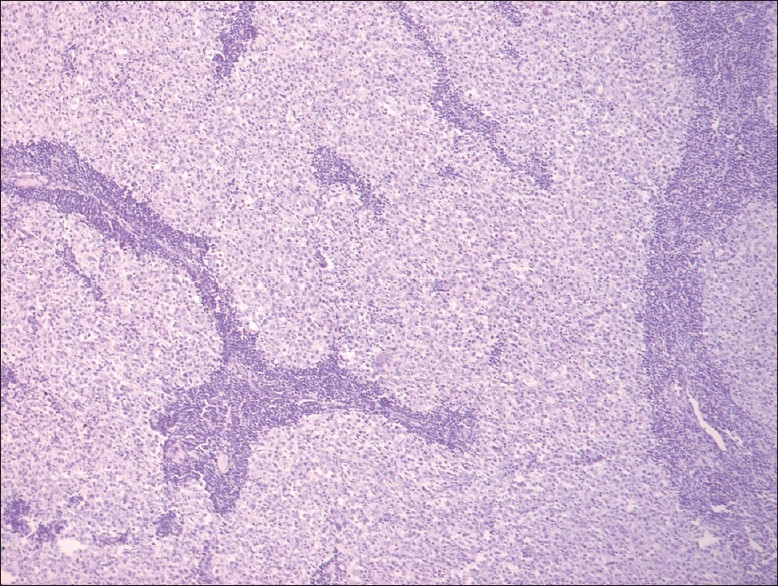
Negative immunoreactivity for CD10 (original magnification, ×10)
Figure 10.
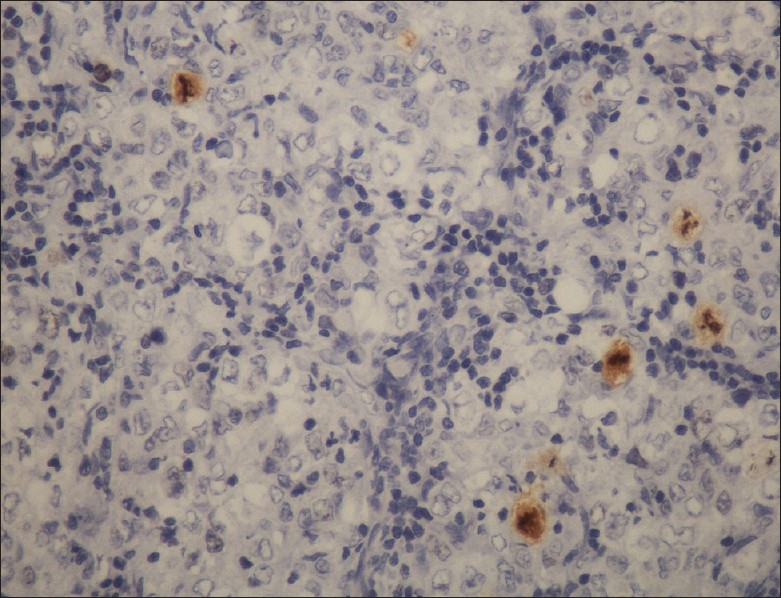
Immunohistochemistry profile showing very low Ki67 proliferative index (original magnification, ×20)
Figure 8.
