Abstract
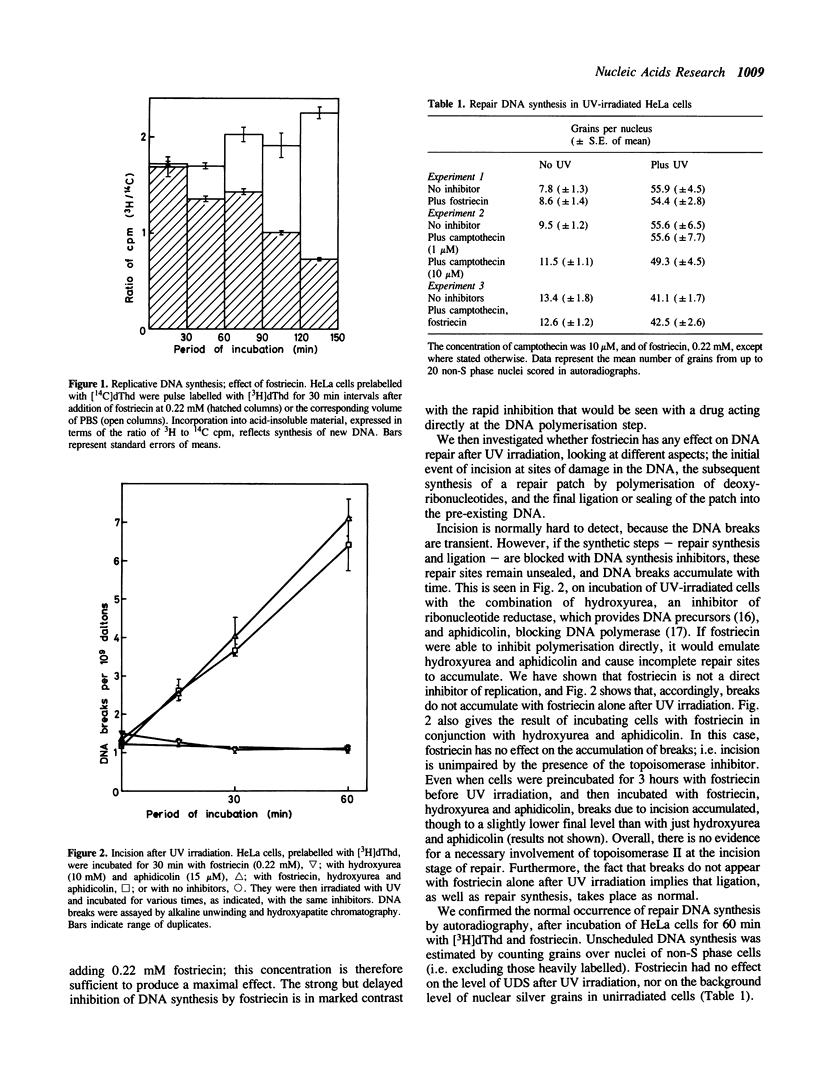
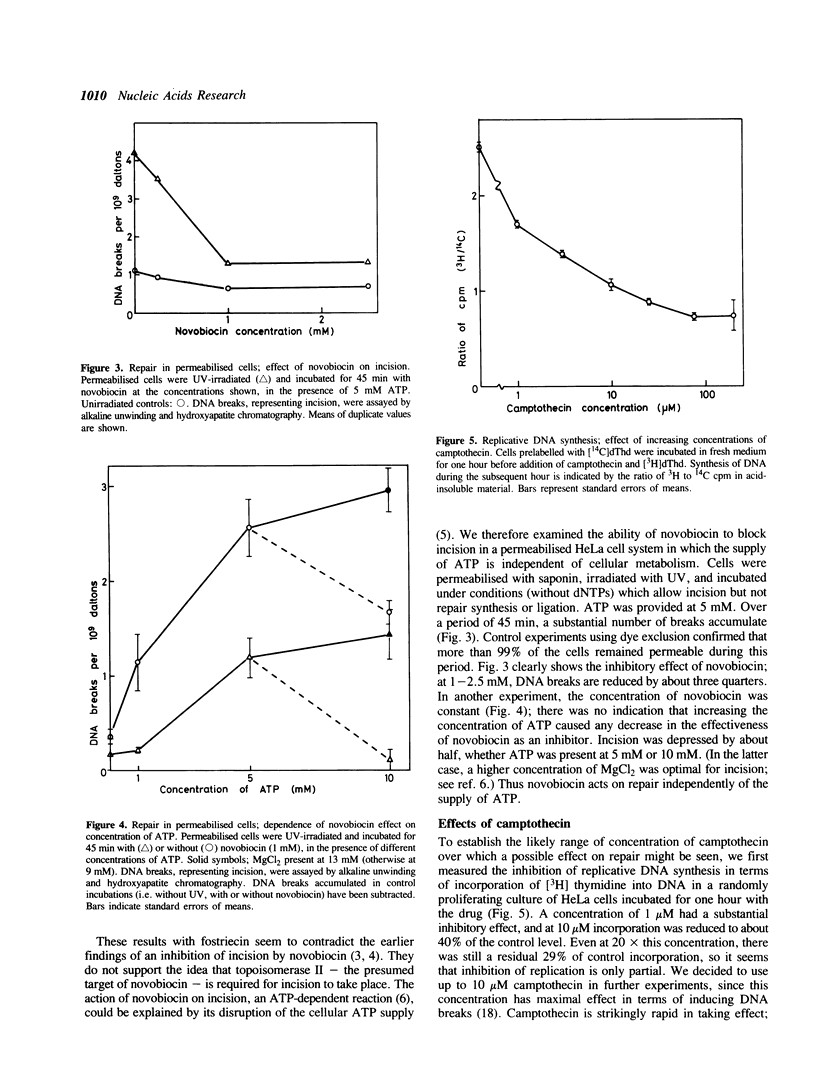
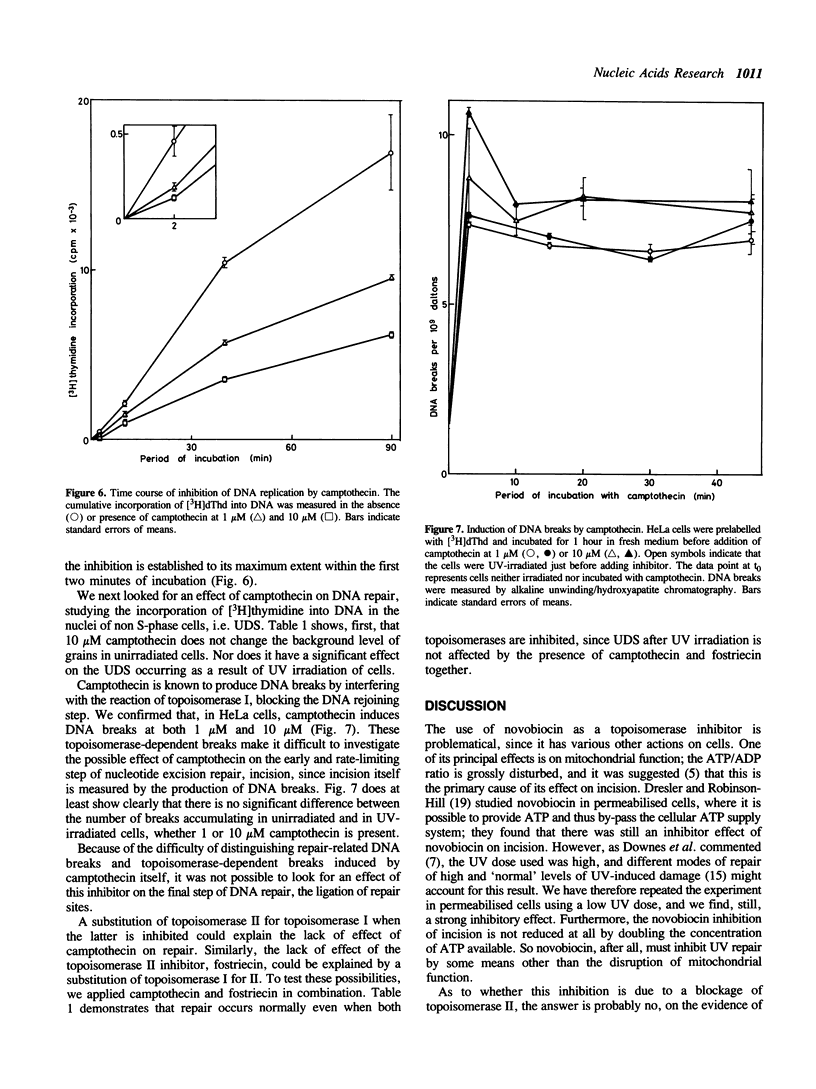
Fostriecin causes a delayed inhibition of replicative DNA synthesis in human cells, consistent with a role for DNA topoisomerase II (its target enzyme) at a late stage in replication. Fostriecin does not inhibit UV-induced excision repair. The less specific inhibitor novobiocin blocks repair in permeabilised cells given a low dose of UV, presumably through a mechanism other than the inhibition of topoisomerase II. Its effect cannot be accounted for by a depletion of the ATP required for incision. Camptothecin, an inhibitor of DNA topoisomerase I, blocks replicative DNA synthesis immediately but incompletely, suggesting a participation of topoisomerase I at the replication fork, but it, too, has no influence on DNA repair. We thus find no evidence for involvement of either topoisomerase I or II in the response of cells to UV damage.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andoh T., Ishii K., Suzuki Y., Ikegami Y., Kusunoki Y., Takemoto Y., Okada K. Characterization of a mammalian mutant with a camptothecin-resistant DNA topoisomerase I. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5565–5569. doi: 10.1073/pnas.84.16.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avemann K., Knippers R., Koller T., Sogo J. M. Camptothecin, a specific inhibitor of type I DNA topoisomerase, induces DNA breakage at replication forks. Mol Cell Biol. 1988 Aug;8(8):3026–3034. doi: 10.1128/mcb.8.8.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boritzki T. J., Wolfard T. S., Besserer J. A., Jackson R. C., Fry D. W. Inhibition of type II topoisomerase by fostriecin. Biochem Pharmacol. 1988 Nov 1;37(21):4063–4068. doi: 10.1016/0006-2952(88)90096-2. [DOI] [PubMed] [Google Scholar]
