Abstract
The main objective was to investigate whether low-molecular-weight fraction of edible mushroom shiitake extract (Lentinus edodes) possesses caries-preventive properties. The study was designed as a double-blind, three-leg, cross-over, randomized, controlled clinical trial carried out on two series of volunteers at the University of Gothenburg, and the Academic Centre for Dentistry Amsterdam. Volunteers rinsed twice daily with a solution containing low-molecular-weight fraction of edible mushroom, placebo (negative control without active ingredients), or Meridol (positive control, AmF-SnF2) for two weeks, with a two-week washout period between each rinsing period. Changes in the acidogenicity of dental plaque before and after a sucrose challenge, shifts in microbial composition, and plaque scores were determined. Frequent rinses with shiitake reduced the metabolic activity of dental plaque. No reduction of plaque scores and no inhibition of the production of organic acids in plaque was found. Minor differences in microbial composition between test sessions were found. To conclude, the results indicate that shiitake extract has anticariogenic potential, but not to the same extent as the positive control.
1. Introduction
Dental caries constitutes a multifactorial disease with a complex origin where the acidogenicity of dental plaque as a consequence may affect dental hard tissues [1–3]. The dental plaque is a complex multispecies biofilm. A reduction in plaque pH occurs following the release of organic acids, primarily lactate, acetate, and propionate, by oral microorganisms as fermentation products.
Over the years, different approaches have been designed intended to prevent this disease from occurring. Apart from strengthening the tooth mineral using fluoride, pronounced changes in environmental factors such as diet, oral hygiene measures, and the use of antimicrobials have been suggested in order to induce ecological shifts in biofilm composition [3, 4]. The latter are designed to inhibit the fermentation activity of cariogenic microorganisms, particularly those harboured in the oral biofilm, which will in turn determine a shift from a diseased to a healthy state [5].
Several possible mechanisms by agents of this kind have been suggested. They include the prevention of bacterial adhesion, a reduction in plaque formation, and interference with the bacterial metabolism. The most commonly used antimicrobial agent is chlorhexidine, a bisbiguanid, with known strong antimicrobial activity [6], but xylitol, fluoride, and essential oils are also known to possess similar, albeit weaker, activity [7, 8]. Chemical components able to produce one or more of the actions on different kinds of biological activity have also been shown to be present in a number of foods [9, 10]. The presence of compounds with antibacterial activity on different pathogens, as well as antiadhesive activity and inhibitory activity on matrix formation, has been demonstrated by different food products [11].
When it comes to different food products and their constituents, interest has recently focused on an edible mushroom, shiitake (Lentinus edodes). The extract from L. edodes has been studied in rats and an inhibitory effect on one of the virulence factors of Streptococcus mutans has been demonstrated [12]. There are few reports related to the general antimicrobial effects of different compounds obtained from shiitake. An aqueous extract from L. edodes displayed high antimicrobial activity on food-borne pathogenic bacterial strains [13]. A diet containing 5% of dried L. edodes has been found to reduce the viable counts of the total number of microorganisms, streptococci, Escherichia coli, and lactic acid bacteria in the intestinal flora of piglets [14]. In a series of in vitro studies, a number of biological activities relevant to caries prevention have been identified, the most prominent of which are the induction of the detachment of cariogenic microorganisms from hydroxyapatite, changes in cell surface hydrophobicity, bactericidal activity against cariogenic microorganisms, the prevention of the coaggregation of microorganisms, and the disruption of signal transduction in Streptococcus mutans [15, 16].
The hypothesis was that frequent mouth rinses with low-molecular-weight fraction of edible mushroom shiitake extract may reduce plaque metabolic activity, change plaque cariogenic microflora towards a healthier oral flora, and reduce plaque amount. Thus, the aim of the present study was to conduct a short-term clinical trial to determine the in vivo potential of rinsing with a low molecular weight extract (<5.000 Da) of shiitake (Lentinula edodes) on the acidogenicity of dental plaque, microbial composition, and plaque index score.
2. Materials and Methods
2.1. Study Design
This study was carried out as a double-blind, randomized, placebo-controlled, three-leg, cross-over clinical trial. Two sets of data were obtained—one at the Department of Cariology, University of Gothenburg (GOT) and one at the Department of Preventive Dentistry in collaboration with the Department of Periodontology, Academic Centre for Dentistry Amsterdam (ACTA). Despite being very similar, the two substudies were not identical in study design and the data that were obtained and, for this reason, the protocol cannot be regarded as a multicentre approach. The study was performed within an International EU Sixth Framework Programme Consortium project (NUTRIDENT, FOOD-CT-2006-036210), which was granted in order to identify beverage/food constituents that are able to reduce the risk of dental caries and gingivitis. This study focused on the opportunity to prevent dental caries and inhibit dental plaque formation. The two series were approved by the Ethical Committee at the University of Gothenburg (Dnr 102–09) and by the institutional review board at ACTA (METc VUmc, protocol number BL21480.029-08), respectively.
