Summary
We previously reported that the frequency of polyploidy aortic vascular smooth muscle cells (VSMC) serves as a biomarker of aging. Cellular senescence of somatic cells is another marker of aging that is characterized by the inability to undergo cell division. Here, we examined whether polyploidy is associated with the development of cellular senescence in vivo. Analysis of aortic tissue preparations from young and old Brown Norway rats showed that expression of senescence markers such as p16INK4a and senescence-associated β-galactosidase activity are detected primarily in the old tissues. VSMC from p16INK4a knockout and control mice display similar levels of polyploid cells. Intriguingly, senescence markers are expressed in most, but not all, polyploid VSMC. Moreover, the polyploid cells exhibit limited proliferative capacity in comparison to their diploid counterparts. This study is the first to demonstrate in vivo that polyploid VSMC adopt a senescent phenotype.
Keywords: aging, cellular senescence, polyploidy, senescence-associated β-galactosidase, vascular smooth muscle
Polyploidy represents greater than diploid DNA content (Ravid et al., 2002). In previous studies, we showed that in old Brown Norway rats the majority of aortic vascular smooth muscle cells (VSMC) are polyploid (Jones & Ravid, 2004). Studies with cultured cells suggested that polyploidy may be associated with cellular senescence (Sigal et al., 1999; Wagner et al., 2001; Uryvaeva et al., 2004). This, however, has never been demonstrated in vivo. As to the significance of senescence for cellular physiology in early life, cell cycle arrest and senescence represent an anticancer mechanism (Campisi, 2005); however, in later life, senescent cells acquire gene expression/phenotypic changes that may contribute to aging and detrimental pathologies (Patil et al., 2005).
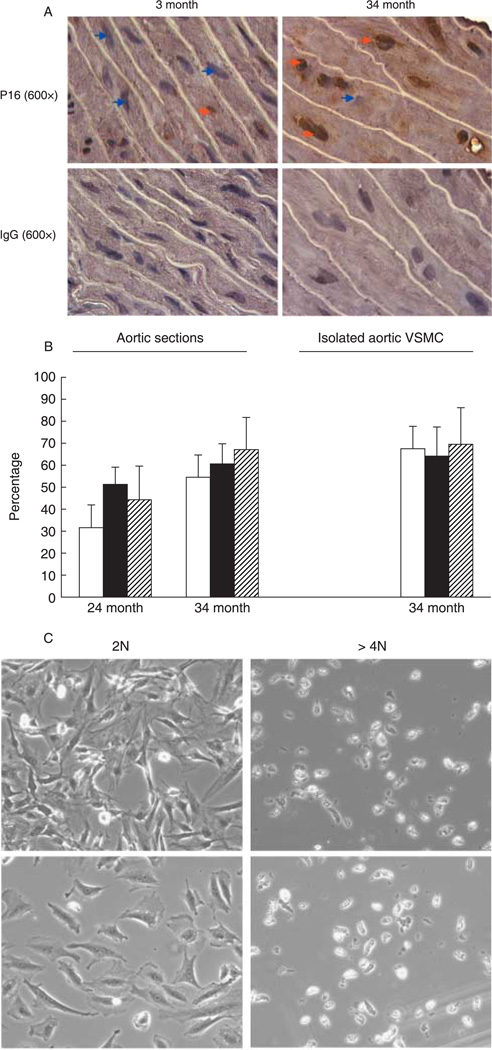
In the current study, we examined the contention that polyploidy aortic VSMC develop a senescence phenotype in vivo. Increased incidence of p16INK4a expression and in some cases of p21CIP1/WAF1 (p21) are considered to be biomarkers of senescence (Herbig et al., 2004, 2006). Aortas derived from old Brown Norway rats are marked by a large fraction of polyploid aortic VSMC, as also indicated by a high frequency of cells with large nuclei (Fig. 1A) (Jones & Ravid, 2004). Immunohistochemistry indicated that p16INK4a is virtually not detected in aortic VSMC of young Brown Norway rats, while frequently noted in tissues of old rats (Fig. 1A and Supplementary Fig. S1). Similar results were obtained with individual VSMC dispersed from whole aortas (Supplementary Fig. S2). About 50–60% of the polyploid cells are positive for p16INK4a in aortas derived from old rats, and approximately 30% of the p16INK4a-positive cells are diploid (Fig. 1B), suggesting that p16INK4a up-regulation alone is not a sufficient inducer of poly-ploidy. Of interest, p16INK4a overexpression in smooth muscle cells does not inhibit cell proliferation (Tanner et al., 2000). In contrast to p16INK4a, p21 is detected at low levels in VSMC from both young and old aortas, with no particular distribution among diploid and polyploid cells (Supplementary Fig. S3A). Analysis of commercially available p21 knockout mice (Deng et al., 1995) or p16INK4a knockout mice (Sharpless et al., 2001) indicated no change in aortic VSMC ploidy compared to control (Supplementary Fig. S3B,C).