- Collins A. R., Squires S., Johnson R. T. Inhibitors of repair DNA synthesis. Nucleic Acids Res. 1982 Feb 25;10(4):1203–1213. doi: 10.1093/nar/10.4.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins A., Johnson R. Novobiocin; an inhibitor of the repair of UV-induced but not X-ray-induced damage in mammalian cells. Nucleic Acids Res. 1979 Nov 10;7(5):1311–1320. doi: 10.1093/nar/7.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey J. M., Jaxel C., Kohn K. W., Pommier Y. Protein-linked DNA strand breaks induced in mammalian cells by camptothecin, an inhibitor of topoisomerase I. Cancer Res. 1989 Sep 15;49(18):5016–5022. [PubMed] [Google Scholar]
- DiNardo S., Voelkel K., Sternglanz R. DNA topoisomerase II mutant of Saccharomyces cerevisiae: topoisomerase II is required for segregation of daughter molecules at the termination of DNA replication. Proc Natl Acad Sci U S A. 1984 May;81(9):2616–2620. doi: 10.1073/pnas.81.9.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. S., Mullinger A. M., Johnson R. T. Action of etoposide (VP-16-123) on human cells: no evidence for topoisomerase II involvement in excision repair of u.v.-induced DNA damage, nor for mitochondrial hypersensitivity in ataxia telangiectasia. Carcinogenesis. 1987 Nov;8(11):1613–1618. doi: 10.1093/carcin/8.11.1613. [DOI] [PubMed] [Google Scholar]
- Downes C. S., Ord M. J., Mullinger A. M., Collins A. R., Johnson R. T. Novobiocin inhibition of DNA excision repair may occur through effects on mitochondrial structure and ATP metabolism, not on repair topoisomerases. Carcinogenesis. 1985 Sep;6(9):1343–1352. doi: 10.1093/carcin/6.9.1343. [DOI] [PubMed] [Google Scholar]