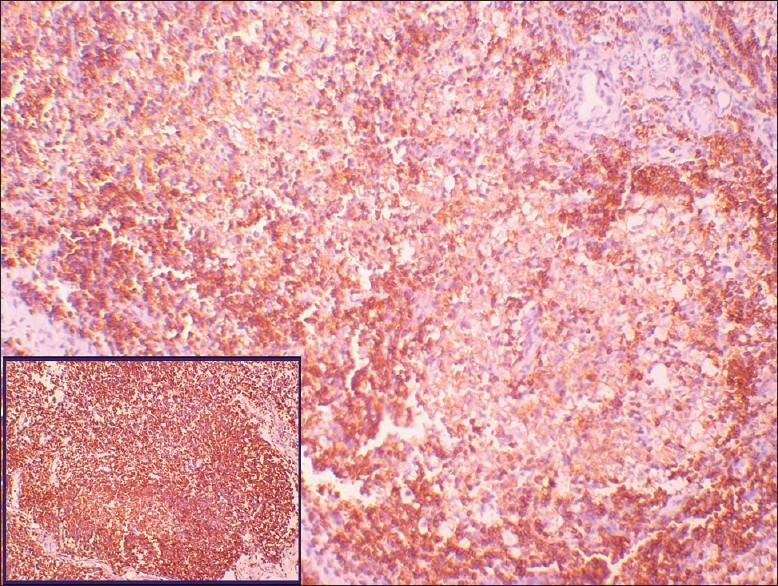
The peripheral small lymphocytes show positive immunoreactivity for Bcl2, whereas the central large cells are negative. Inset shows control stain (original magnification, ×10)
The patient was referred to a general physician for systemic evaluation and was found to be free of other systemic manifestations. There were no palpable lymph nodes found anywhere in the body. A final diagnosis of low-grade B-cell lymphoma of mucosa-associated lymphoid tissue (MALT) was accorded based on the clinical, radiographic, histopathologic, and immunohistochemical investigations.
DISCUSSION
Lymphoma constitutes a diverse and complex group of malignancies of lymphoid histogenesis. According to the revised European–American Classification of Lymphoid Neoplasms (REAL), classification the category of marginal zone B-cell lymphoma includes lymphomas that were originally designated as monocytoid B-cell lymphoma and low-grade B-cell lymphoma of MALT. Both these neoplasms were thought to represent different clinical presentations of a B-cell lymphoma believed to arise from normal marginal zone B-cells in the lymph node or their extranodal counterparts, respectively.[8]
The most frequent location of extranodal lymphoma in the head and neck is the palate. Lymphoma usually appear as a nontender diffuse mass and are rarely ulcerated. Many salivary gland lymphocytic infiltrates of the palate are usually non-Hodgkin B-cell lymphomas of MALT.[9]
Extranodal marginal B-cell lymphomas of MALT (B-MALT) are known to show histologic features similar to benign lymphoid hyperplasias (BLH). In the past, many low-grade lymphomas were probably misdiagnosed and inappropriately categorized as BLH. Extranodal infiltration composed of small round or slightly irregular lymphoid cells—often admixed with plasma cells, histiocytes and lymphoid follicle—were classified as ‘pseudolymphomas’ since clinical studies showed that in patients with these lesions, the disease pursued an indolent clinical course.[10]
The term pseudolymphoma/benign lymphoid hyperplasia should be restricted to tumefactive lesions with prominent lymphoid infiltrate that by routine morphology appears to be reactive and that on ancillary immunophenotypic and molecular genetic analysis lacks evidence of clonality.[11] There appears to be no appreciable clinical difference between BLH and the palatal lymphomas. Both can present as unilateral or bilateral swellings that may be soft or firm. Both lesions usually have intact surface mucosa. Our case also had no mucosal ulceration. Even histopathologically, palatal lymphoma is very similar to BLH. The subtle distinctions in interpretation of the histological characteristics between benign and malignant lymphoid proliferations makes the diagnosis of these lesions difficult. Several papers have dealt with the histologic features of BLH and nodular lymphoma.[12–14] Fortunately, with the advent of advanced immunophenotyping and molecular genetic techniques, it is now possible to differentiate the two lesions. Table 2 lists out the essential features that help us distinguish BLH from B-cell MALT lymphomas.[11,15]
Table 2.
