Abstract
Age-associated endothelium dysfunction is a major risk factor for the development of cardiovascular diseases. Endothelium-synthesized prostaglandins and thromboxane are local hormones, which mediate vasodilation and vasoconstriction and critically maintain vascular homeostasis. Accumulating evidence indicates that the age-related changes in endothelial eicosanoids contribute to decline in endothelium function and are associated with pathological dysfunction. In this review we summarize currently available information on aging-shifted prostaglandin profiles in endothelium and how these shifts are associated with cardiovascular disorders, providing one molecular mechanism of age-associated endothelium dysfunction and cardiovascular diseases.
1. Introduction
Cardiovascular disorders, including atherosclerosis, coronary artery disease, heart failure, and hypertension, remain the leading cause of death worldwide [1]. These diseases are among several pathological conditions that are associated with aging [2–4], and age is a primary risk factor for their development [5, 6]. Endothelium is a thin layer of epithelial cells which line the interior of lymph and blood vessels and is a major component of the vascular wall. One important contributor to the development of cardiovascular diseases is a dysfunctional endothelium. Endothelial dysfunction is considered a fair predictor of cardiovascular diseases [4, 7–11].
Furchgott and Zawadzki unequivocally demonstrated that the endothelium is required for normal vessel relaxation [12]. Besides inducing relaxation, normal and healthy endothelium regulates vessel wall permeability, blood flow, vascular tone, and structure and exerts anticoagulant and fibrinolytic properties [13]. Aging adversely affects these normal functions of the endothelium, enhancing vasospasm and thrombosis, leading to eventual cardiovascular diseases [4, 14–16]. Age-impaired vascular relaxation has been shown in different human vascular beds including brachial artery, aorta, coronary artery, carotid, and mesenteric microvessels [14–21]. In line with these reports, additional evidence has been obtained in different vascular beds of animals including dogs [2, 22], rats [2, 23–32] and mice [33, 34]. This reduced relaxation is accompanied with increased blood pressure [35–39]. Elevated blood pressure is an important cardiovascular risk factor that can eventually lead to heart failure.
Normal endothelial function is regulated by a controlled balance between endothelium-dependent relaxing factors and endothelium-dependent contracting factors. The main vasoactive factors released by endothelial cells are nitric oxide (NO) and cyclooxygenase- (COX-) derived eicosanoids [4, 40, 41]. NO production has been shown to be reduced with aging [42–45]. There is less information on how eicosanoids change in the endothelium with age. It is also not well understood how changes in eicosanoid profile might contribute to endothelium dysfunction. Nevertheless, accumulating evidence indicates that the age-related changes in endothelial eicosanoids contribute to endothelium dysfunction and to the development of age-associated cardiovascular diseases.
In endothelium, there are six primary cyclooxygenase-(COX-) derived eicosanoids, prostaglandin H2 (PGH2), prostaglandin I2 (PGI2, prostacyclin), prostaglandin E2 (PGE2), prostaglandin F2α (PGF2α), prostaglandin D2 (PGD2), and thromboxane A2 (TxA2) (Figure 1). These eicosanoids are local hormones that are synthesized by virtually all mammalian tissues [46] and act at or near their sites of synthesis in both autocrine and paracrine fashion. They trigger a vast array of biological signals, among which are vasodilation, vasoconstriction, and platelet aggregation [47–49]. In fact, the eicosanoids were the first identified endothelium-derived vasoactive factors [50, 51]. Although there is conflicting evidence [52–54], the majority of the literature shows that PGI2 and PGD2 are vasodilators [55–59], whereas PGH2, PGF2α, and TxA2 are vasoconstrictors and/or platelet aggregation inducers [53, 54, 60–66]. PGE2 can induce vasodilation [47, 67–70] or vasoconstriction [53, 54, 71–73], depending on the vascular bed and concentration [74, 75]. In healthy endothelium, these vasodilators and vasoconstrictors, coexisting with other vasoactive factors, are held in balance to maintain normal vascular functions. The aging process shifts this balanced profile toward a proconstrictive mediator profile [76, 77]. In this paper, we summarize and discuss how endothelium-derived eicosanoid profile changes with age and how those changes might contribute to age-associated endothelium dysfunction.
Figure 1.
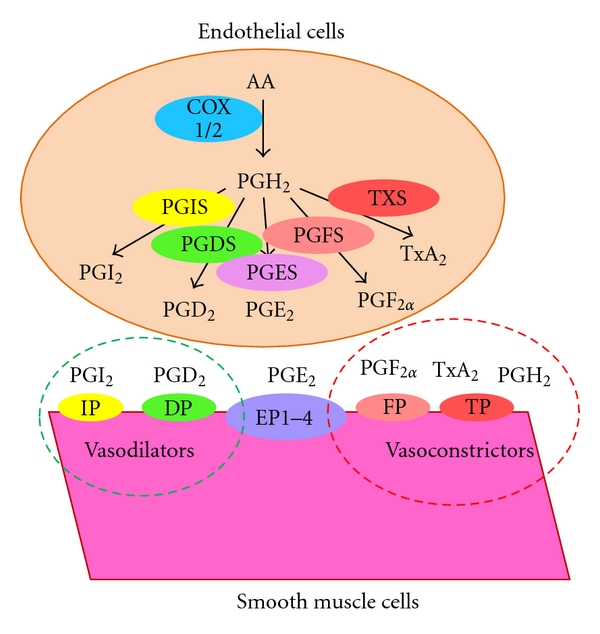
Synthesis and signaling of PGs in the vascular system. Upon stimulation, AA is released from the endothelial cell membrane to the cytosol where it is enzymatically converted to PGH2 by COX1 and COX2. Subsequently, PGH2 is transformed to PGI2, PGE2, PGD2, PGF2α, and TxA2. These substances, as well as untransformed PGH2, are released out of endothelial cells and into the circulation, where they interact with their receptors localized on the smooth muscle cell surface and trigger vasoactive signals.
There is limited data on how eicosanoids change in humans [4], and most experiments have been conducted in animal models and most commonly in rat [2]. Rats of 1.5–2 months or less are considered immature, rats of 3–6 months are considered young adult, and rats of approximately 24 months or more are considered aged, though there are differences between strains [2].
2. Cyclooxygenases and PGH2
There are two isoforms of the cyclooxygenases (COX1 and COX2) encoded by two different genes. Both COX1 and COX2 are expressed in the endothelial and vascular smooth muscle cells, and the expression levels are 20-fold higher in endothelial cells than in smooth muscle cells [78]. In endothelium, both of the COX enzymes are constitutively expressed [79, 80]. However, they are also inducible, for instance, by shear stress [79–81]. Endothelial cells express COX1 preferentially over COX2 [82, 83].
In human mesenteric microvessels of individuals greater than 80 years of age, COX1 levels are 50% increased, while COX2 levels are slightly decreased [21]. In normotensive rats, both COX1 and COX2, in either whole vascular tissue or endothelial cells from vasculatures, are increased with aging from 1-fold to 5-fold [29, 42, 63, 84–86]. Comparable effects of aging on COX1 and COX2 expression levels have been observed in mice [33, 34]. At similar ages, COX1 or COX2 expression, measured at the mRNA or protein levels, is almost doubled in the aorta of spontaneous hypertensive rats (SHRs) as compared to normotensive control Wistar-Kyoto (WKY) rats [63, 84, 87, 88]. Similar increases in COX1 and COX2 were observed in Nω-nitro-L-arginine methyl ester-(L-NAME-) induced hypertensive rats as compared to control Sprague-Dawley rats [89]. Increased COX2 was also reported in the renal artery of hypertensive patients [89]. These data indicate that there are age-associated increases in COX1 and COX2 levels, as well as an association between elevated COX1/COX2 levels, in both animal models and human studies, and clinical cardiovascular disorders.
Upon stimulation, arachidonic acid (AA) is released from the cell membrane to the cytosol where it is enzymatically converted to PGH2 by COX1 and COX2 (also referred to as synthase, PGHS1, and synthase 2, PGHS2, resp.). As shown in Figure 1, PGH2 is the common precursor of other prostaglandins and TxA2. It is transformed to various PGs and TxA2 by a corresponding specific terminal synthase. Besides serving as a common precursor, untransformed PGH2 can trigger signals such as vasoconstriction and platelet aggregation by interacting with TxA2 receptor (TP) [25, 26, 90–99]. Although no evidence has directly shown the attenuation of untransformed and bioactive PGH2 during aging or in cardiovascular pathology, likely due to instability and difficulty in measurement [25, 26, 91, 92, 95, 96], increased COX1 and COX2 associated with aging [21, 29, 42, 63, 84–86] and hypertension [63, 84, 87, 88] would be predicted to result in increases in untransformed PGH2. Indirect evidence is provided by reports of reduced vasoconstriction of aortas of aged and/or hypertensive rats by inhibitors of PGH2 synthases, rather than TxA2 synthase (TXS) [25, 26, 91, 92, 96]. Although the vascular contraction induced by AA has mainly been attributed to TxA2 [100, 101], the efficacy of PGHS inhibition, but poor efficacy for a TXS inhibitor, in inducing relief from vasoconstriction provides evidence for PGH2 as a vasoconstrictor [91, 92, 96].
3. PGI2
PGI2 (prostacyclin) is the first described metabolite of arachidonic acid, and endothelium is the major site of its biosynthesis [51, 57]. In endothelium, both COX1 and COX2 are the upstream contributors of PGI2 synthesis [80, 102–104]. PGI2 is synthesized by its terminal specific PGI2 synthase (PGIS) [105, Figure 1]. PGIS colocalizes with COX1 in endothelial cells [106]. In endothelium, PGIS is by far the most abundant PG terminal synthase, with its expression level 5–100-fold higher than the other PG terminal synthases [54, 64, 65, 84]. Accordingly, PGI2 is the most abundant endothelial eicosanoid, with expression levels 10–100-fold higher than that of the other eicosanoids in humans [107, 108] and in animals [54, 97, 109, 110].
PGI2 triggers potent vasodilation [51, 57] by interacting with the PGI2 receptor (IP) (Figure 1), which located in smooth muscle cells [108, 111]. The vasodilation effect of PGI2 has also been shown in pig coronary arteries at low concentrations [58]. At higher concentrations PGI2 may induce vasoconstriction [32, 54, 64]. PGI2 cannot cause vasoconstriction until its concentration reached 1 μM or higher. 1 μM is 1000-fold higher than the endogenous concentration of PGI2, which is in the 0.2–1 nM range [112]. Even at elevated concentrations, PGI2 is a weak vasoconstrictor and induces modest tension in the rat aorta [32, 54, 64]. Modest vasoconstrictive effects of PGI2 may emanate from weak cross-activation of TP, which can induce vasoconstriction [49]. At lower concentrations, PGI2, especially endogenous PGI2, is a vasodilator. In addition, PGI2 is the most potent endogenous anticoagulation agent [113]. The vasodilation and anticoagulation effects of PGI2 have been confirmed by a recent report showing that IP deletion in mice results in hypertension and reduced anticoagulation activity [114].
In human blood, PGI2, measured as PGF1α, is 400 pg/mL in new born infants, 230 pg/mL in infants, 150 pg/mL in adolescents, and 85 pg/mL in adults [112]. Age-associated PGI2 decline is also observed in urine of humans [115, 116]. The endothelium is the main site for PGI2 synthesis [50, 51]. Although there has been no report on PGI2 production in isolated human vessels, PGI2 levels were reported to decline in cultured human vascular endothelial cells during serial passage [117–119]. Based on these reports, one would expect that PGIS in endothelium decreases with age. Yet there have been no reports evaluating age-associated PGIS changes in the human endothelium. In the endothelial cells from rat aorta, there is a slight and insignificant age-associated decrease in PGIS mRNA [84]. However, additional evidence shows that mRNA or protein of PGIS is 2–4-fold higher in aorta or coronary arteries of aged normotensive rats [85, 86, 110, 120] suggesting that lower PGI levels may be caused by increased PGI2 degradation with age, rather than the change in PGI2 synthesis. In fact, there is no apparent correlation between circulating PGI2 level with level of endothelial PGIS, suggesting the necessity of investigation of the effects of age on the metabolism/degradation of PGI2. More work is needed to determine whether circulating PGI2 correlates to endothelial PGI2 and to clarify the effects of age on PGI2 in the endothelium and in the circulation. Age-associated reduction in IP level has been consistently reported in rats [84, 85]. The reduced IP is expected to lead to reduced sensitivity to PGI2 effects. Consistently, dilation in response to PGI2 is significantly blunted in aged humans as determined by forearm blood flow measurements [121].
Reports on the change in PGI2 or PGIS under pathological conditions, such as hypertension, are contradictory. While one group reported a 50% reduction in PGI2 in SHR aorta as compared to WKY aorta [96], another group reported insignificant differences in PGI2 levels in SHR and WYK rats [64, 65]. In addition, Tang and Vanhoutte reported that PGI2 mRNA is 4-fold higher in the endothelial cells of SHR aorta than in WKY aorta [84]. These limited and inconsistent reports indicate a need for more complete and thorough investigations into how aging affects PGI2, its synthase, receptor, and metabolism. Moreover, clarifying PGI2 effects in the development of cardiovascular disorders in animal models and in humans could be of potential therapeutic significance.