Fig. 1.
Polyploid vascular smooth muscle cells (VSMC) express p16INK4a and lose replication potential. (A) Aortic tissue sections derived from young and old Brown Norway rats were fixed with paraformaldehyde and subjected to immunostaining as detailed under the Supplementary material. The orange arrows point to p16INK4a-positive cells (brown staining localized to the nucleus) and the blue ones to p16INK4a-negative cells. Staining with anti-IgG was used as control. Shown are phase-contrast images at ×600 magnification, representative of three rats in each group. Staining was also carried out in cryosections, showing similar results (not shown). (B) The filled bars depict the percentage of polyploid cells identified based on nuclear size (for tissue sections), or 4′,6-diamindino-2-phenylindole (DAPI) staining (for isolated VSMC), the empty bars represent the percentage of polyploid cells that are also p16INK4a-positive, and the striped bars represent the percentage of p16INK4a-positive cells that are polyploid. Five slides per rat were analyzed, and a total of two or three rats were examined in each age group. Shown are average percentages ± standard deviations. (C) VSMC were isolated from 34-month-old aortas, and sorted by flow cytometer to obtain diploid (2N) and ≥ 4N populations, as described in Jones & Ravid (2004). Equal numbers of cells were cultured and monitored as in Nagata et al. (2005). Shown are phase-contrast microscopy images of the cells at 2 (lower panels) and 7 days after culturing. A small fraction of the polyploid population proliferated, but this also corresponds to the number of contaminating diploid cells in this pool (up to 10%).
Another hallmark of cellular senescence is the inability of cells to re-enter the cell cycle. Diploid aortic VSMC isolated by flow cytometry, as in Nagata et al. (2005), readily attach to the tissue-culture flasks and proliferate. In contrast, the majority of polyploid aortic VSMC remain permanently growth-arrested (Fig. 1C).
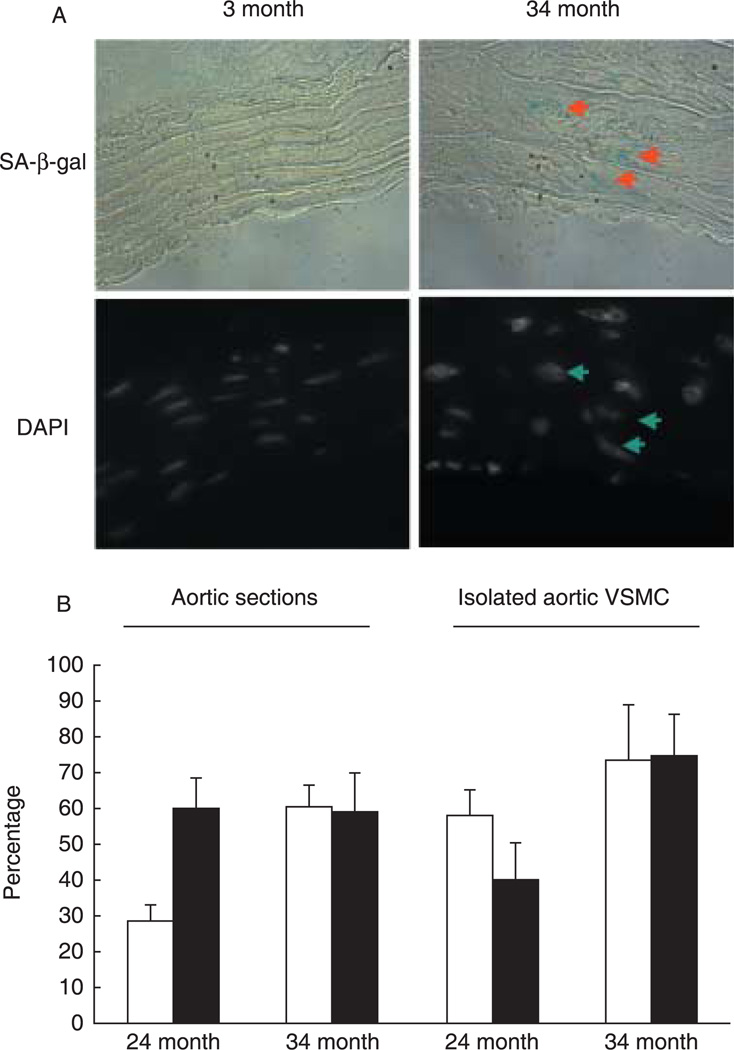
A traditionally accepted marker for this phenotype is senescence-associated β-galactosidase (SA-β-gal) activity (Dimri & Campisi, 1994; Dimri et al., 1995). A recent study identified this activity to be contributed to in part by lysosomal β-galactosidase, and although it is a marker of aging cells, it is not required for senescence (Lee et al., 2006). Analysis of aortic sections and of freshly isolated aortic VSMC showed that SA-β-gal-positive cells are polyploid, but not all polyploid VSMC are SA-β-gal-positive (Fig. 2 and Supplementary Fig S4). In comparing aortic sections to isolated cells, lower percentages of SA-β-gal-positive cells were found, perhaps due to weak SA-β-gal staining, which is likely more challenging to detect in tissues. Interestingly, about 70% of the p16INK4a-positive VSMC in 34-month-old samples are also positive for SA-β-gal (Supplementary Fig. S5).