All volunteers made eight visits to the laboratory (GOT) respective clinic (ACTA), for a first visit, when a clinical examination was carried out and information about the study was given, and for a total of seven subsequent test visits. In all, there were four washout periods. The study started with a two (GOT) and a three-week (ACTA) preexperimental washout period followed by three two-week periods with daily mouth rinsing with the assigned product intermitted with two-week washout periods. The total duration of the study was, therefore, 14 (GOT) or 15 (ACTA) weeks.
At the screening (GOT) or at the first visit (ACTA), a medical questionnaire was completed and the oral health status of the participants was determined by an intraoral examination. Professional oral hygiene for GOT was performed at the start of each test period as well as directly after each test period (prior to washout) and for ACTA before the start of the first washout (before the baseline samples).
At each of the following visits, the subjects underwent the following data collection in the order mentioned: (1) collection of resting and fermented plaque for protein/acid analyses, (2) plaque acidogenicity (only GOT), (3) collection of plaque for microbiological analyses, and (4) assessment of plaque score. At the end of each two-week test period, the subjects were asked to fill in a questionnaire with questions related to the usage and experience of the product used. The investigators involved in plaque sampling and pH measurements were blinded with respect to the treatment allocation of the subjects.
2.2. Study Population
The research population at GOT was made up of students and staff at the Institute of Odontology, as well as individuals in the nearby vicinity recruited via advertisements on bulletin boards. A total of 65 volunteers were screened. For ACTA, recruitment was performed using the existing database (approximately 600 entries being not-dental students) at the Department of Periodontology. The inclusion criteria were healthy adults, possessing at least three premolars/molars in each quadrant, who were able to reduce their plaque pH by at least one pH unit after a mouth rinse with 10% sucrose solution for 1 min (only GOT), no metal fillings in the premolar/molar region (only GOT), and a stimulated saliva secretion rate of > 0.7 mL/min (only GOT). The exclusion criteria were subjects with untreated caries or periodontal disease, wearing partial dentures, wearing orthodontic bands, and the use of antibiotics less than three months prior to the start of the study. All the subjects were given verbal and written information about the study and signed an informed consent form prior to the start of the study.
Sample size calculations were made by using the results of plaque acidogenicity relating to the effect of Meridol mouthwash (AmF-SnF2) on the amount of lactate in sucrose-fermenting plaque [17] where an effect size of 0.759 had been found. Since no data were available on the effect of shiitake extract rinse on lactate production, a more conservative effect (75%) was used for calculation. An a priori two-tailed analysis of the required sample size with an alpha-error probability of 0.05, a power of 0.8, and an effect size of 0.569 was performed. This gave a minimum sample size of 27. In GOT, 30 subjects who fulfilled the inclusion criteria were enrolled, while at ACTA 35 subjects were enrolled compensating for potential dropouts in order to complete the study with at least 30 individuals. The subjects were randomised using a computer-generated allocation schedule, and the subjects were not informed of their allocation.
Apart from the specific instructions given to participants in GOT/ACTA for each test period, the volunteers were asked to refrain from any oral hygiene procedures during the last 72 hours (GOT) and 48 hours (ACTA), respectively, prior to each visit, as well as eating/drinking during the last two hours prior to the test. A toothpaste containing 1450 ppm F as NaF was distributed to all subjects to be used twice daily throughout the entire study: Pepsodent Super Fluor, Unilever Sverige AB, Stockholm, Sweden (GOT) and Prodent, Sara Lee, the Netherlands (ACTA), respectively.
2.3. Test Products
The following three products were tested: (1) shiitake (low-molecular-weight fraction of shiitake mushroom (Lentinula edodes) extract), (2) placebo (negative vehicle control without active ingredients), and (3) Meridol (AmF-SnF2, positive control). The active product was produced and shipped by the subcontractor MicroPharm Ltd (UK) in 20 mL aliquots. The product was prepared at MicroPharm Ltd according to the GMP guidelines at the company. In addition, the placebo formulation (negative control) was distributed by MicroPharm Ltd in identical vials containing 20 mL aliquots. The active and placebo solutions contained identical flavouring and preservative agents. The positive control (Meridol, GABA International AB, Münchenstein, Switzerland) contained 125 ppm AmF + 125 ppm SnF2. Prior to the start of the study, the solution was aseptically distributed in 20 mL aliquots into empty vials identical to those used for the active and placebo solutions. At the start of each test period, the subjects received a total of 30 vials (28 vials + 2 extra) to be used during the 14-day test period.