4. PGE2
Prostaglandin E2 (PGE2) is the most abundant prostaglandin in the human body. In endothelium, however, its level is lower than that of PGI2, in line with a lower expression level of the corresponding synthases, which are 5–100-fold lower than PGIS [54, 64, 65, 84]. There are three types of known PGE2 synthases (PGESs), the cytosolic PGES (cPGES) and two forms of membrane PGES, mPGES1 and mPGES2 [122, Figure 1]. cPGES is constitutively expressed and functionally coupled to COX1 [122, 123]. mPGES1 is inducible and functionally coupled with COX2 [124] and is the major PGE2 synthase responsible for PGE2 production [123]. In endothelium, the expression levels of the PGESs are comparable to other PG synthases [54, 64, 65, 84]. Consistently, the amount of PGE2 in endothelium is comparable to other PGs, but lower than the amount of PGI2 [54, 97, 107–110, 125]. In further accord, the contribution of PGE2 to endothelium-dependent vasoaction is marginal [84, 125]. Chen et al. showed that deletion of mPGES1 in mice resulted in abolished production of PGE2 but did not affect blood pressure [114]. Yang, on the other hand, showed that mPGES1 deletion in mice resulted in exaggerated hypertensive in response to high salt and angiotensin II infusion [126], suggesting that mPGES1 may be an important physiological regulator of blood pressure. While the role of mPGES1 in blood pressure regulation is debatable, mPGES1 is implicated in atherosclerosis. Deletion of mPGES1 in mice retards atherosclerosis development [127].
PGE2 acts through four PGE2 receptors (EP1, EP2, EP3, and EP4), which are mainly located in the smooth muscle cells in the vessels [125, 128]. Activation of EP1 and EP3 receptors induces calcium mobilization/release and inhibits adenylyl cyclase release, which triggers vasoconstrictions [111, 129]. In contrast, activation of EP2 and EP4 receptors stimulates adenylyl cyclase and induces cyclic adenosine monophosphate release, which triggers vasorelaxation [111, 129]. The vascular actions of PGE2 are complex due to the opposing vasoactions triggered by the binding of PGE2 to the variant PGE2 receptors. Depending on the circumstances, PGE2 may be vasodilating [47, 67–70] or vasoconstricting [53, 54, 71–73]. In addition to the distributions of different PGE2 receptors expressed in the vascular system, PGE2 concentration is also important. This complexity likely explains the reported inconsistent effects of mPGES1 deletion on blood pressure [114, 126]. PGE2 has a biphasic effect on human blood platelet aggregation. At low concentrations (0.01–1 μM), it potentiates platelet aggregation, and, at higher concentrations (10 μM), it inhibits ADP- and collagen-induced aggregation in platelet rich plasma [71, 130–132]. The endogenous PGE2 concentration is below 1 μM [133], making PGE2 a stimulator of atherosclerosis. Thus, reduced PGE2 level by mPGES1 deletion retards atherosclerosis development [127].
There is little information available on age-related changes in any of the PGESs, PGE2, or EPs. A recent report by Tang and Vanhoutte revealed that while cPGES and mPGES1 in the aorta endothelial cells are insignificantly higher in aged rats, mRNA of mPGES2 is 5-fold higher [84], which can presumably result in higher level of PGE2. PGE2 secreted from coronary arteries is increased in aged rat as compared to young rats [120]. Expression of EP1–4 increased with age, with EP4 elevated 2-fold in endothelial cells from rats of 72 weeks as compared with rats of 36 weeks [84]. Since vasoaction depends on the ligand and the type of receptors, age-increased PGE2 and EP4 are assumed to predispose to increased vasodilation. Further investigation is required to determine the effect of age-related changes in PGE2 and its synthases and receptors in different vascular beds and on relaxation/constriction of vasculatures.
5. PGF2α
There are two isomers of prostaglandin F2α. One is PGF2α, and the other is 9α, 11β-PGF2 [134–137]. They are transformed from PGH2 by the membrane-associated 9,11-endoperoxide reductase and from PGD2/PGE2 by cytosolic PGD2 11-ketoreductase/PGE2 9-ketoreductase, respectively [138, Figure 1]. In endothelium, the level of PGF2α is similar to that of PGE2, but much lower than that of PGI2 [54, 97, 107–110, 125], corresponding to low abundance of PGF2α cognate synthase (PGFS) in the endothelium [54, 64, 65, 84].
PGF2α has its own specific receptor (FP), which is expressed in endothelium and in vascular smooth muscle cells [139–143]. PGF2α can also interact with TP [54]. Interaction between PGF2α and its receptor generates calcium release and triggers potent vasoconstriction [144–148]. Deletion of FP reduces arterial blood pressure and delays atherogenesis in hyperlipidemic mice [149]. PGF2α has also been indicated in promoting cardiac hypertrophy [150–152]. Although PGF2α is a potent vasoconstrictor, the contribution of PGF2α to endothelium-dependent contractions is minimal in most cases due to its relatively low abundance in the endothelium [54, 97, 107–110, 125].
Information on the effects of aging on PGF2α is limited. PGFS mRNA was doubled in the endothelial cells from aged rat aorta as compared to that from young rat aorta [84]. Consistently, PGF2α is 2-fold higher in the aorta of aged rats versus young rats [110, 148]. Change in FP mRNA in the endothelial cells of rat aorta with age, however, is insignificant [84]. Basal PGF2α is slightly higher in the aorta of SHRs than that of WKY rats, but the difference is increased upon acetylcholine stimulation [54]. Research needs to be conducted to obtain more complete information on age-associated changes in PGF2α in humans and the effects of those changes on the development of cardiovascular disorders.
6. PGD2
PGD2 is synthesized by two PGD2 synthases (PGDSs) encoded by two unrelated genes. One is hematopoietic PGDS (H-PGDS), and the other is lipocalin-type enzyme (L-PGDS) [138, Figure 1]. Both can be upregulated in response to an increase in fluid shear stress [153]. In most of the vasculatures, the level of PGD2 is very low or undetectable in some vascular beds [74], due to the low level of PGDSs [54, 64, 65, 84].
PGD2 has multiple receptors [154]. However, two PGD2 receptors (DP1 and DP2) have been most widely studied (Figure 1). Besides playing an important role in the central nervous and immune systems [154], PGD2 has functions in the vasculature. PGD2 can elicit endothelium-dependent relaxation through receptor activation [59] and acts as a vasodilator [155, 156]. On the other hand, it can also act as a bronchoconstrictor [157–159]. Finally, PGD2 is an anticoagulant [160–163].
There is only one report on the effect of aging on PGDS and DP. While aging had no effect on L-PGDS, it caused a 5-fold increase in H-PGDS mRNA in aged rat aorta endothelial cells [84]. Age had no apparent effect on DP [84]. H-PGDS is 3-fold higher in aorta endothelial cells from SHRs versus WKY rats, whereas L-PGDS is decreased in these cells in SHRs versus WKY rats [84]. In the smooth muscle cells from the same aorta preparations, DP mRNA was measured to be 3-fold higher in SHRs as compared with WKY rats [84].
7. TxA2
TxA2 is mainly produced in the platelets [100, 101]. It is also synthesized in the vasculature, the endothelium, and smooth muscles by TxA2 synthase (TXS) [49, Figure 1]. However, the amount of TxA2 in the endothelium is much lower than the amount of PGI2 [54, 97, 107–110, 125]. Consistently, the expression level of the TXS is much lower than that of PGIS [54, 64, 65, 84].
There are two types of TxA2 receptors (TP) denoted, TPa and TPb. TP interacts with TxA2 and other PGs, although TxA2 is the most potent agonist [54, 164]. TP appears to be the main receptor of PGH2 [25, 26, 90–97]. Deletion of TP receptors has provided insights into their physiological function. For example, TP knochout mice exhibit decreased vascular proliferation and platelet activation in response to intimal lesions [165]. These animals also experience delays in atherogenesis [166]. TP deletion also prevents angiotensin-II- and L-NAME-induced hypertension and associated cardiac hypertrophy [167].
TxA2 elicits diverse physiological/pathophysiological reactions, including platelet aggregation and vascular smooth muscle contraction [49]. Activation of platelet aggregation is thought to be the dominant biological function of TxA2. TxA2 causes platelet shape change, aggregation, and secretion, which promotes thrombus formation and thrombosis [168–171]. Thrombosis can cause acute myocardial infarction and atherogenesis [166, 171–174]. TxA2-induced contraction effects are variable, depending on the specific vascular beds examined and the agent used to induce contraction [116, 175, 176]. The majority of reports coincide with the view that the contraction induced by endothelium-derived TxA2 is weak, because inhibitors of TXS do not induce relaxation [91, 92, 96, 176]. Contraction effects are likely mediated by TP activated by PGH2 because inhibitors of PGHSs and TP induce relaxation [91, 92, 96, 175, 176].
Several publications reported a 2–5-fold increase in TxA2 in aorta or mesenteric arteries of aged rats as compared to that of young rats [42, 86, 172]. Consistently, Tang and Vanhoutte reported a 4-fold increase in TXS mRNA [84]. In contrast, a single investigation of age-dependence of TxA2 did not find any significant difference in TxA2 between young and aged rat aortas [110]. Aging did not show any significant effect on rat aorta TP mRNA [84].
An increased production of TxA2 has been found in patients and animal models of several cardiovascular diseases including unstable angina [177], experimental myocardial ischemia and infarction [178], cerebral vasospasm, pregnancy induced hypertension [179, 180], and congenital heart disease [116]. TxA2 levels reported in those studies are systemic, rather than endothelial. In endothelium, there is no difference in aorta TxA2 between SHRs and WKY rats [54, 64, 65, 87]. However, TXS mRNA is doubled in the aorta endothelium of SHRs versus WKY rats [84]. Age-related changes in TP have not been found [84, 181].
In summary (Table 1), aging has been consistently shown to cause severalfold increase in COXs, that is, the synthesis of PGH2 [29, 42, 63, 84–86]. Aging probably reduces PGI2, the predominant PG in the endothelium [112, 115–118, 182], though it is not certain and requires more work. Aging has been shown, or has the potential, to change other PGs in the endothelium. However, because the level of PGI2 is 10–100-fold higher than that of the rest of PGs, the shift of PG profile in the endothelium during aging will be predominantly determined by PGI2 and untransformed PGH2. PGI2 and PGH2 have opposing effects on vessels and platelets. The net result of the effects of aging will be a shift toward a proconstrictive mediator profile, as shown in Figure 2.
Table 1.
Age-associated changes in PGs and TxA2 and their synthases and receptors.
| Entity | Tissue | Age | Change | References |
|---|---|---|---|---|
| COX1/2 (hum, r, m) | Mesenteric microvessels | Adult, aged | Increase | [21, 29, 33, 34, 42, 63, 84–86] |
| PGI2 (hum) | Blood | Adolescent, aged | Decrease | [112, 116] |
| PGIS (r) | Aorta, coronary artery in heart | Adults, aged | Increase | [85, 86, 110, 120] |
| IP (r) | Aorta | Adults, aged | Decrease | [84, 85, 121] |
| PGE2 (r) | Coronary artery in heart | Aged | Increase | [120] |
| cPGES (r), | Aorta | Old adult | N/S | [84] |
| mPGES-1 (r) | Aorta | Old adult | N/S | [84] |
| mPGES-2 (r) | Aorta | Old adult | Increase | [84] |
| EP1–3 (r) | Aorta | Old adult | N/S | [84] |
| EP4 (r) | Aorta | Old adult | Increase | [84] |
| PGF2α (ham, r) | Aorta | Aged | Increase | [110, 148] |
| PGFS (r) | Aorta | Old adult | Increase | [84] |
| FP (r) | Aorta | Old adult | N/S | [84] |
| PGDS (r) | Aorta | Old adult | Increase | [84] |
| DP (r) | Aorta | Old adult | N/S | [84] |
| TxA2 (r) | Aorta or mesenteric artery | Increase | [42, 86, 172] | |
| TXS (r) | Aorta | Old adult | Increase | [84] |
| TP (r) | Aorta | Old adult | N/S | [84] |
hum: human; ham: hamster; r: rat; m: mouse; N/S: not significant.
Definition of age groups: human, adolescent, 13–19 years; adult, 20–60 years; aged, >60 years. Hamster, aged, >18 months. Rat, young adult, 3–6 months; old adult, 6–18 months; aged >24 months.
Figure 2.
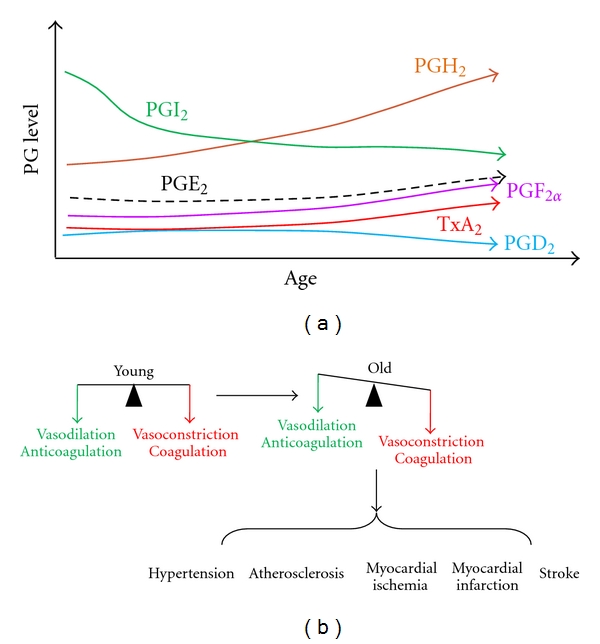
Age-shifted PG profile (a) and vasoaction (b). (a) As age advances, most of the PGs and TxA2 increase, whereas PGI2 decreases. (b) The age shifts PG profile toward vasoconstriction and coagulation causing several cardiovascular disorders.