Lymphoid hyperplasia vs B.cell MALT lymphoma
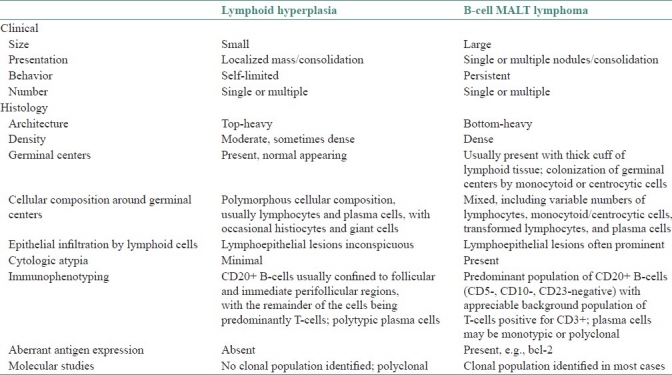
BLH is characterized by numerous well-demarcated germinal centers of varying size and shape often rimmed by a mantle of small lymphocytes. While many B-cell lymphomas have a nodular growth pattern, these nodules are neoplastic and should not be considered germinal centers.[16,17] In addition, in lymphomas, these neoplastic nodules tend to be ovoid to round and show little variation in size. In BLH, the cells demonstrate all stages of follicular center cell transformation, including plasma cells. Mitosis may be abundant, although never atypical and never seen outside the germinal centers. Nuclear debris and evidence of phagocytosis are often prominent. The cells comprising malignant lymphomas, on the other hand, are neoplastic and do not show the wide range of cell types seen in reactive lesions. Nuclear atypia as well as mitosis (occasionally atypical) may be seen, but the mitotic rate rarely approaches that seen in benign lesions.
In our case, the follicle-like structures showed variation in size and were composed of central large cells surrounded by a thin rim of small, round lymphocytes. Mitotic activity was found to be quite low. Hence, it was quite difficult to give a confirmative diagnosis solely on the basis of routine histopathologic examination. Therefore we performed immunohistochemical analysis on the lesional tissue for the following markers: CD20, CD5, CD10, Bcl2, and Ki-67. The result was positive for CD20 and negative for CD5, CD10, and bcl2, while Ki-67 showed positivity of 1%. Immunophenotypic studies have shown that low-grade B-cell lymphomas of MALT typically do not express the B cell-associated antigens CD10, CD21, or Cd23, or the T-cell antigens (including CD5).[8,18] These findings were consistent with the immunohistochemistry profile of our case and thus confirmed our diagnosis of low-grade B-cell lymphoma of MALT.
MANAGEMENT
Patients with localized low-grade B-cell lymphomas follow a very indolent clinical course. Therapeutic strategies for this kind of disease are not standardized yet due to the small number of MALT lymphomas described in the head and neck region. Remedial measures include combined radiochemotherapy for advanced cases and either radiation therapy or surgery for small lesions. Our patient underwent surgical excision of the lesion and continues to be well after 6 months of follow-up.[19]
CONCLUSION
A number of factors can make the diagnosis of oral lymphoma difficult, despite the fact that the histological features are the same as at other sites. Many lymphomas are extranodal and there is almost always a prominent superimposed, nonspecific, inflammatory response. Clinically obvious lymphomas present primarily to the medical rather than the dental practitioner or oral surgeons. Hence to see a spectrum of lymphomas in oral pathology practice is unusual. Early recognition and biopsy are extremely important because the lymphoma may be confined entirely to the palate in the early stages and such localized palatal lymphomas respond well to irradiation, whereas disseminated disease necessitates chemotherapy.