- Dresler S. L., Lieberman M. W. Requirement of ATP for specific incision of ultraviolet-damaged DNA during excision repair in permeable human fibroblasts. J Biol Chem. 1983 Oct 25;258(20):12269–12273. [PubMed] [Google Scholar]
- Dresler S. L., Robinson-Hill R. M. Direct inhibition of u.v.-induced DNA excision repair in human cells by novobiocin, coumermycin and nalidixic acid. Carcinogenesis. 1987 Jun;8(6):813–817. doi: 10.1093/carcin/8.6.813. [DOI] [PubMed] [Google Scholar]
- Drlica K., Franco R. J. Inhibitors of DNA topoisomerases. Biochemistry. 1988 Apr 5;27(7):2253–2259. doi: 10.1021/bi00407a001. [DOI] [PubMed] [Google Scholar]
- Erixon K., Ahnström G. Single-strand breaks in DNA during repair of UV-induced damage in normal human and xeroderma pigmentosum cells as determined by alkaline DNA unwinding and hydroxylapatite chromatography: effects of hydroxyurea, 5-fluorodeoxyuridine and 1-beta-D-arabinofuranosylcytosine on the kinetics of repair. Mutat Res. 1979 Feb;59(2):257–271. doi: 10.1016/0027-5107(79)90164-7. [DOI] [PubMed] [Google Scholar]
- Fry D. W., Besserer J. A., Boritzki T. J. Transport of the antitumor antibiotic Cl-920 into L1210 leukemia cells by the reduced folate carrier system. Cancer Res. 1984 Aug;44(8):3366–3370. [PubMed] [Google Scholar]
- Fry D. W., Boritzki T. J., Jackson R. C. Studies on the biochemical mechanism of the novel antitumor agent, CI-920. Cancer Chemother Pharmacol. 1984;13(3):171–175. doi: 10.1007/BF00269023. [DOI] [PubMed] [Google Scholar]
- Gilmour D. S., Pflugfelder G., Wang J. C., Lis J. T. Topoisomerase I interacts with transcribed regions in Drosophila cells. Cell. 1986 Feb 14;44(3):401–407. doi: 10.1016/0092-8674(86)90461-7. [DOI] [PubMed] [Google Scholar]
- Hamlin J. L. Effect of damage to early, middle, and late-replicating DNA on progress through the S period in Chinese hamster ovary cells. Exp Cell Res. 1978 Mar 15;112(2):225–232. doi: 10.1016/0014-4827(78)90204-5. [DOI] [PubMed] [Google Scholar]