8. Association of Prostaglandin and Cardiovascular Disorders in Aging
Associated with this shift are several cardiovascular disorders including hypertension, atherosclerosis, myocardial ischemia, myocardial infarction, and stroke (Figure 2(b)). Reduced ratio of PGI2/TxA2 was observed in elderly hypertensive patients [183–186]. Age impaired PGI2 synthesis [84, 187] is associated with hypertension [84], progression of atherosclerotic lesions [188], and increased thrombotic risk and heart failure [189, 190]. In addition, aging not only reduces the expression of IP [84], but also reduces the sensitivity of IP [182, 191]. These factors might contribute to the progression of atherosclerosis, as mice with deleted IP [192, 193] and human patients with a dysfunctional prostacyclin IP receptor mutation [194] show accelerated atherothrombosis [97].
On the other hand, aging induces TXS [84]. Higher concentrations of TxA2 are observed in serum or urine in several age-related and hypertensive diseases [185, 186, 195]. In the atherosclerotic coronary artery, the density of TP receptor is increased [171]. Aging-increased TxA2, together with induced TP in the atherosclerotic coronary artery, accelerates arterial atherosclerosis, leading to myocardial infarction [191]. The TP-mediated signaling can also be triggered by PGH2. Age increases COX1/2 in animals and human [21, 29, 33, 34, 42, 63, 84–86] and thereby increases PGH2 production. Age-increased expression of COX-2 in coronary, carotid, and femoral arteries is associated with human atherosclerosis [196–199].
9. Therapeutics That Modulate Prostaglandins in Cardiovascular Disorders
Because prostaglandins and thromboxane are such important factors in endothelium functions and therefore in the physiology and pathology of the vascular system, numerous pharmacological agents that target these factors have been developed to mitigate cardiovascular diseases. As listed in Table 2, prostacyclin (PGI2) and analogues are used clinically to treat hypertension, especially pulmonary hypertension [75, 200–202]. They are also used to inhibit arterial thrombosis and ameliorate myocardial ischemia [203–207]. Although the vascular actions of PGE2 are complex, PGE2 and analogues are used to reduce blood pressure and to alleviate congestive heart failure [208–210], owing to their ability to stimulate renin release and natriuresis and diuresis [211–213]. PGE2, PGE1, and their analogues are more often used to maintain the patency of the ductus arteriosus in infants with congenital heart disease [214–217]. Antagonists of TXS and TP are potent antithrombosis agents and used to treat atherosclerosis, myocardial ischemia, and stroke [218–227].
Table 2.
Prostaglandin-related pharmacological agents in the treatment of cardiovascular diseases.
| Modulator | Drugs (trade name) | Clinical application | References |
|---|---|---|---|
| PGI2 and its stable analogues | Epoprostenol sodium (Flolan), Beraprost sodium (Procyclin), Iloprost (Ventavis), Treprostinil (Remodulin) | Primary pulmonary hypertension, pulmonary arterial hypertension | [200–207] |
| PGE2 and its analogues | Dinoprostone, Viprostol | Congenital heart disease | [208–210, 214–217] |
| TXS inhibitors | Dazoxiben, Camonagrel, Picotamide (Dusodril) | Thrombosis, atherosclerosis, arrhythmias | [218–226] |
| TP inhibitors | Picotamide, S18886 (Triplion) | Thrombosis, atherosclerosis, ischemic stroke, myocardial infarction | [84, 223–227] |
| COX1 inhibitor | Aspirin | Thrombosis, atherosclerosis, ischemic stroke, myocardial infarction | [231–235] |
The underlying principle of the design of these drugs is to selectively increase the effects of vasodilators and anticoagulators and to selectively reduce the effects of vasoconstrictors and coagulators by modulating the amount of ligands, synthases, or receptors of a specific eicosanoid. Because prostaglandins and thromboxane A2 are from the same precursor but elicit opposing effects, selectivity is crucial in the design of these therapeutics. Nonselective inhibition of the upstream synthases, COX1 and COX2, can result in undesirable side effects including hypertension, manifestation of myocardial ischemia, and increased incidents of acute myocardial infarction and stroke, which occur more often in the elderly [104, 228–230].
Intriguingly, low dose of aspirin, an inhibitor of COX1, is popularly used in the prevention of cardiovascular diseases [231–233]. Aspirin covalently acetylates a specific serine moiety (serine 530 of COX-1 and serine 516 of COX-2) [234, 235], and its binding to COX1 is about 170-fold stronger than that to COX-2 [236]. Thus, aspirin is a covalent inhibitor of COX1 inactivating it irreversibly. TxA2 is mainly produced in platelets [100, 101], whereas PGI2 is mainly produced by endothelial cells [51, 57]. Different from most other cell types, platelets do not possess nuclei, which are required for protein synthesis. While COX1 can be regenerated in other cells, such as endothelial cells, COX1 cannot be regenerated in platelets. Nor can COX1 activity be recovered after inactivation by aspirin. Therefore, low dose of aspirin irreversibly and selectively inhibits TxA2 production in platelets.
However, new platelets are constantly formed, and TxA2 is persistently produced [237], which leads to a need for continuous dosing to constantly inhibit COX1. Aspirin resistance is a common clinical phenomenon [238] and has been observed for more than twenty five years [239]. Aspirin resistant patients, partially due to inherited polymorphisms in COX1 [240, 241], have a nearly 4-fold increase in risk of suffering a vascular event compared with aspirin responders [242–244]. As an alternative to aspirin therapy, antagonists of TXS and TP, which can also be combined with aspirin, have been applied to ameliorate thrombosis and prevent cardiovascular diseases [226].
10. Conclusion and Perspective
The incidence and prevalence of cardiovascular diseases increase with advancing age, to the extent that age has been identified as the dominant risk factor for these pathologies [2, 4–6]. It is well established that PGs are powerful endogenous vasodilators and vasoconstrictors and platelet aggregators, playing important roles in regulating homeostasis in vascular systems. Although limited, the current analysis of the literature suggests that there is a modified PG profile associated with age and indicates that age has significant effects on the abundance of PGs, their synthesis, as well as their signaling transduction pathways. Aging-modulated PG profile offers a potentially important molecular mechanism underlying age-dependent endothelial dysfunction and age-associated cardiovascular diseases. Knowledge of age-associated PGs profile changes can be important for designing new pharmacological interventions to prevent or slow down age-associated cardiovascular diseases. Given their biological roles, improved investigation of age-associated changes in PG synthesis, metabolism, and signaling in all major vascular beds is needed.
It is clearly difficult to obtain human vascular tissues to determine age associated changes. Surrogate tissues and fluids such as human blood or urine are plentiful but are of limited value for assessing tissue-specific effects. Defining the relationship between PGs, particularly PGI2 and PGH2, in vascular tissues and the amounts in blood or urine in animal models could be helpful to interpret PG profiles in humans. Technical challenges exist due to metabolite instability. For example, PGH2 is transformed to other PGs and is biologically important in its own right, but untransformed PGH2 is difficult to measure [98, 245]. The development of user-friendly methods could facilitate acquiring these measurements [91, 98, 245]. For example, PGH2 can be instantly reduced to 12-heptadecatrienoic acid (12-HHT) by FeCl2 [91, 98, 245]. 12-HHT is stable and inactive and measurable [91, 98, 245]. Therefore, total PGH2 can be measured as 12-HHT. A relatively mild reducing agent, SnCl2, can reduce untransformed PGH2 to PGF2α. Untransformed PGH2 can be calculated by subtracting the estimate of PGF2α in samples without SnCl2 from the corresponding estimate in samples with SnCl2 [91, 98, 125]. Alternatively, epidemiological approaches could avoid these technical difficulties and offer valuable genetic information. Haplotype analyses have revealed that several polymorphisms in COX, PGIS, and IP are associated with age and cardiovascular diseases [246–250].
Research on an important aspect of age-associated changes in PGs is largely absent in the literature; that of age-associated effects on PG metabolism. One of the most important features of PGs is rapid clearance. Most PGs are metabolized to inactive forms within 1–3 minutes [119, 251], and consequently their signaling is terminated within that time frame. This is due to an effective and efficient metabolism system mainly composed of prostaglandin transporter (PGT) and 15-hydroxyprostaglandin dehydrogenase (15-PGDH) [252]. Both PGT and 15-PGDH have been shown to regulate PG degradation [245, 253, 254]. Thus far, there have been no reports on the influence of age on PG metabolism.
In conclusion, PGs and TxA2 play critical roles in many important events involved in the normal functions of vascular system, including vasodilation, vasoconstriction, platelet aggregation, and inflammation. Although these eicosanoids were discovered in the 1970s, the research into age-associated shifts of the PG profile has just begun. Age-associated alterations in PG profiles are not only interesting, but also important in defining the molecular mechanisms of age-associated cardiovascular pathological conditions and informing strategic and personalized prevention and cure of those diseases.
Acknowledgment
Support for this project was provided by the American Heart Association (0735066N).
References
- 1. http://www.who.int/mediacentre/factsheets/fs317/en/index.html.
- 2.Docherty JR. Cardiovascular responses in ageing: a review. Pharmacological Reviews. 1990;42(2):103–125. [PubMed] [Google Scholar]
- 3.Zeiher AM, Drexler H, Saurbier B, Just H. Endothelium-mediated coronary blood flow modulation in humans: effects of age, atherosclerosis, hypercholesterolemia, and hypertension. Journal of Clinical Investigation. 1993;92(2):652–662. doi: 10.1172/JCI116634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandes RP, Fleming I, Busse R. Endothelial aging. Cardiovascular Research. 2005;66(2):286–294. doi: 10.1016/j.cardiores.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 5.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a "set up" for vascular disease. Circulation. 2003;107(1):139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 6.Rothwell PM, Coull AJ, Silver LE, et al. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study) Lancet. 2005;366(9499):1773–1783. doi: 10.1016/S0140-6736(05)67702-1. [DOI] [PubMed] [Google Scholar]
- 7.Jayakody L, Kappagoda T, Senaratne MP, Thomson AB. Impairment of endothelium-dependent relaxation: an early marker for atherosclerosis in the rabbit. British Journal of Pharmacology. 1988;94(2):335–346. doi: 10.1111/j.1476-5381.1988.tb11535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elliott HL. Endothelial dysfunction in cardiovascular disease: risk factor, risk marker, or surrogate end point? Journal of Cardiovascular Pharmacology. 1998;32(supplement 3):S74–S77. [PubMed] [Google Scholar]
- 9.Fathi R, Haluska B, Isbel N, Short L, Marwick TH. The relative importance of vascular structure and function in predicting cardiovascular events. Journal of the American College of Cardiology. 2004;43(4):616–623. doi: 10.1016/j.jacc.2003.09.042. [DOI] [PubMed] [Google Scholar]
- 10.Mancini GB. Vascular structure versus function: is endothelial dysfunction of independent prognostic importance or not? Journal of the American College of Cardiology. 2004;43(4):624–628. doi: 10.1016/j.jacc.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 11.Herrera MD, Mingorance C, Rodríguez-Rodríguez R, Alvarez de Sotomayor M. Endothelial dysfunction and aging: an update. Ageing Research Reviews. 2010;9(2):142–152. doi: 10.1016/j.arr.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 13.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109(23):III27–III32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- 14.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. Journal of the American College of Cardiology. 1994;24(2):471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 15.Taddei S, Virdis A, Mattei P, et al. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation. 1995;91(7):1981–1987. doi: 10.1161/01.cir.91.7.1981. [DOI] [PubMed] [Google Scholar]