Malignant B-cell lymphoma mimics BLH, both clinically and histologically. The pathologist must be familiar with the features that distinguish between these two diseases. Light microscopy supported by immunohistochemistry is essential for providing the final diagnosis.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Eversole LR. Clinical Outline of Oral Pathology: Diagnosis and Treatment 3/e. Hamilton, Ontario: Decker BC Inc; 2002. pp. 112–53. [Google Scholar]
- 2.Dias DK. Short notes for Maxillofacial Surgery and Clinical Dentistry. New Delhi: Jaypee Brothers Medical Publishers (P) Ltd; 2002. pp. 84–5. [Google Scholar]
- 3.Rooney N, Ramsay AD. Lymphomas of the head and neck 2: The B-cell lymphomas. Eur J Cancer B Oral Oncol. 1994;30B:155–9. doi: 10.1016/0964-1955(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 4.Ugboko VI, Oginni FO, Adelusola KA, Durosinmi MA. Orofacial non-Hodgkin's lymphoma in Nigerians. J Oral Maxillofac Surg. 2004;62:1347–50. doi: 10.1016/j.joms.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Mawardi H, Cutler C, Treister N. Medical management update: Non-Hodgkin lymphoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:e19–33. doi: 10.1016/j.tripleo.2008.08.054. [DOI] [PubMed] [Google Scholar]
- 6.Wolvius EB, van der Valk P, van der Wal JE, van Diest PJ, Huijgens PC, van der Waal I, et al. Primary extranodal non-Hodgkin's lymphoma of the oral cavity. An analysis of 34 cases. Eur J Cancer B Oral Oncol. 1994;30B:121–5. doi: 10.1016/0964-1955(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 7.Saltzstein SL. Extranodal malignant lymphomas and pseudolymphoma. In: Sommers SC, Rosen RP, Fechner RE, editors. Pathology annual. New York: Appleton-Century-Crafts; 1969. p. 159. [Google Scholar]
- 8.Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, et al. A revised European-American classification of lymphoid-neoplasms: A proposal from International lymphoma study group. Blood. 1994;84:1361–92. [PubMed] [Google Scholar]
- 9.Makepeace AR, Fermont DC, Bennett MH. Non-Hodgkin's lymphoma of the nasopharynx, para-nasal sinus and palate. Clin Radiol. 1989;40:144–6. doi: 10.1016/s0009-9260(89)80073-x. [DOI] [PubMed] [Google Scholar]
- 10.Medeiros LJ, Harmon DC, Linggood RM, Harris NL. Immunohistologic features predict clinical behavior of orbital and conjunctival lymphoid infiltrates. Blood. 1989;74:2121–9. [PubMed] [Google Scholar]
- 11.Morice GW, Colby TV. Lymphoproliferative Diseases. In: Tomashefski JF Jr, Cagle PT, Farver CF, Fraire AE, editors. Dail and Hammar's Pulmonary Pathology. 3rd ed. Vol. 2. New York: Springer Science+Business Media, LLC; 2008. p. 7. Neoplastic Lung Disease. [Google Scholar]
- 12.Adkins KF. Lymphoid hyperplasia in the Oral Mucosa. Aust Dent J. 1973;18:38–40. doi: 10.1111/j.1834-7819.1973.tb02354.x. [DOI] [PubMed] [Google Scholar]
- 13.Rappaport H. In Atlas of tumor pathology, Section III, Fascicle8. Washington D.C: Armed forces Institute of pathology; 1966. Tumors of the Hematopoietic system; pp. 426–42. [Google Scholar]
- 14.Bernard CW, Cossman J, Jaffe ES. Malignant lymphomas as Tumours of the Immune system. Br J Cancer. 1980;42:1–20. doi: 10.1038/bjc.1980.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilliam AC, Wood GS. Cutaneous lymphoid hyperplasias. Semin Cutan Med Surg. 2000;19:133–41. doi: 10.1016/s1085-5629(00)80011-5. [DOI] [PubMed] [Google Scholar]
- 16.Jaffe ES, Shevach EM, Frank MM, Berard CW, Green I. Nodular lymphoma–evidence for origin from follicular B lymphocytes. N Engl J Med. 1974;290:813–9. doi: 10.1056/NEJM197404112901501. [DOI] [PubMed] [Google Scholar]
- 17.Warnke R, Levy R. Immunopathology of follicular lymphomas. A model of B-lymphocyte homing. N Engl J Med. 1978;298:481–6. doi: 10.1056/NEJM197803022980903. [DOI] [PubMed] [Google Scholar]
- 18.Freedman AS, Nadler LM. Non-Hodgkin's Lymphomas. In: Holland Jk, Bast RC, Morton DL, Frei E, Kufe DW, Weichelbaum RR., editors. Cancer Medicine. 4th ed. Baltimore: Williams and Wilkins; 1997. pp. 2757–95. [Google Scholar]
- 19.Tauber S, Nerlich A, Lang S. MALT lymphoma of the paranasal sinuses and the hard palate: Report of two cases and review of the literature. Eur Arch Otorhinolaryngol. 2006;263:19–22. doi: 10.1007/s00405-003-0654-3. [DOI] [PubMed] [Google Scholar]