- Hand R. Eucaryotic DNA: organization of the genome for replication. Cell. 1978 Oct;15(2):317–325. doi: 10.1016/0092-8674(78)90001-6. [DOI] [PubMed] [Google Scholar]
- Horwitz S. B., Chang C. K., Grollman A. P. Studies on camptothecin. I. Effects of nucleic acid and protein synthesis. Mol Pharmacol. 1971 Nov;7(6):632–644. [PubMed] [Google Scholar]
- Hsiang Y. H., Lihou M. G., Liu L. F. Arrest of replication forks by drug-stabilized topoisomerase I-DNA cleavable complexes as a mechanism of cell killing by camptothecin. Cancer Res. 1989 Sep 15;49(18):5077–5082. [PubMed] [Google Scholar]
- Ikegami S., Taguchi T., Ohashi M., Oguro M., Nagano H., Mano Y. Aphidicolin prevents mitotic cell division by interfering with the activity of DNA polymerase-alpha. Nature. 1978 Oct 5;275(5679):458–460. doi: 10.1038/275458a0. [DOI] [PubMed] [Google Scholar]
- Kaufmann W. K., Briley L. P. Reparative strand incision in saponin-permeabilized human fibroblasts. Mutat Res. 1987 Nov;184(3):237–243. doi: 10.1016/0167-8817(87)90022-8. [DOI] [PubMed] [Google Scholar]
- Mattern M. R., Paone R. F., Day R. S., 3rd Eukaryotic DNA repair is blocked at different steps by inhibitors of DNA topoisomerases and of DNA polymerases alpha and beta. Biochim Biophys Acta. 1982 Apr 26;697(1):6–13. doi: 10.1016/0167-4781(82)90038-0. [DOI] [PubMed] [Google Scholar]
- Reichard P. Control of deoxyribonucleotide synthesis in vitro and in vivo. Adv Enzyme Regul. 1972;10:3–16. doi: 10.1016/0065-2571(72)90003-9. [DOI] [PubMed] [Google Scholar]
- Schor S. L., Johnson R. T., Waldren C. A. Changes in the organization of chromosomes during the cell cycle: response to ultraviolet light. J Cell Sci. 1975 May;17(3):539–565. doi: 10.1242/jcs.17.3.539. [DOI] [PubMed] [Google Scholar]