- 16.Taddei S, Virdis A, Mattei P, et al. Hypertension causes premature aging of endothelial function in humans. Hypertension. 1997;29(3):736–743. doi: 10.1161/01.hyp.29.3.736. [DOI] [PubMed] [Google Scholar]
- 17.Egashira K, Inou T, Hirooka Y, et al. Effects of age on endothelium-dependent vasodilation of resistance coronary artery by acetylcholine in humans. Circulation. 1993;88(1):77–81. doi: 10.1161/01.cir.88.1.77. [DOI] [PubMed] [Google Scholar]
- 18.Küng CF, Lüscher TF. Different mechanisms of endothelial dysfunction with aging and hypertension in rat aorta. Hypertension. 1995;25(2):194–200. doi: 10.1161/01.hyp.25.2.194. [DOI] [PubMed] [Google Scholar]
- 19.Gerhard M, Roddy MA, Creager SJ, Creager MA. Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension. 1996;27(4):849–853. doi: 10.1161/01.hyp.27.4.849. [DOI] [PubMed] [Google Scholar]
- 20.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. Journal of Physiology. 2004;556(1):315–324. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodríguez-Mañas L, Vallejo S, López-Dóriga P, et al. Endothelial dysfunction in aged humans is related with oxidative stress and vascular inflammation. Aging Cell. 2009;8(3):226–238. doi: 10.1111/j.1474-9726.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- 22.Shimizu I, Toda N. Alterations with age of the response to vasodilator agents in isolated mesenteric arteries of the beagle. British Journal of Pharmacology. 1986;89(4):769–778. doi: 10.1111/j.1476-5381.1986.tb11181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moritoki H, Hosoki E, Ishida Y. Age-related decrease in endothelium-dependent dilator response to histamine in rat mesenteric artery. European Journal of Pharmacology. 1986;126(1-2):61–67. doi: 10.1016/0014-2999(86)90738-7. [DOI] [PubMed] [Google Scholar]
- 24.Hongo K, Nakagomi T, Kassell NF, et al. Effects of aging and hypertension on endothelium-dependent vascular relaxation in rat carotid artery. Stroke. 1988;19(7):892–897. doi: 10.1161/01.str.19.7.892. [DOI] [PubMed] [Google Scholar]
- 25.Hynes MR, Duckles SP. Effect of increasing age on the endothelium-mediated relaxation of rat blood vessels in vitro. Journal of Pharmacology and Experimental Therapeutics. 1987;241(2):387–392. [PubMed] [Google Scholar]
- 26.Koga T, Takata Y, Kobayashi K, Takishita S, Yamashita Y, Fujishima M. Age and hypertension promote endothelium-dependent contractions to acetylcholine in the aorta of the rat. Hypertension. 1989;14(5):542–548. doi: 10.1161/01.hyp.14.5.542. [DOI] [PubMed] [Google Scholar]
- 27.Atkinson J, Tatchum-Talom R, Capdeville-Atkinson C. Reduction of endothelial function with age in the mesenteric arterial bed of the normotensive rat. British Journal of Pharmacology. 1994;111(4):1184–1188. doi: 10.1111/j.1476-5381.1994.tb14870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tominaga M, Fujii K, Abe I, Takata Y, Kobayashi K, Fujishima M. Hypertension and ageing impair acetylcholine-induced vasodilation in rats. Journal of Hypertension. 1994;12(3):259–268. [PubMed] [Google Scholar]
- 29.Stewart KG, Zhang Y, Davidge ST. Aging increases PGHS-2-dependent vasoconstriction in rat mesenteric arteries. Hypertension. 2000;35(6):1242–1247. doi: 10.1161/01.hyp.35.6.1242. [DOI] [PubMed] [Google Scholar]
- 30.Abeywardena MY, Jablonskis LT, Head RJ. Age- and hypertension-induced changes in abnormal contractions in rat aorta. Journal of Cardiovascular Pharmacology. 2002;40(6):930–937. doi: 10.1097/00005344-200212000-00015. [DOI] [PubMed] [Google Scholar]
- 31.Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. American Journal of Physiology. 2002;283(4):H1662–H1672. doi: 10.1152/ajpheart.00004.2002. [DOI] [PubMed] [Google Scholar]
- 32.Gomez E, Schwendemann C, Roger S, et al. Aging and prostacyclin responses in aorta and platelets from WKY and SHR rats. American Journal of Physiology. 2008;295(5):H2198–H2211. doi: 10.1152/ajpheart.00507.2008. [DOI] [PubMed] [Google Scholar]
- 33.Gendron MÈ, Thorin-Trescases N, Villeneuve L, Thorin E. Aging associated with mild dyslipidemia reveals that COX-2 preserves dilation despite endothelial dysfunction. American Journal of Physiology. 2007;292(1):H451–H458. doi: 10.1152/ajpheart.00551.2006. [DOI] [PubMed] [Google Scholar]
- 34.Gendron MÈ, Thorin E. A change in the redox environment and thromboxane A2 production precede endothelial dysfunction in mice. American Journal of Physiology. 2007;293(4):H2508–H2515. doi: 10.1152/ajpheart.00352.2007. [DOI] [PubMed] [Google Scholar]
- 35.Haudenschild CC, Prescott MF, Chobanian AV. Aortic endothelial and subendothelial cells in experimental hypertension and aging. Hypertension. 1981;3(3):148–153. doi: 10.1161/01.hyp.3.3_pt_2.i148. [DOI] [PubMed] [Google Scholar]
- 36.Haudenschild CC, Chobanian AV. Blood pressure lowering diminishes age-related changes in the rat aortic intima. Hypertension. 1984;6(2):I-62–I-68. doi: 10.1161/01.hyp.6.2_pt_2.i62. [DOI] [PubMed] [Google Scholar]
- 37.Soltis EE. Effect of age on blood pressure and membrane-dependent vascular responses in the rat. Circulation Research. 1987;61(6):889–897. doi: 10.1161/01.res.61.6.889. [DOI] [PubMed] [Google Scholar]
- 38.Koga T, Takata Y, Kobayashi K, Takishita S, Yamashita Y, Fujishima M. Ageing suppresses endothelium-dependent relaxation and generates contraction mediated by the muscarinic receptors in vascular smooth muscle of normotensive Wistar-Kyoto and spontaneously hypertensive rats. Journal of Hypertension. 1988;6(4):S243–S245. doi: 10.1097/00004872-198812040-00073. [DOI] [PubMed] [Google Scholar]
- 39.Iwama Y, Kato T, Muramatsu M, et al. Correlation with blood pressure of the acetylcholine-induced endothelium- derived contracting factor in the rat aorta. Hypertension. 1992;19(4):326–332. doi: 10.1161/01.hyp.19.4.326. [DOI] [PubMed] [Google Scholar]
- 40.Ferrari AU, Radaelli A, Centola M. Aging and the cardiovascular system. Journal of Applied Physiology. 2003;95(6):2591–2597. doi: 10.1152/japplphysiol.00601.2003. [DOI] [PubMed] [Google Scholar]
- 41.Matz RL, Andriantsitohaina R. Age-related endothelial dysfunction: potential implications for pharmacotherapy. Drugs and Aging. 2003;20(7):527–550. doi: 10.2165/00002512-200320070-00005. [DOI] [PubMed] [Google Scholar]
- 42.Matz RL, de Sotomayor MA, Schott C, Stoclet JC, Andriantsitohaina R. Vascular bed heterogeneity in age-related endothelial dysfunction with respect to NO and eicosanoids. British Journal of Pharmacology. 2000;131(2):303–311. doi: 10.1038/sj.bjp.0703568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barton M, Cosentino F, Brandes RP, Moreau P, Shaw S, Lüscher TF. Anatomic heterogeneity of vascular aging: role of nitric oxide and endothelin. Hypertension. 1997;30(4):817–824. doi: 10.1161/01.hyp.30.4.817. [DOI] [PubMed] [Google Scholar]
- 44.Fleming I, Busse R. NO: the primary EDRF. Journal of Molecular and Cellular Cardiology. 1999;31(1):5–14. doi: 10.1006/jmcc.1998.0839. [DOI] [PubMed] [Google Scholar]
- 45.Tschudi MR, Barton M, Bersinger NA, et al. Effect of age on kinetics of nitric oxide release in rat aorta and pulmonary artery. Journal of Clinical Investigation. 1996;98(4):899–905. doi: 10.1172/JCI118872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith WL. Localization of enzymes responsible for prostaglandin formation. In: Wmlis AL, editor. Handbook of Eicosanoids: Prostaglandins and Related Lipids. IA. Boca Raton, Fla, USA: CRC Press; 1987. pp. 175–184. [Google Scholar]
- 47.Weeks JR. Prostaglandins. Annual Review of Pharmacology. 1972;12:317–336. doi: 10.1146/annurev.pa.12.040172.001533. [DOI] [PubMed] [Google Scholar]
- 48.Clyman RI, Mauray F, Roman C, Rudolph AM. PGE2 is a more potent vasodilator of the lamb ductus arteriosus than is either PGI2 or 6 keto PGF(1α) Prostaglandins. 1978;16(2):259–264. doi: 10.1016/0090-6980(78)90028-x. [DOI] [PubMed] [Google Scholar]
- 49.Nakahata N. Thromboxane A2: physiology/pathophysiology, cellular signal transduction and pharmacology. Pharmacology and Therapeutics. 2008;118(1):18–35. doi: 10.1016/j.pharmthera.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 50.Moncada S, Gryglewski R, Bunting S, Vane JR. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976;263(5579):663–665. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- 51.Moncada S, Herman AG, Higgs EA, Vane JR. Differential formation of prostacyclin (PGX or PGI2) by layers of the arterial wall. An explanation for the anti-thrombotic properties of vascular endothelium. Thrombosis Research. 1977;11(3):323–344. doi: 10.1016/0049-3848(77)90185-2. [DOI] [PubMed] [Google Scholar]
- 52.Williams SP, Dorn GW, 2nd, Rapoport RM. Prostaglandin I2 mediates contraction and relaxation of vascular smooth muscle. American Journal of Physiology. 1994;267(2):H796–H803. doi: 10.1152/ajpheart.1994.267.2.H796. [DOI] [PubMed] [Google Scholar]
- 53.Rapoport RM, Williams SP. Role of prostaglandins in acetylcholine-induced contraction of aorta from spontaneously hypertensive and Wistar-Kyoto rats. Hypertension. 1996;28(1):64–75. doi: 10.1161/01.hyp.28.1.64. [DOI] [PubMed] [Google Scholar]
- 54.Gluais P, Lonchampt M, Morrow JD, Vanhoutte PM, Feletou M. Acetylcholine-induced endothelium-dependent contractions in the SHR aorta: the Janus face of prostacyclin. British Journal of Pharmacology. 2005;146(6):834–845. doi: 10.1038/sj.bjp.0706390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bunting S, Gryglewski S, Moncada S, Vane JR. Arterial walls generate from prostaglandin endoperoxides a substance (prostaglandin X) which relaxes strips of mesenteric and coeliac arteries and inhibits platelet aggregation. Prostaglandins. 1976;12(6):897–913. doi: 10.1016/0090-6980(76)90125-8. [DOI] [PubMed] [Google Scholar]
- 56.Gorman RR, Fitzpatrick FA, Miller OV. Reciprocal regulation of human platelet cAMP levels by thromboxane A2 and prostacyclin. Advances in Cyclic Nucleotide Research. 1978;9:597–609. [PubMed] [Google Scholar]
- 57.Moncada S, Vane JR. The role of prostacyclin in vascular tissue. Desty's Federal Procedure. 1979;38(1):66–71. [PubMed] [Google Scholar]
- 58.Shimokawa H, Flavahan NA, Lorenz RR, Vanhoutte PM. Prostacyclin releases endothelium-derived relaxing factor and potentiates its action in coronary arteries of the pig. British Journal of Pharmacology. 1988;95(4):1197–1203. doi: 10.1111/j.1476-5381.1988.tb11756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abran D, Varma DR, Chemtob S. Regulation of prostanoid vasomotor effects and receptors in choroidal vessels of newborn pigs. American Journal of Physiology. 1997;272(3):R995–R1001. doi: 10.1152/ajpregu.1997.272.3.R995. [DOI] [PubMed] [Google Scholar]
- 60.Katusic ZS, Shepherd JT, Vanhoutte PM. Endothelium-dependent contractions to calcium ionophore A23187, arachidonic acid and acetylcholine in canine basilar arteries. Stroke. 1988;19(4):476–479. doi: 10.1161/01.str.19.4.476. [DOI] [PubMed] [Google Scholar]
- 61.Shirahase H, Usui H, Kurahashi K, Fujiwara M, Fukui K. Endothelium-dependent contraction induced by nicotine in isolated canine basilar artery-possible involvement of a thromboxane A2 (TXA2) like substance. Life Sciences. 1988;42(4):437–445. doi: 10.1016/0024-3205(88)90082-3. [DOI] [PubMed] [Google Scholar]
- 62.Taddei S, Vanhoutte PM. Role of endothelium in endothelin-evoked contractions in the rat aorta. Hypertension. 1993;21(1):9–15. doi: 10.1161/01.hyp.21.1.9. [DOI] [PubMed] [Google Scholar]
- 63.Ge T, Hughes H, Junquero DC, Wu KK, Vanhoutte PM, Boulanger CM. Endothelium-dependent contractions are associated with both augmented expression of prostaglandin H synthase-1 and hypersensitivity to prostaglandin H2 in the SHR aorta. Circulation Research. 1995;76(6):1003–1010. doi: 10.1161/01.res.76.6.1003. [DOI] [PubMed] [Google Scholar]
- 64.Gluais P, Paysant J, Badier-Commander C, Verbeuren T, Vanhoutte PM, Félétou M. In SHR aorta, calcium ionophore A-23187 releases prostacyclin and thromboxane A2 as endothelium-derived contracting factors. American Journal of Physiology. 2006;291(5):H2255–H2264. doi: 10.1152/ajpheart.01115.2005. [DOI] [PubMed] [Google Scholar]