Fig. 2.
Increased senescence-associated β-galactosidase (SA-β-gal) activity is noted in aortic vascular smooth muscle cells (VSMC) of old rats, and predominantly in the polyploid cells. (A) The SA-β-gal staining assay (blue color; upper panel) was performed on 5-µm cryosectioned aortas and counterstained with 4′,6-diamindino-2-phenylindole (DAPI) to display nuclei (lower panel) (see Supplementary material). The upper panels show aortic sections viewed with phase-contrast microscopy and the bottom panels display respective cell nuclei stained with DAPI (examined with Olympus microscope; ×100 objective). Arrows point to SA-β-gal–positive cells (upper panels) and to corresponding large nuclei (lower panels). (B) Tissue sections or isolated VSMC prepared from 24- or 34-month-old Brown Norway rats as described in the Supplementary material were quantitated. The empty bars depict the percentage of polyploid cells identified based on nuclear size (for tissue sections) or DAPI staining (for isolated VSMC), and the filled bars represent the percentage of polyploid cells that are also SA-β-gal-positive. Three to five slides per rat were analyzed, and a total of two to three rats were examined in each age group. Shown are average percentages ± standard deviations.
In summary, our study is the first to show that senescence markers in aging aortas are primarily detected in polyploid cells, but not in all of them. We propose that the ploidy state along with other signals gradually trigger a complete senescent phenotype and potential vascular pathology.
Supplementary Material
Acknowledgments
This work was supported by grant AG022623 to K.R. K.R. is an established investigator with the American Heart Association. R.A.D. is an American Cancer Society Research Professor and Ellison Medical Foundation Senior Scholar.
Footnotes
Supplementary material
The following experimental procedures and figures are available as supplementary material for this article:
Experimental Procedures: Senescence-associated β-galactosidase (SA-β-gal) staining, immunohistochemistry.
Fig. S1 Validation of the immunohistochemistry method used to stain aortic tissue sections with anti-p16INK4a
Fig. S2 Immunohistochemistry of isolated aortic vascular smooth muscle cells (VSMC) stained with anti-p16INK4a.
Fig. S3 (A) Immunohistochemistry of aortic sections with anti-p21. (B) Ploidy analysis of VSMC derived from p21 knockout mice. (C) Ploidy analysis of VSMC derived from p16INK4a knockout mice.
Fig. S4 SA-β-gal activity assay in isolated aortic vascular smooth muscle cells.
Fig. S5 Isolated aortic VSMC subjected to immunohistochemistry with anti-p16INK4a and to SA-β-gal activity assay.
These materials are available as part of the online article from: http://www.blackwell-synergy.com/doi/abs/10.1111/j.1474-9726.2007.00274.x
(This link will take you to the article abstract).