- Snyder R. D. Is DNA topoisomerase involved in the UV excision repair process? New evidence from studies with DNA intercalating and non-intercalating antitumor agents. Photochem Photobiol. 1987 Jan;45(1):105–111. doi: 10.1111/j.1751-1097.1987.tb08410.x. [DOI] [PubMed] [Google Scholar]
- Squires S., Johnson R. T., Collins A. R. Initial rates of DNA incision in UV-irradiated human cells: differences between normal, xeroderma pigmentosum and tumour cells. Mutat Res. 1982 Aug;95(2-3):389–404. doi: 10.1016/0027-5107(82)90273-1. [DOI] [PubMed] [Google Scholar]
- Stewart A. F., Schütz G. Camptothecin-induced in vivo topoisomerase I cleavages in the transcriptionally active tyrosine aminotransferase gene. Cell. 1987 Sep 25;50(7):1109–1117. doi: 10.1016/0092-8674(87)90177-2. [DOI] [PubMed] [Google Scholar]
- Uemura T., Yanagida M. Isolation of type I and II DNA topoisomerase mutants from fission yeast: single and double mutants show different phenotypes in cell growth and chromatin organization. EMBO J. 1984 Aug;3(8):1737–1744. doi: 10.1002/j.1460-2075.1984.tb02040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B., Pardoll D. M., Coffey D. S. Supercoiled loops and eucaryotic DNA replicaton. Cell. 1980 Nov;22(1 Pt 1):79–85. doi: 10.1016/0092-8674(80)90156-7. [DOI] [PubMed] [Google Scholar]
- Wood R. D., Robins P., Lindahl T. Complementation of the xeroderma pigmentosum DNA repair defect in cell-free extracts. Cell. 1988 Apr 8;53(1):97–106. doi: 10.1016/0092-8674(88)90491-6. [DOI] [PubMed] [Google Scholar]
- Yang L., Wold M. S., Li J. J., Kelly T. J., Liu L. F. Roles of DNA topoisomerases in simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1987 Feb;84(4):950–954. doi: 10.1073/pnas.84.4.950. [DOI] [PMC free article] [PubMed] [Google Scholar]