- 65.Gluais P, Vanhoutte PM, Félétou M. Mechanisms underlying ATP-induced endothelium-dependent contractions in the SHR aorta. European Journal of Pharmacology. 2007;556(1–3):107–114. doi: 10.1016/j.ejphar.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 66.Hirao A, Kondo K, Inui N, Umemura K, Ohashi K, Watanabe H. Cyclooxygenase-dependent vasoconstricting factor(s) in remodelled rat femoral arteries. Cardiovascular Research. 2008;79(1):161–168. doi: 10.1093/cvr/cvn111. [DOI] [PubMed] [Google Scholar]
- 67.Nakano J, McCloy RB, Prancan AV. Circulatory and pulmonary airway responses to different mixtures of prostaglandins E2 and F2α in dogs. European Journal of Pharmacology. 1973;24(1):61–66. doi: 10.1016/0014-2999(73)90114-3. [DOI] [PubMed] [Google Scholar]
- 68.Dusting GJ, Vane JR. Some cardiovascular properties of prostacyclin (PGI2) which are not shared by PGE2. Circulation Research. 1980;46(6):I183–I187. [PubMed] [Google Scholar]
- 69.Carter J, Reynoldson JA, Thorburn GD. The effects of certain vasodilating prostaglandins on the coronary and hindlimb vascular beds of the conscious sheep. Comparative Biochemistry and Physiology. 1986;83(2):401–406. doi: 10.1016/0742-8413(86)90143-x. [DOI] [PubMed] [Google Scholar]
- 70.Neisius U, Olsson R, Rukwied R, Lischetzki G, Schmelz M. Prostaglandin E2 induces vasodilation and pruritus, but no protein extravasation in atopic dermatitis and controls. Journal of the American Academy of Dermatology. 2002;47(1):28–32. doi: 10.1067/mjd.2002.120462. [DOI] [PubMed] [Google Scholar]
- 71.Gray SJ, Heptinstall S. The effects of PGE2 and CL 115,347, an antihypertensive PGE2 analogue, on human blood platelet behaviour and vascular contractility. European Journal of Pharmacology. 1985;114(2):129–137. doi: 10.1016/0014-2999(85)90620-x. [DOI] [PubMed] [Google Scholar]
- 72.Förstermann U, Mügge A, Alheid U, Bode SM, Frölich JC. Endothelium-derived relaxing factor (EDRF): a defence mechanism against platelet aggregation and vasospasm in human coronary arteries. European Heart Journal. 1989;10(supplement F):36–43. doi: 10.1093/eurheartj/10.suppl_f.36. [DOI] [PubMed] [Google Scholar]
- 73.Fabre JE, Nguyen M, Athirakul K, et al. Activation of the murine EP3 receptor for PGE2 inhibits cAMP production and promotes platelet aggregation. Journal of Clinical Investigation. 2001;107(5):603–610. doi: 10.1172/JCI10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smith WL. Prostaglandin biosynthesis and its compartmentation in vascular smooth muscle and endothelial cells. Annual Review of Physiology. 1986;48:251–262. doi: 10.1146/annurev.ph.48.030186.001343. [DOI] [PubMed] [Google Scholar]
- 75.Norel X. Prostanoid receptors in the human vascular wall. The Scientific World Journal. 2007;7:1359–1374. doi: 10.1100/tsw.2007.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Singh N, Prasad S, Singer DR, MacAllister RJ. Ageing is associated with impairment of nitric oxide and prostanoid dilator pathways in the human forearm. Clinical Science. 2002;102(5):595–600. [PubMed] [Google Scholar]
- 77.Wang M, Zukas AM, Hui Y, Ricciotti E, Puré E, FitzGerald GA. Deletion of microsomal prostaglandin E synthase-1 augments prostacyclin and retards atherogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(39):14507–14512. doi: 10.1073/pnas.0606586103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.DeWitt DL, Day JS, Sonneburg WK, Smith WL. Concentrations of prostaglandin endoperoxide synthase and prostaglandin I2 synthase in the endothelium and smooth muscle of bovine aorta. Journal of Clinical Investigation. 1983;72(6):1882–1888. doi: 10.1172/JCI111151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Doroudi R, Gan LM, Selin Sjögren L, Jern S. Effects of shear stress on eicosanoid gene expression and metabolite production in vascular endothelium as studied in a novel biomechanical perfusion model. Biochemical and Biophysical Research Communications. 2000;269(1):257–264. doi: 10.1006/bbrc.2000.2279. [DOI] [PubMed] [Google Scholar]
- 80.Funk CD, FitzGerald GA. COX-2 inhibitors and cardiovascular risk. Journal of Cardiovascular Pharmacology. 2007;50(5):470–479. doi: 10.1097/FJC.0b013e318157f72d. [DOI] [PubMed] [Google Scholar]
- 81.Topper JN, Cai J, Falb D, Gimbrone MA., Jr. Identification of vascular endothelial genes differentially responsive to fluid mechanical stimuli: cyclooxygenase-2, manganese superoxide dismutase, and endothelial cell nitric oxide synthase are selectively up-regulated by steady laminar shear stress. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(19):10417–10422. doi: 10.1073/pnas.93.19.10417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Onodera M, Morita I, Mano Y, Murota S. Differential effects of nitric oxide on the activity of prostaglandin endoperoxide H synthase-1 and -2 in vascular endothelial cells. Prostaglandins Leukotrienes and Essential Fatty Acids. 2000;62(3):161–167. doi: 10.1054/plef.2000.0136. [DOI] [PubMed] [Google Scholar]
- 83.Kawka DW, Ouellet M, Hétu PO, Singer II, Riendeau D. Double-label expression studies of prostacyclin synthase, thromboxane synthase and COX isoforms in normal aortic endothelium. Biochimica et Biophysica Acta. 2007;1771(1):45–54. doi: 10.1016/j.bbalip.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 84.Tang EH, Vanhoutte PM. Gene expression changes of prostanoid synthases in endothelial cells and prostanoid receptors in vascular smooth muscle cells caused by aging and hypertension. Physiological Genomics. 2008;32(3):409–418. doi: 10.1152/physiolgenomics.00136.2007. [DOI] [PubMed] [Google Scholar]
- 85.Numaguchi Y, Harada M, Osanai H, et al. Altered gene expression of prostacyclin synthase and prostacyclin receptor in the thoracic aorta of spontaneously hypertensive rats. Cardiovascular Research. 1999;41(3):682–688. doi: 10.1016/s0008-6363(98)00239-9. [DOI] [PubMed] [Google Scholar]
- 86.Kang KB, Rajanayagam MA, van der Zypp A, Majewski H. A role for cyclooxygenase in aging-related changes of β-adrenoceptor- mediated relaxation in rat aortas. Naunyn-Schmiedeberg's Archives of Pharmacology. 2007;375(4):273–281. doi: 10.1007/s00210-007-0153-y. [DOI] [PubMed] [Google Scholar]
- 87.Graham DA, Rush JW. Cyclooxygenase and thromboxane/prostaglandin receptor contribute to aortic endothelium-dependent dysfunction in aging female spontaneously hypertensive rats. Journal of Applied Physiology. 2009;107(4):1059–1067. doi: 10.1152/japplphysiol.90785.2008. [DOI] [PubMed] [Google Scholar]
- 88.Wong WT, Tian XY, Leung FP, et al. Bone morphogenic protein-4 impairs endothelial function through oxidative stress-dependent cyclooxygenase-2 upregulation: implications on hypertension. Circulation Research. 2010;107(8):984–991. doi: 10.1161/CIRCRESAHA.110.222794. [DOI] [PubMed] [Google Scholar]
- 89.Qu C, Leung SW, Vanhoutte PM, Man RY. Chronic inhibition of nitric-oxide synthase potentiates endothelium-dependent contractions in the rat aorta by augmenting the expression of cyclooxygenase-2. Journal of Pharmacology and Experimental Therapeutics. 2010;334(2):373–380. doi: 10.1124/jpet.110.167098. [DOI] [PubMed] [Google Scholar]
- 90.Kato T, Iwama Y, Okumura K, Hashimoto H, Ito T, Satake T. Prostaglandin H2 may be the endothelium-derived contracting factor released by acetylcholine in the aorta of the rat. Hypertension. 1990;15(5):475–481. doi: 10.1161/01.hyp.15.5.475. [DOI] [PubMed] [Google Scholar]
- 91.Pagano PJ, Lin L, Sessa WC, Nasjletti A. Arachidonic acid elicits endothelium-dependent release from the rabbit aorta of a constrictor prostanoid resembling prostaglandin endoperoxides. Circulation Research. 1991;69(2):396–405. doi: 10.1161/01.res.69.2.396. [DOI] [PubMed] [Google Scholar]
- 92.Dai FX, Skopec J, Diederich A, Diederich D. Prostaglandin H2 and thromboxane A2 are contractile factors in intrarenal arteries of spontaneously hypertensive rats. Hypertension. 1992;19(6):795–798. doi: 10.1161/01.hyp.19.6.795. [DOI] [PubMed] [Google Scholar]
- 93.Shimizu K, Muramatsu M, Kakegawa Y, et al. Role of prostaglandin H2 as an endothelium-derived contracting factor in diabetic state. Diabetes. 1993;42(9):1246–1252. doi: 10.2337/diab.42.9.1246. [DOI] [PubMed] [Google Scholar]
- 94.Kent KC, Collins LJ, Schwerin FT, Raychowdhury MK, Ware JA. Identification of functional PGH2/TxA2 receptors on human endothelial cells. Circulation Research. 1993;72(5):958–965. doi: 10.1161/01.res.72.5.958. [DOI] [PubMed] [Google Scholar]
- 95.Asano H, Shimizu K, Muramatsu M, et al. Prostaglandin H2 as an endothelium-derived contracting factor modulates endothelin-1-induced contraction. Journal of Hypertension. 1994;12(4):383–390. [PubMed] [Google Scholar]
- 96.Lin L, Balazy M, Pagano PJ, Nasjletti A. Expression of prostaglandin H2-mediated mechanism of vascular contraction in hypertensive rats: relation to lipoxygenase and prostacyclin synthase activities. Circulation Research. 1994;74(2):197–205. doi: 10.1161/01.res.74.2.197. [DOI] [PubMed] [Google Scholar]
- 97.Félétou M, Verbeuren TJ, Vanhoutte PM. Endothelium-dependent contractions in SHR: a tale of prostanoid TP and IP receptors. British Journal of Pharmacology. 2009;156(4):563–574. doi: 10.1111/j.1476-5381.2008.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hamberg M, Samuelsson B. Prostaglandin endoperoxides. Novel transformations of arachidonic acid in human platelets. Proceedings of the National Academy of Sciences of the United States of America. 1974;71(9):3400–3404. doi: 10.1073/pnas.71.9.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hamberg M, Svensson J, Wakabayashi T, Samuelsson B. Isolation and structure of two prostaglandin endoperoxides that cause platelet aggregation. Proceedings of the National Academy of Sciences of the United States of America. 1974;71(2):345–349. doi: 10.1073/pnas.71.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Moncada S, Needleman P, Bunting S, Vane JR. Prostaglandin endoperoxide and thromboxane generating systems and their selective inhibition. Prostaglandins. 1976;12(3):323–335. doi: 10.1016/0090-6980(76)90014-9. [DOI] [PubMed] [Google Scholar]
- 101.Needleman P, Moncada S, Bunting S, Vane JR, Hamberg M, Samuelsson B. Identification of an enzyme in platelet microsomes which generates thromboxane A2 from prostaglandin endoperoxides. Nature. 1976;261(5561):558–560. doi: 10.1038/261558a0. [DOI] [PubMed] [Google Scholar]
- 102.Flavahan NA. Balancing prostanoid activity in the human vascular system. Trends in Pharmacological Sciences. 2007;28(3):106–110. doi: 10.1016/j.tips.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 103.Mcadam BF, Catella-Lawson F, Mardini IA, Kapoor S, Lawson JA, Fitzgerald GA. Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: the human pharmacology of a selective inhibitor of COX-2. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(1):272–277. doi: 10.1073/pnas.96.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rovati GE, Sala A, Capra V, Dahlén SE, Folco G. Dual COXIB/TP antagonists: a possible new twist in NSAID pharmacology and cardiovascular risk. Trends in Pharmacological Sciences. 2010;31(3):102–107. doi: 10.1016/j.tips.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 105.Wu KK, Liou JY. Cellular and molecular biology of prostacyclin synthase. Biochemical and Biophysical Research Communications. 2005;338(1):45–52. doi: 10.1016/j.bbrc.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 106.Liou JY, Shyue SK, Tsai MJ, Chung CL, Chu KY, Wu KK. Colocalization of prostacyclin synthase with prostaglandin H synthase-1 (PGHS-1) but not phorbol ester-induced PGHS-2 in cultured endothelial cells. Journal of Biological Chemistry. 2000;275(20):15314–15320. doi: 10.1074/jbc.275.20.15314. [DOI] [PubMed] [Google Scholar]
- 107.Brandt R, Dembinska-Kiec A, Gryglewski RJ, Nowak J. Release of prostacyclin from the human pulmonary vascular bed in response to cholinergic stimulation. Naunyn-Schmiedeberg's Archives of Pharmacology. 1984;325(1):69–75. doi: 10.1007/BF00507056. [DOI] [PubMed] [Google Scholar]
- 108.Norel X, Haye-Legrand I, Labat C, Benveniste J, Brink C. Antigen-induced contraction of human isolated lung preparations passively sensitized with monoclonal IgE: effects of indomethacin. International Archives of Allergy and Applied Immunology. 1991;96(4):368–375. doi: 10.1159/000235524. [DOI] [PubMed] [Google Scholar]
- 109.Buzzard CJ, Pfister SL, Campbell WB. Endothelium-dependent contractions in rabbit pulmonary artery are mediated by thromboxane A2 . Circulation Research. 1993;72(5):1023–1034. doi: 10.1161/01.res.72.5.1023. [DOI] [PubMed] [Google Scholar]