Please note: Blackwell Publishing are not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Deng C, Zhang P, Harper JW, Elledge SJ, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- Dimri GP, Campisi J. Molecular and cell biology of replicative senescence. Cold Spring Harb. Symp. Quant. Biol. 1994;59:67–73. doi: 10.1101/sqb.1994.059.01.010. [DOI] [PubMed] [Google Scholar]
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, Peacocke M, Campisi J. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl Acad. Sci. USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbig U, Ferreira M, Condel L, Carey D, Sedivy JM. Cellular senescence in aging primates. Science. 2006;311:1257. doi: 10.1126/science.1122446. [DOI] [PubMed] [Google Scholar]
- Herbig U, Jobling WA, Chen BP, Chen DJ, Sedivy JM. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21CIp1, but not p16INK4a. Mol. Cell. 2004;14:510–513. doi: 10.1016/s1097-2765(04)00256-4. [DOI] [PubMed] [Google Scholar]
- Jones MR, Ravid K. Vascular smooth muscle polyploidization as a biomarker for aging and its impact on differential gene expression. J. Biol. Chem. 2004;279:5306–5313. doi: 10.1074/jbc.M308406200. [DOI] [PubMed] [Google Scholar]
- Lee BY, Han JA, Im JS, Morrone A, Johung K, Goodwin EC, Kleijer WJ, DiMaio D, Hwang ES. Senescence-associated β-galactosidase is lysosomal β-galactosidase. Aging Cell. 2006;5:187–195. doi: 10.1111/j.1474-9726.2006.00199.x. [DOI] [PubMed] [Google Scholar]
- Nagata Y, Jones MR, Nguyen HG, McCrann DJ, St Hilaire C, Schreiber BM, Hashimoto A, Inagaki M, Earnshaw WC, Todokoro K, Ravid K. Vascular smooth muscle cell polyploidization involves changes in chromosome passenger proteins and an endomitotic cell cycle. Exp. Cell Res. 2005;305:277–291. doi: 10.1016/j.yexcr.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Patil CK, Mian IS, Campisi J. The thorny path linking cellular senescence to organismal aging. Mech. Ageing Dev. 2005;126:1040–1045. doi: 10.1016/j.mad.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Ravid K, Lu J, Zimmet JM, Jones MR. Roads to polyploidy: the megakaryocyte example. J. Cell Physiol. 2002;190:7–12. doi: 10.1002/jcp.10035. [DOI] [PubMed] [Google Scholar]
- Sharpless NE, Bardeesy N, Lee KH, Carrasco D, Castrillon DH, Aguirre AJ, Wu EA, Horner JW, DePinho RA. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature. 2001;413:86–91. doi: 10.1038/35092592. [DOI] [PubMed] [Google Scholar]
- Sigal SH, Rajvanshi P, Gorla GR, Sokhi RP, Saxena R, Gebhard DRJ, Reid LM, Gupta S. Partial hepatectomy-induced polyploidy attenuates hepatocyte replication and activates cell aging events. Am. J. Physiol. 1999;276(5 Part 1):G1260–G1272. doi: 10.1152/ajpgi.1999.276.5.G1260. [DOI] [PubMed] [Google Scholar]
- Tanner FC, Boehm M, Akyurek LM, San H, Yang ZY, Tashiro J, Nabel GJ, Nabel EG. Differential effects of the cyclin-dependent kinase inhibitors p27Kip1, p21Cip1, and p16Ink4 on vascular smooth muscle cell proliferation. Circulation. 2000;101:2022–2025. doi: 10.1161/01.cir.101.17.2022. [DOI] [PubMed] [Google Scholar]
- Uryvaeva IV, Delone GV, Marshak TL, Semenova ML, Zakhidov ST. Changes of chromosomes and cell cycle (endoreduplication, somatic crossing-over, and robertsonian fusions) in hepatocytes of senescence accelerated SAMR1 mice. Dokl. Biol. Sci. 2004;395:173–176. doi: 10.1023/b:dobs.0000025251.17344.c5. [DOI] [PubMed] [Google Scholar]
- Wagner M, Hampel B, Bernhard D, Hala M, Zwerschke W, Jansen-Durr P. Replicative senescence of human endothelial cells in vitro involves G1 arrest, polyploidization and senescence-associated apoptosis. Exp. Gerontol. 2001;36:1327–1347. doi: 10.1016/s0531-5565(01)00105-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.