- 110.Heymes C, Habib A, Yang D, et al. Cyclo-oxygenase-1 and -2 contribution to endothelial dysfunction in ageing. British Journal of Pharmacology. 2000;131(4):804–810. doi: 10.1038/sj.bjp.0703632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Coleman RA, Smith WL, Narumiya S. VIII. International union of pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacological Reviews. 1994;46(2):205–229. [PubMed] [Google Scholar]
- 112.Kääpä P, Viinikka L, Ylikorkala O. Plasma prostacyclin from birth to adolescence. Archives of Disease in Childhood. 1982;57(6):459–461. doi: 10.1136/adc.57.6.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Armstrong JM, Lattimer N, Moncada S, Vane JR. Comparison of the vasodepressor effects of prostacyclin and 6-oxo-prostaglandin F1α with those of prostaglandin E2 in rats and rabbits. British Journal of Pharmacology. 1978;62(1):125–130. doi: 10.1111/j.1476-5381.1978.tb07014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cheng Y, Wang M, Yu Y, Lawson J, Funk CD, FitzGerald GA. Cyclooxygenases, microsomal prostaglandin E synthase-1, and cardiovascular function. Journal of Clinical Investigation. 2006;116(5):1391–1399. doi: 10.1172/JCI27540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fischer S, Bernutz C, Meier H, Weber PC. Formation of prostacyclin and thromboxane in man as measured by the main urinary metabolites. Biochimica et Biophysica Acta. 1986;876(2):194–199. doi: 10.1016/0005-2760(86)90274-2. [DOI] [PubMed] [Google Scholar]
- 116.Adatia I, Barrow SE, Stratton PD, Miall-Allen VM, Ritter JM, Haworth SG. Thromboxane A2 and prostacyclin biosynthesis in children and adolescents with pulmonary vascular disease. Circulation. 1993;88(5 I):2117–2122. doi: 10.1161/01.cir.88.5.2117. [DOI] [PubMed] [Google Scholar]
- 117.Tokunaga O, Yamada T, Fan JI, Watanabe T. Age-related decline in prostacyclin synthesis by human aortic endothelial cells: qualitative and quantitative analysis. American Journal of Pathology. 1991;138(4):941–949. [PMC free article] [PubMed] [Google Scholar]
- 118.Neubert K, Haberland A, Kruse I, Schimke I. The ratio of formation of prostacyclin/thromboxane A2 in HUVEC decreased in each subsequent passage. Prostaglandins. 1997;54(1):447–462. doi: 10.1016/s0090-6980(97)00063-4. [DOI] [PubMed] [Google Scholar]
- 119.Ferreira SH, Vane JR. Prostaglandins: their disappearance from and release into the circulation. Nature. 1967;216(5118):868–873. doi: 10.1038/216868a0. [DOI] [PubMed] [Google Scholar]
- 120.Ishihata A, Ogaki T, Aita T, Katano Y. Role of prostaglandins in urotensin II-induced vasodilatation in the coronary arteries of aged rats. European Journal of Pharmacology. 2005;523(1–3):119–126. doi: 10.1016/j.ejphar.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 121.Nicholson WT, Vaa B, Hesse C, Eisenach JH, Joyner MJ. Aging Is Associated with reduced prostacyclin-mediated dilation in the human forearm. Hypertension. 2009;53(6):973–978. doi: 10.1161/HYPERTENSIONAHA.108.121483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hara S, Kamei D, Sasaki Y, Tanemoto A, Nakatani Y, Murakami M. Prostaglandin E synthases: understanding their pathophysiological roles through mouse genetic models. Biochimie. 2010;92(6):651–659. doi: 10.1016/j.biochi.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 123.Samuelsson B, Morgenstern R, Jakobsson PJ. Membrane prostaglandin E synthase-1: a novel therapeutic target. Pharmacological Reviews. 2007;59(3):207–224. doi: 10.1124/pr.59.3.1. [DOI] [PubMed] [Google Scholar]
- 124.Sampey AV, Monrad S, Crofford LJ. Microsomal prostaglandin E synthase-1: the inducible synthase for prostaglandin E2 . Arthritis Research and Therapy. 2005;7(3):114–117. doi: 10.1186/ar1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Camacho M, López-Belmonte J, Vila L. Rate of vasoconstrictor prostanoids released by endothelial cells depends on cyclooxygenase-2 expression and prostaglandin I synthase activity. Circulation Research. 1998;83(4):353–365. doi: 10.1161/01.res.83.4.353. [DOI] [PubMed] [Google Scholar]
- 126.Yang T. Microsomal prostaglandin e synthase-1 and blood pressure regulation. Kidney International. 2007;72(3):274–278. doi: 10.1038/sj.ki.5002326. [DOI] [PubMed] [Google Scholar]
- 127.Wang M, Zukas AM, Hui Y, Ricciotti E, Puré E, FitzGerald GA. Deletion of microsomal prostaglandin E synthase-1 augments prostacyclin and retards atherogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(39):14507–14512. doi: 10.1073/pnas.0606586103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sugimoto Y, Narumiya S. Prostaglandin E receptors. Journal of Biological Chemistry. 2007;282(16):11613–11617. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 129.Alfranca A, Iñiguez MA, Fresno M, Redondo JM. Prostanoid signal transduction and gene expression in the endothelium: role in cardiovascular diseases. Cardiovascular Research. 2006;70(3):446–456. doi: 10.1016/j.cardiores.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 130.Shio H, Shaw J, Ramwell P. Relation of cyclic AMP to the release and actions of prostaglandins. Annals of the New York Academy of Sciences. 1971;185:327–335. doi: 10.1111/j.1749-6632.1971.tb45257.x. [DOI] [PubMed] [Google Scholar]
- 131.Salzman EW, Kensler PC, Levine L. Cyclic 3',5'-adenosine monophosphate in human blood platelets. IV. Regulatory role of cyclic amp in platelet function. Annals of the New York Academy of Sciences. 1972;201:61–71. doi: 10.1111/j.1749-6632.1972.tb16287.x. [DOI] [PubMed] [Google Scholar]
- 132.Weiss HJ, Willis AL, Kuhn D, Brand H. Prostaglandin E2 potentiation of platelet aggregation induced by LASS endoperoxide: absent in storage pool disease, normal after aspirin ingestion. British Journal of Haematology. 1976;32(2):257–272. doi: 10.1111/j.1365-2141.1976.tb00929.x. [DOI] [PubMed] [Google Scholar]
- 133.Morimoto A, Morimoto K, Watanabe T, Sakata Y, Murakami N. Does an increase in prostaglandin E2 in the blood circulation contribute to a febrile response in rabbits? Brain Research Bulletin. 1992;29(2):189–192. doi: 10.1016/0361-9230(92)90025-s. [DOI] [PubMed] [Google Scholar]
- 134.Bergstorem S, Ryhage R, Samuelsson B, Sjoevall J. Prostaglandins and related factors. 15. The structures of prostaglandin E1, F1-α, and F1-β . The Journal of Biological Chemistry. 1963;238:3555–3564. [PubMed] [Google Scholar]
- 135.Dunn MJ, Liard JF, Dray F. Basal and stimulated rates of renal secretion and excretion of prostaglandings E2, Falpha, and 13, 14-dihydro-15-keto Falpha in the dog. Kidney International. 1978;13(2):36–43. doi: 10.1038/ki.1978.20. [DOI] [PubMed] [Google Scholar]
- 136.Liston TE, Roberts LJ., II Transformation of prostaglandin D2 to 9α,11β-(15S)-trihydroxyprosta-(5Z,13E)-dien-1-oic acid (9α,11β-prostaglandin F2): a unique biologically active prostaglandin produced enzymatically in vivo in humans. Proceedings of the National Academy of Sciences of the United States of America. 1985;82(18):6030–6034. doi: 10.1073/pnas.82.18.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Beasley CR, Robinson C, Featherstone RL. 9α,11β-prostaglandin F2, a novel metabolite of prostaglandin D2 is a potent contractile agonist of human and guinea pig airways. Journal of Clinical Investigation. 1987;79(3):978–983. doi: 10.1172/JCI112909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Helliwell RJ, Adams LF, Mitchell MD. Prostaglandin synthases: recent developments and a novel hypothesis. Prostaglandins Leukotrienes and Essential Fatty Acids. 2004;70(2):101–113. doi: 10.1016/j.plefa.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 139.Fukunaga M, Makita N, Roberts LJ, 2nd, Morrow JD, Takahashi K, Badr KF. Evidence for the existence of F2-isoprostane receptors on rat vascular smooth muscle cells. American Journal of Physiology. 1993;264(6):C1619–C1624. doi: 10.1152/ajpcell.1993.264.6.C1619. [DOI] [PubMed] [Google Scholar]
- 140.Abramovitz M, Boie Y, Nguyen T, et al. Cloning and expression of a cDNA for the human prostanoid FP receptor. Journal of Biological Chemistry. 1994;269(4):2632–2636. [PubMed] [Google Scholar]
- 141.Lake S, Gullverg H, Wahlqvist J, et al. Cloning of the rat and human prostaglandin F2 alpha receptors and the expression of the rat prostaglandin F2 alpha receptor. FEBS Letters. 1994;355(3):317–325. doi: 10.1016/0014-5793(94)01198-2. [DOI] [PubMed] [Google Scholar]
- 142.Sakamoto K, Ezashi T, Miwa K, et al. Molecular cloning and expression of a cDNA of the bovine prostaglandin F2 alpha receptor. Advances in Prostaglandin, Thromboxane, and Leukotriene Research. 1995;23:259–261. [PubMed] [Google Scholar]
- 143.Sugimoto Y, Hasumoto K, Namba T, et al. Cloning and expression of a cDNA for mouse prostaglandin F receptor. Journal of Biological Chemistry. 1994;269(2):1356–1360. [PubMed] [Google Scholar]
- 144.Csepli J, Csapo AI. The effect of the prostaglandin F2α analogue ICI 81008 on uterine small arteries and on blood pressure. Prostaglandins. 1975;10(4):689–697. doi: 10.1016/s0090-6980(75)80017-7. [DOI] [PubMed] [Google Scholar]
- 145.Hata AN, Breyer RM. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacology and Therapeutics. 2004;103(2):147–166. doi: 10.1016/j.pharmthera.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 146.Smyth EM, Grosser T, Wang M, Yu Y, FitzGerald GA. Prostanoids in health and disease. Journal of Lipid Research. 2009;50:S423–428. doi: 10.1194/jlr.R800094-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wong SL, Leung FP, Vanhoutte P, Huang Y. Endothelium-dependent contractions in hamster aorta: the essential role of COX-2 and prostaglandin-2α . Basic, Basic and Clinical Pharmacology and Toxicology. 2008;102:15–15. [Google Scholar]
- 148.Wong SL, Leung FP, Lau CW, et al. Cyclooxygenase-2-derived prostaglandin F2α mediates endothelium-dependent contractions in the aortae of hamsters with increased impact during aging. Circulation Research. 2009;104(2):228–235. doi: 10.1161/CIRCRESAHA.108.179770. [DOI] [PubMed] [Google Scholar]
- 149.Yu Y, Lucitt MB, Stubbe J, et al. Prostaglandin F2α elevates blood pressure and promotes atherosclerosis. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(19):7985–7990. doi: 10.1073/pnas.0811834106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Adams JW, Migita DS, Yu MK, et al. Prostaglandin F2α stimulates hypertrophic growth of cultured neonatal rat ventricular myocytes. Journal of Biological Chemistry. 1996;271(2):1179–1186. doi: 10.1074/jbc.271.2.1179. [DOI] [PubMed] [Google Scholar]
- 151.Lai J, Jin H, Yang R, et al. Prostaglandin F2α induces cardiac myocyte hypertrophy in vitro and cardiac growth in vivo. American Journal of Physiology. 1996;271(6):H2197–H2208. doi: 10.1152/ajpheart.1996.271.6.H2197. [DOI] [PubMed] [Google Scholar]
- 152.Pönicke K, Giessler C, Grapow M, et al. FP-receptor mediated trophic effects of prostanoids in rat ventricular cardiomyocytes. British Journal of Pharmacology. 2000;129(8):1723–1731. doi: 10.1038/sj.bjp.0703243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Taba Y, Sasaguri T, Miyagi M, et al. Fluid shear stress induces lipocalin-type prostaglandin D2 synthase expression in vascular endothelial cells. Circulation Research. 2000;86(9):967–973. doi: 10.1161/01.res.86.9.967. [DOI] [PubMed] [Google Scholar]
- 154.Jones RL, Giembycz MA, Woodward DF. Prostanoid receptor antagonists: development strategies and therapeutic applications. British Journal of Pharmacology. 2009;158(1):104–145. doi: 10.1111/j.1476-5381.2009.00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.VanderEnde DS, Morrow JD. Release of markedly increased quantities of prostaglandin D2 from the skin in vivo in humans after the application of cinnamic aldehyde. Journal of the American Academy of Dermatology. 2001;45(1):62–67. doi: 10.1067/mjd.2001.113694. [DOI] [PubMed] [Google Scholar]
- 156.Walch L, Labat C, Gascard JP, de Montpreville V, Brink C, Norel X. Prostanoid receptors involved in the relaxation of human pulmonary vessels. British Journal of Pharmacology. 1999;126(4):859–866. doi: 10.1038/sj.bjp.0702393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wasserman MA, DuCharme DW, Griffin RL. Bronchopulmonary and cardiovascular effects of prostaglandin D2 in the dog. Prostaglandins. 1977;13(2):255–269. doi: 10.1016/0090-6980(77)90007-7. [DOI] [PubMed] [Google Scholar]
- 158.Hardy CC, Robinson C, Tattersfield AE, Holgate ST. The bronchoconstrictor effect of inhaled prostaglandin D2 in normal and asthmatic men. New England Journal of Medicine. 1984;311(4):209–213. doi: 10.1056/NEJM198407263110401. [DOI] [PubMed] [Google Scholar]
- 159.Johnston SL, Freezer NJ, Ritter W, O'Toole S, Howarth PH. Prostaglandin D2-induced bronchoconstriction is mediated only in part by the thromboxane prostanoid receptor. European Respiratory Journal. 1995;8(3):411–415. doi: 10.1183/09031936.95.08030411. [DOI] [PubMed] [Google Scholar]
- 160.Whittle BJ, Moncada S, Vane JR. Comparison of the effects of prostacyclin (PGI2), prostaglandin E1 and O2 on platelet aggregation in different species. Prostaglandins. 1978;16(3):373–388. doi: 10.1016/0090-6980(78)90216-2. [DOI] [PubMed] [Google Scholar]
- 161.Cooper B. Diminished platelet adenylate cyclase activation by prostaglandin D2 in acute thrombosis. Blood. 1979;54(3):684–693. [PubMed] [Google Scholar]
- 162.Siegl AM. Receptors for PGI2 and PGD2 on human platelets. Methods in Enzymology. 1982;86:179–192. doi: 10.1016/0076-6879(82)86189-2. [DOI] [PubMed] [Google Scholar]
- 163.Thierauch KH, Stürzebecher CS, Schillinger E, et al. Stable 9 beta- or 11 alpha-halogen-15-cyclohexyl-prostaglandins with high affinity to the PGD2-receptor. Advances in Prostaglandin, Thromboxane, and Leukotriene Research. 1989;19:655–658. [PubMed] [Google Scholar]
- 164.Kiriyama M, Ushikubi F, Kobayashi T, Hirata M, Sugimoto Y, Narumiya S. Ligand binding specificities of the eight types and subtypes of the mouse prostanoid receptors expressed in Chinese hamster ovary cells. British Journal of Pharmacology. 1997;122(2):217–224. doi: 10.1038/sj.bjp.0701367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cheng Y, Austin SC, Rocca B, et al. Role of prostacyclin in the cardiovascular response to thromboxane A2 . Science. 2002;296(5567):539–541. doi: 10.1126/science.1068711. [DOI] [PubMed] [Google Scholar]
- 166.Kobayashi T, Tahara Y, Matsumoto M, et al. Roles of thromboxane A2 and prostacyclin in the development of atherosclerosis in apoE-deficient mice. Journal of Clinical Investigation. 2004;114(6):784–794. doi: 10.1172/JCI21446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Francois H, Athirakul K, Mao L, Rockman H, Coffman TM. Role for thromboxane receptors in angiotensin-II-induced hypertension. Hypertension. 2004;43(2):364–369. doi: 10.1161/01.HYP.0000112225.27560.24. [DOI] [PubMed] [Google Scholar]
- 168.Ally AI, Horrobin DF. Thromboxane A2 in blood vessel walls and its physiological significance: relevance to thrombosis and hypertension. Prostaglandins and Medicine. 1980;4(6):431–438. doi: 10.1016/0161-4630(80)90051-8. [DOI] [PubMed] [Google Scholar]
- 169.Dorn GW, DeJesus A. Human platelet aggregation and shape change are coupled to separate thromboxane A2-prostaglandin H2 receptors. American Journal of Physiology. 1991;260(2):H327–H334. doi: 10.1152/ajpheart.1991.260.2.H327. [DOI] [PubMed] [Google Scholar]
- 170.Spurney RF, Bernstein RJ, Ruiz P, Pisetsky DS, Coffman TM. Physiologic role for enhanced renal thromboxane production in murine lupus nephritis. Prostaglandins. 1991;42(1):15–28. doi: 10.1016/0090-6980(91)90090-3. [DOI] [PubMed] [Google Scholar]
- 171.Katugampola SD, Davenport AP. Thromboxane receptor density is increased in human cardiovascular disease with evidence for inhibition at therapeutic concentrations by the AT1 receptor antagonist losartan. British Journal of Pharmacology. 2001;134(7):1385–1392. doi: 10.1038/sj.bjp.0704416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Martin W. The combined role of atheroma, cholesterol, platelets, the endothelium and fibrin in heart attacks and strokes. Medical Hypotheses. 1984;15(3):305–322. doi: 10.1016/0306-9877(84)90021-5. [DOI] [PubMed] [Google Scholar]
- 173.Dorn GW, Liel N, Trask JL, Mais DE, Assey ME, Halushka PV. Increased platelet thromboxane A2/prostaglandin H2 receptors in patients with acute myocardial infarction. Circulation. 1990;81(1):212–218. doi: 10.1161/01.cir.81.1.212. [DOI] [PubMed] [Google Scholar]
- 174.Dogne JM, Hanson J, Pratico D. Thromboxane, prostacyclin andisoprostanes: therapeutic targets in atherogenesis. Trends in Pharmacological Sciences. 2005;26(12):639–644. doi: 10.1016/j.tips.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 175.Lüscher TF, Boulanger CM, Dohi Y, Yang ZH. Endothelium-derived contracting factors. Hypertension. 1992;19(2):117–130. doi: 10.1161/01.hyp.19.2.117. [DOI] [PubMed] [Google Scholar]
- 176.Félétou M, Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture) American Journal of Physiology. 2006;291(3):H985–H1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- 177.Fitzgerald DJ, Roy L, Catella F, Fitzderald GA. Platelet activation in unstable coronary disease. New England Journal of Medicine. 1986;315(16):983–989. doi: 10.1056/NEJM198610163151602. [DOI] [PubMed] [Google Scholar]
- 178.Shinmura K, Tamaki K, Sato T, Ishida H, Bolli R. Prostacyclin attenuates oxidative damage of myocytes by opening mitochondrial ATP-sensitive K+ channels via the EP3 receptor. American Journal of Physiology. 2005;288(5):H2093–H2101. doi: 10.1152/ajpheart.01003.2004. [DOI] [PubMed] [Google Scholar]
- 179.Fitzgerald DJ, Rocki W, Murray R, Mayo G, FitzGerald GA. Thromboxane A2 synthesis in pregnancy-induced hypertension. Lancet. 1990;335(8692):751–754. doi: 10.1016/0140-6736(90)90869-7. [DOI] [PubMed] [Google Scholar]
- 180.Keith JC, Jr., Spitz B, van Assche FA. Thromboxane synthetase inhibition as a new therapy for preeclampsia: animal and human studies minireview. Prostaglandins. 1993;45(1):3–13. doi: 10.1016/0090-6980(93)90085-l. [DOI] [PubMed] [Google Scholar]
- 181.Shams G, Wallace LJ, Miller DD, Feller DR. Effects of thromboxane A2 on thoracic aorta of young and old rats: use of selective thromboxane receptor antagonists. Pharmacology. 1990;40(1):27–32. doi: 10.1159/000138635. [DOI] [PubMed] [Google Scholar]
- 182.Vanhoutte PM, Tang EH. Endothelium-dependent contractions: when a good guy turns bad! Journal of Physiology. 2008;586(22):5295–5304. doi: 10.1113/jphysiol.2008.161430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hornych A, Forette F, Bariety J, Krief C, Aumont J, Paris M. The influence of age on renal prostaglandin synthesis in man. Prostaglandins Leukotrienes and Essential Fatty Acids. 1991;43(3):191–195. doi: 10.1016/0952-3278(91)90168-5. [DOI] [PubMed] [Google Scholar]
- 184.Hou J, Abe K, Yoshinaga K. The investigation about age-related changes in vasoactive substances of normal subjects and of patients with essential hypertension. Nippon Jinzo Gakkai Shi. 1992;34(3):287–293. [PubMed] [Google Scholar]
- 185.Lüscher TF. Imbalance of endothelium-derived relaxing and contracting factors: a new concept in hypertension? American Journal of Hypertension. 1990;3(4):317–330. doi: 10.1093/ajh/3.4.317. [DOI] [PubMed] [Google Scholar]
- 186.Christman BW, McPherson CD, Newman JH, et al. An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. New England Journal of Medicine. 1992;327(2):70–75. doi: 10.1056/NEJM199207093270202. [DOI] [PubMed] [Google Scholar]
- 187.Woodman CR, Price EM, Laughlin MH. Selected Contribution: aging impairs nitric oxide and prostacyclin mediation of endothelium-dependent dilation in soleus feed arteries. Journal of Applied Physiology. 2003;95(5):2164–2170. doi: 10.1152/japplphysiol.01073.2002. [DOI] [PubMed] [Google Scholar]
- 188.Zou MH, Ullrich V. Peroxynitrite formed by simultaneous generation of nitric oxide and superoxide selectively inhibits bovine aortic prostacyclin synthase. FEBS Letters. 1996;382(1-2):101–104. doi: 10.1016/0014-5793(96)00160-3. [DOI] [PubMed] [Google Scholar]
- 189.Santilli F, Davì G, Basili S, et al. Thromboxane and prostacyclin biosynthesis in heart failure of ischemic origin: effects of disease severity and aspirin treatment. Journal of Thrombosis and Haemostasis. 2010;8(5):914–922. doi: 10.1111/j.1538-7836.2010.03820.x. [DOI] [PubMed] [Google Scholar]
- 190.Sellers MM, Stallone JN. Sympathy for the devil: the role of thromboxane in the regulation of vascular tone and blood pressure. American Journal of Physiology. 2008;294(5):H1978–H1986. doi: 10.1152/ajpheart.01318.2007. [DOI] [PubMed] [Google Scholar]
- 191.Félétou M, Vanhoutte PM, Verbeuren TJ. The thromboxane/endoperoxide receptor (TP): the common villain. Journal of Cardiovascular Pharmacology. 2010;55(4):317–332. doi: 10.1097/fjc.0b013e3181d8bc8a. [DOI] [PubMed] [Google Scholar]
- 192.Xiao CY, Hara A, Yuhki K, et al. Roles of prostaglandin I2 and thromboxane A2 in cardiac ischemia-reperfusion injury: a study using mice lacking their respective receptors. Circulation. 2001;104(18):2210–2215. doi: 10.1161/hc4301.098058. [DOI] [PubMed] [Google Scholar]
- 193.Cheng Y, Austin SC, Rocca B, et al. Role of prostacyclin in the cardiovascular response to thromboxane A2 . Science. 2002;296(5567):539–541. doi: 10.1126/science.1068711. [DOI] [PubMed] [Google Scholar]
- 194.Arehart E, Stitham J, Asselbergs FW, et al. Acceleration of cardiovascular disease by a dysfunctional prostacyclin receptor mutation: potential implications for cyclooxygenase-2 inhibition. Circulation Research. 2008;102(8):986–993. doi: 10.1161/CIRCRESAHA.107.165936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 196.Baker CS, Hall RJC, Evans TJ, et al. Cyclooxygenase-2 is widely expressed in atherosclerotic lesions affecting native and transplanted human coronary arteries and colocalizes with inducible nitric oxide synthase and nitrotyrosine particularly in macrophages. Arteriosclerosis, Thrombosis, and Vascular Biology. 1999;19(3):646–655. doi: 10.1161/01.atv.19.3.646. [DOI] [PubMed] [Google Scholar]
- 197.Hong BK, Kwon HM, Lee BK, et al. Coexpression of cyclooxygenase-2 and matrix metalloproteinases in human aortic atherosclerotic lesions. Yonsei Medical Journal. 2000;41(1):82–88. doi: 10.3349/ymj.2000.41.1.82. [DOI] [PubMed] [Google Scholar]
- 198.Schonbeck U, Sukhova GK, Graber P, Coulter S, Libby P. Augmented expression of cyclooxygenase-2 in human atherosclerotic lesions. American Journal of Pathology. 1999;155(4):1281–1291. doi: 10.1016/S0002-9440(10)65230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Stemme V, Swedenborg J, Claesson HE, Hansson GK. Expression of cyclo-oxygenase-2 in human atherosclerotic carotid arteries. European Journal of Vascular and Endovascular Surgery. 2000;20(2):146–152. doi: 10.1053/ejvs.2000.1145. [DOI] [PubMed] [Google Scholar]
- 200.Chen YF, Jowett S, Barton P, et al. Clinical and cost-effectiveness of epoprostenol, iloprost, bosentan, sitaxentan and sildenafil for pulmonary arterial hypertension within their licensed indications: a systematic review and economic evaluation. Health Technology Assessment. 2009;13(49):1–320. doi: 10.3310/hta13490. [DOI] [PubMed] [Google Scholar]
- 201.Peter CS, Peter C. Prostaglandins, prostacyclin, and thromboxane in cardiovascular diseases. Drug Development Research. 1986;7(4):341–359. [Google Scholar]
- 202.McLaughlin VV, Gaine SP, Barst RJ, et al. Efficacy and safety of treprostinil: an epoprostenol analog for primary pulmonary hypertension. Journal of Cardiovascular Pharmacology. 2003;41(2):293–299. doi: 10.1097/00005344-200302000-00019. [DOI] [PubMed] [Google Scholar]
- 203.Toda N. Beraprost sodium. Cardiovascular Drug Reviews. 1988;6(3):222–238. [Google Scholar]
- 204.Umetsu T, Murata T, Tanaka Y, Osada E, Nishio S. Antithrombotic effect of TRK-100, a novel, stable PGI2 analogue. Japanese Journal of Pharmacology. 1987;43(1):81–90. doi: 10.1254/jjp.43.81. [DOI] [PubMed] [Google Scholar]
- 205.Umetsu T, Murata T, Nishio S. Studies on the antiplatelet effect of the stable epoprostenol analogue beraprost sodium and its mechanism of action in rats. Arzneimittel-Forschung. 1989;39(1):68–73. [PubMed] [Google Scholar]
- 206.Murai T, Muraoka K, Saga K, et al. Effect of beraprost sodium on peripheral circulation insufficiency in rats and rabbits. Arzneimittel-Forschung. 1989;39(8):856–859. [PubMed] [Google Scholar]
- 207.Ma XL, Johnson G, 3rd, Lefer AM. Low doses of superoxide dismutase and a stable prostacyclin analogue protect in myocardial ischemia and reperfusion. Journal of the American College of Cardiology. 1992;19(1):197–204. doi: 10.1016/0735-1097(92)90073-v. [DOI] [PubMed] [Google Scholar]
- 208.Chan PS, Cervoni P, Scully PA, Ronsberg MA, Accomando RC. Mechanism of action of a new prostaglandin antihypertensive, viprostol [CL 115 347; (dl)-15-deoxy-16-hydroxy-16(α/β)-vinyl-prostaglandin E2 methyl ester]: (I) Vasodilation. Journal of Hypertension. 1986;4(6):741–747. doi: 10.1097/00004872-198612000-00009. [DOI] [PubMed] [Google Scholar]
- 209.Chan PS, Cervoni P, Accomando RC, Quirk GJ, Ronsgerg MA. Mechanism of action of a new prostaglandin antihypertensive, viprostol [CL 115 347; (dl)-15-deoxy-16-hydroxy-16(α/β)-vinyl prostaglandin E2 methyl ester]: (II) Effects on the adrenergic nervous system. Journal of Hypertension. 1986;4(6):749–757. doi: 10.1097/00004872-198612000-00010. [DOI] [PubMed] [Google Scholar]
- 210.Olivari MT, Levine TB, Cohn JN. Evidence for a direct renal stimulating effect of prostaglandin E2 on renin release in patients with congestive heart failure. Circulation. 1986;74(6):1203–1207. doi: 10.1161/01.cir.74.6.1203. [DOI] [PubMed] [Google Scholar]
- 211.Haas JA, Hammond TG, Granger JP, Blaine EH, Knox FG. Mechanism of natriuresis during intrarenal infusion of prostaglandins. The American Journal of Physiology. 1984;247(3):F475–479. doi: 10.1152/ajprenal.1984.247.3.F475. [DOI] [PubMed] [Google Scholar]
- 212.Katayama S, Attallah AA, Stahl RA, Bloch DL, Lee JB. Mechanism of furosemide-induced natriuresis by direct stimulation of renal prostaglandin E2 . The American Journal of Physiology. 1984;247(4):F555–561. doi: 10.1152/ajprenal.1984.247.4.F555. [DOI] [PubMed] [Google Scholar]
- 213.Stokes JB. Integrated actions of renal medullary prostaglandins in the control of water excretion. The American Journal of Physiology. 1981;240(6):F471–F480. doi: 10.1152/ajprenal.1981.240.6.F471. [DOI] [PubMed] [Google Scholar]
- 214.Olley PM, Coceani F, Bodach E. E-type prostaglandins: a new emergency therapy for certain cyanotic congenital heart malformations. Circulation. 1976;53(4):728–731. doi: 10.1161/01.cir.53.4.728. [DOI] [PubMed] [Google Scholar]
- 215.Heymann MA. Pharmacologic use of prostaglandin E1 in infants with congenital heart disease. American Heart Journal. 1981;101(6):837–843. doi: 10.1016/0002-8703(81)90622-0. [DOI] [PubMed] [Google Scholar]
- 216.Silove ED, Roberts DGV, de Giovanni JV. Evaluation of oral and low dose intravenous prostaglandin E2 in management of ductus dependent congenital heart disease. Archives of Disease in Childhood. 1985;60(11):1025–1030. doi: 10.1136/adc.60.11.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Silove ED, Coe JY, Shiu MF, et al. Oral prostaglandin E2 in ductus-dependent pulmonary circulation. Circulation. 1981;63(3):682–688. doi: 10.1161/01.cir.63.3.682. [DOI] [PubMed] [Google Scholar]
- 218.Fiddler GI, Lumley P. Preliminary clinical studies with thromboxane synthase inhibitors and thromboxane receptor blockers. A review. Circulation. 1990;81(1) supplement I:I -69–I -78. [PubMed] [Google Scholar]
- 219.Jones EW, Cockbill SR, Cowley AJ, et al. Effects of dazoxiben and low-dose aspirin on platelet behaviour in man. British Journal of Clinical Pharmacology. 1983;15(supplement 1):39S–44S. doi: 10.1111/j.1365-2125.1983.tb02105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Villalobos MA, de La Cruz JP, Escalante R, Arrebola MM, Guerrero A, Sánchez de la Cuesta F. Effects of camonagrel, a selective inhibitor of platelet thromboxane synthase, on the platelet-subendothelium interaction. Pharmacology. 2003;69(1):44–50. doi: 10.1159/000071266. [DOI] [PubMed] [Google Scholar]
- 221.Coker SJ, Parratt JR. The effects of dazoxihen on arrhythmias and ventricular fibrillation induced by coronary, artery occlusion and reperfusion. British Journal of Clinical Pharmacology. 1983;15(supplement 1):87S–95S. doi: 10.1111/j.1365-2125.1983.tb02115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Coker SJ. Further evidence that thromboxane exacerbates arrhythmias: effects of UK38485 during coronary artery occlusion and reperfusion in anaesthetized greyhounds. Journal of Molecular and Cellular Cardiology. 1984;16(7):633–641. doi: 10.1016/s0022-2828(84)80627-6. [DOI] [PubMed] [Google Scholar]
- 223.Neri Serneri GG, Coccheri S, Marubini E, Violi F. Picotamide, a combined inhibitor of thromboxane A2 synthase and receptor, reduces 2-year mortality in diabetics with peripheral arterial disease: the DAVID study. European Heart Journal. 2004;25(20):1845–1852. doi: 10.1016/j.ehj.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 224.Vetrano A, Milani M, Corsini G. Effects of aspirin or picotamide, an antithromboxane agent, in combination with low-intensity oral anticoagulation in patients with acute myocardial infarction: a controlled randomized pilot trial. Giornale Italiano di Cardiologia Journal. 1999;29(5):524–528. [PubMed] [Google Scholar]
- 225.Cocozza M, Picano T, Oliviero U, Russo N, Coto V, Milani M. Effects of picotamide, an antithromboxane agent, on carotid atherosclerotic evolution: a two-year, double-blind, placebo-controlled study in diabetic patients. Stroke. 1995;26(4):597–601. doi: 10.1161/01.str.26.4.597. [DOI] [PubMed] [Google Scholar]
- 226.Hennerici MG, Bots ML, Ford I, Laurent S, Touboul PJ. Rationale, design and population baseline characteristics of the PERFORM Vascular Project: an ancillary study of the Prevention of cerebrovascular and cardiovascular events of ischemic origin with terutroban in patients with a history of ischemic stroke or transient ischemic attack (PERFORM) trial. Cardiovascular Drugs and Therapy. 2010;24(2):175–180. doi: 10.1007/s10557-010-6231-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Simonet S, Descombes JJ, Vallez MO, et al. S 18886, a new thromboxane (TP)-receptor antagonist is the active isomer of S 18204 in all species, except in the guinea-pig. Advances in Experimental Medicine and Biology. 1997;433:173–176. doi: 10.1007/978-1-4899-1810-9_35. [DOI] [PubMed] [Google Scholar]
- 228.Johnson AG, Nguyen TV, Day RO. Do nonsteroidal anti-inflammatory drugs affect blood pressure? A meta-analysis. Annals of Internal Medicine. 1994;121(4):289–300. doi: 10.7326/0003-4819-121-4-199408150-00011. [DOI] [PubMed] [Google Scholar]
- 229.Swan SK, Rudy DW, Lasseter KC, et al. Effect of cyclooxygenase-2 inhibition on renal function in elderly persons receiving a low-salt diet: a randomized, controlled trial. Annals of Internal Medicine. 2000;133(1):1–9. doi: 10.7326/0003-4819-133-1-200007040-00002. [DOI] [PubMed] [Google Scholar]
- 230.Hawkey CJ, Hawkey GM, Everitt S, Skelly MM, Stack WA, Gray D. Increased risk of myocardial infarction as first manifestation of ischaemic heart disease and nonselective nonsteroidal anti-inflammatory drugs. British Journal of Clinical Pharmacology. 2006;61(6):730–737. doi: 10.1111/j.1365-2125.2006.02644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Husain S, Andrews NP, Mulcahy D, Panza JA, Quyyumi AA. Aspirin improves endothelial dysfunction in atherosclerosis. Circulation. 1998;97(8):716–720. doi: 10.1161/01.cir.97.8.716. [DOI] [PubMed] [Google Scholar]
- 232.Fuster V, Dyken ML, Vokonas PS, Hennekens C. Aspirin as a therapeutic agent in cardiovascular disease. Special Writing Group. Circulation. 1993;87(2):659–675. doi: 10.1161/01.cir.87.2.659. [DOI] [PubMed] [Google Scholar]
- 233.Antiplatelet Trials Collaboration. Collaborative overview of randomized trials of antiplatelet therapy, I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. British Medical Journal. 1994;308(6921):81–106. [PMC free article] [PubMed] [Google Scholar]
- 234.Roth GJ, Majerus PW. The mechanism of the effect of aspirin on human platelets. I. Acetylation of a particulate fraction protein, Annual Review of Pharmacology and Toxicology. The Journal of Clinical Investigation. 1975;56(3):624–632. doi: 10.1172/JCI108132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Loll PJ, Picot D, Garavito RM. The structural basis of aspirin activity inferred from the crystal structure of inactivated prostaglandin H2 synthase. Nature Structural & Molecular Biology. 1995;2(8):637–643. doi: 10.1038/nsb0895-637. [DOI] [PubMed] [Google Scholar]
- 236.Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annual Review of Pharmacology and Toxicology. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 237.Pignatelli P, di Santo S, Barillá F, Gaudio C, Violi F. Multiple anti-atherosclerotic treatments impair aspirin compliance: effects on aspirin resistance. Journal of Thrombosis and Haemostasis. 2008;6(10):1832–1834. doi: 10.1111/j.1538-7836.2008.03122.x. [DOI] [PubMed] [Google Scholar]
- 238.Dawson J, Quinn T, Rafferty M, et al. Aspirin resistance and compliance with therapy. Cardiovascular Therapeutics. 2011;29(5):301–307. doi: 10.1111/j.1755-5922.2010.00188.x. [DOI] [PubMed] [Google Scholar]
- 239.Di Minno G. Aspirin resistance and platelet turnover: a 25-year old issue. Nutrition, Metabolism and Cardiovascular Diseases. 2011;21(8):542–545. doi: 10.1016/j.numecd.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 240.Szczeklik A, Musiał J, Undas A, Sanak M. Aspirin resistance. Journal of Thrombosis and Haemostasis. 2005;3(8):1655–1662. doi: 10.1111/j.1538-7836.2005.01372.x. [DOI] [PubMed] [Google Scholar]
- 241.Goodman T, Ferro A, Sharma P. Pharmacogenetics of aspirin resistance: a comprehensive systematic review. British Journal of Clinical Pharmacology. 2008;66(2):222–331. doi: 10.1111/j.1365-2125.2008.03183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Krasopoulos G, Brister SJ, Beattie WS, Buchanan MR. Aspirin "resistance" and risk of cardiovascular morbidity: systematic review and meta-analysis. British Medical Journal. 2008;336(7637):195–198. doi: 10.1136/bmj.39430.529549.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Snoep JD, Hovens MM, Eikenboom JC, van der Bom JG, Huisman MV. Association of laboratory-defined aspirin resistance with a higher risk of recurrent cardiovascular events: a systematic review and meta-analysis. Archives of Internal Medicine. 2007;167(15):1593–1599. doi: 10.1001/archinte.167.15.1593. [DOI] [PubMed] [Google Scholar]
- 244.Eikelboom JW, Hirsh J, Weitz JI, Johnston M, Yi Q, Yusuf S. Aspirin-resistant thromboxane biosynthesis and the risk of myocardial infarction, stroke, or cardiovascular death in patients at high risk for cardiovascular events. Circulation. 2002;105(14):1650–1655. doi: 10.1161/01.cir.0000013777.21160.07. [DOI] [PubMed] [Google Scholar]
- 245.Tai HH, Cho H, Tong M, Ding Y. NAD+-linked 15-hydroxyprostaglandin dehydrogenase: structure and biological function. Current Pharmaceutical Design. 2006;12(8):955–962. doi: 10.2174/138161206776055958. [DOI] [PubMed] [Google Scholar]
- 246.Rudock ME, Liu Y, Ziegler JT, et al. Association of polymorphisms in cyclooxygenase (COX)-2 with coronary and carotid calcium in the Diabetes Heart Study. Atherosclerosis. 2009;203(2):459–465. doi: 10.1016/j.atherosclerosis.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Helmersson J, Ärnlöv J, Axelsson T, Basu S. A polymorphism in the cyclooxygenase 1 gene is associated with decreased inflammatory prostaglandin F2α formation and lower risk of cardiovascular disease. Prostaglandins Leukotrienes and Essential Fatty Acids. 2009;80(1):51–56. doi: 10.1016/j.plefa.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 248.Nakayama T. Prostacyclin synthase gene: genetic polymorphisms and prevention of some cardiovascular diseases. Current Medicinal Chemistry: Cardiovascular and Hematological Agents. 2005;3(2):157–164. doi: 10.2174/1568016053544327. [DOI] [PubMed] [Google Scholar]
- 249.Stitham J, Stojanovic A, Hwa J. Impaired receptor binding and activation associated with a human prostacyclin receptor polymorphism. Journal of Biological Chemistry. 2002;277(18):15439–15444. doi: 10.1074/jbc.M201187200. [DOI] [PubMed] [Google Scholar]
- 250.Lemaitre RN, Rice K, Marciante K, et al. Variation in eicosanoid genes, non-fatal myocardial infarction and ischemic stroke. Atherosclerosis. 2009;204(2):e58–e63. doi: 10.1016/j.atherosclerosis.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Hamberg M, Samuelsson B. On the metabolism of prostaglandins E 1 and E 2 in man. Journal of Biological Chemistry. 1971;246(22):6713–6721. [PubMed] [Google Scholar]
- 252.Nomura R, Lu R, Pucci ML, Schuster VL. The two-step model of prostaglandin signal termination: in vitro reconstitution with the prostaglandin transporter and prostaglandin 15 dehydrogenase. Molecular Pharmacology. 2004;65(4):973–978. doi: 10.1124/mol.65.4.973. [DOI] [PubMed] [Google Scholar]
- 253.Chang HY, Locker J, Lu R, Schuster VL. Failure of postnatal ductus arteriosus closure in prostaglandin transporter-deficient mice. Circulation. 2010;121(4):529–536. doi: 10.1161/CIRCULATIONAHA.109.862946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Chi Y, Min J, Jasmin JF, Lisanti MP, Chang YT, Schuster VL. Development of a high affinity inhibitor of the prostaglandin transporter PGT. Journal of Pharmacology and Experimental Therapeutics. 2011;339(2):529–536. doi: 10.1124/jpet.111.181354. [DOI] [PMC free article] [PubMed] [Google Scholar]