The volunteers were asked to rinse with the assigned solution twice daily. On each rinsing occasion, they were instructed to rinse vigorously with 10 mL (1/2 of the volume of the vial) for 30 sec, after which they expectorated the solution. A second identical rinsing procedure with the remaining 10 mL was repeated directly after the first one. The total daily exposure was, therefore, 40 mL for 120 sec. No food or drink intake was allowed for at least one hour after the rinse. To standardise the sampling procedure after two weeks' use of the mouthwash, all the volunteers were asked to rinse exactly three hours before the visit on day 14. No food or drink intake was allowed for at least one hour after the rinse.
2.4. Plaque Acidogenicity
In GOT, changes in plaque acidogenicity were measured before and after a mouth rinse with 10% sucrose using the microtouch method [18]. An iridium microelectrode (Beetrode MEPH-1, WPI Instruments, New Haven, Conn, USA) was inserted into the plaque in an interproximal area in the left and right upper premolar/molar region. The electrode was connected to an Orion SA720 pH/ISE Meter (Orion Research, Boston, Mass, USA) to which a reference electrode was also connected. The reference electrode was placed in a solution of 3 M KCl into which a finger of the volunteer was also inserted in order to create a salt bridge. Prior to and during each test session, the electrode was calibrated against a standard buffer at pH 7 [19]. After baseline registration (0 min), the subjects rinsed with the sucrose solution for 1 min, after which pH was measured at seven different time points up to 45 min.
2.5. Protein and Organic Acid Analyses
Two plaque samples were collected for the protein and acid anion profile, before (resting) and 10 min after the start of rinsing (fermented). The collection of resting plaque was carried out on the buccal surface of the right upper second molar using a sterile carver (GOT) and Teflon spatula (ACTA), respectively. The volunteers then rinsed for 2 min (ACTA) or 1 min (GOT) with 10 mL of 10% sucrose (w/v) solution. Fermented plaque, collected 10 min after the start of the sucrose rinse, was collected from the contralateral buccal surface (left second upper molar). For GOT, the fermented plaque sample was collected at the same time point as the pH measurements.
The plaque was transferred to a precooled Eppendorf tube containing 50 μL of MilliQ water. The samples were immediately spun down by centrifuging the tube for 30 sec at 16.100 ×g and put on ice until they were further processed within one hour. The samples were heated at 80°C for 5 min and cooled on ice. The samples from GOT were sent on dry ice to ACTA for further processing and analyses. The vials with plaque were centrifuged at 16.100 ×g for 15 min at 4°C. The supernatants were transferred into vials with a microspin filter (Ultrafree-MC 0.22 μm, Millipore, Bedford, Mass, USA) and centrifuged at 13.684 ×g for 5 min at 4°C. The supernatants and pellets were then stored at −80°C. Organic acids in resting and fermenting plaque were determined as their anions by capillary electrophoresis on a Beckman P/ACE MDQ system. Sodium salts of formic, acetic, propionic, butyric, succinic, and lactic acid were used to prepare mixture standard solutions in MilliQ water. Calibration curves were made for each acid separately. As an internal standard, oxalate was included in all samples. Formic, butyric, succinic, propionic, acetic, and lactic acid were determined in duplicate samples. Acid data were normalised by protein content of the plaque sample. Protein content was determined according to Bradford [20].
2.6. Microbiological Analyses
In GOT, a stimulated saliva sample was collected by chewing on a piece of paraffin for 5 min. The saliva sample was within one hour handled at the laboratory for microbial analyses. The samples were dispersed on a Whirlimixer, diluted in 10-fold stages in a potassium phosphate buffer and plated in duplicate on MSB agar (mutans streptococci), MS agar (total streptococci), Rogosa SL agar (lactobacilli), blood agar (total viable count). After being incubated in its respective atmosphere, the number of colony-forming units (CFU) was counted. The number of mutans streptococci was identified by their characteristic colony morphology on the MSB agar.
At ACTA, all visible plaque was collected from a buccal surface of the upper first molar using a Teflon spatula. Plaque was put into sterile Eppendorf tubes and kept on ice until stored at −80°C. Samples were sent on dry ice to the Department of Microbial Diseases (UCL Eastman Dental Institute, University College, London, UK) for analyses of microbiological composition. The numbers of Streptococcus sanguinis, Streptococcus mutans, Lactobacillus casei, Veillonella dispar, Neisseria subflava, Actinomyces naeslundii, Prevotella intermedia, Fusobacterium nucleatum, and total bacterial 16SrDNA were determined by using multiplex quantitative PCR (qPCR) [21]. In brief, DNA was extracted from plaque biofilms using a phenol : chloroform : isoamyl alcohol (25 : 24 : 1) bead-beating extraction method [22], which involves physical cell lysis, protein removal, and finally DNA precipitation using polyethylene glycol. Three triplex qPCR assays were then carried out using 2 μL of extracted DNA to enumerate eight oral taxa as well as the total number of organisms. The assays were performed using the Rotor-Gene 6500 (QIAGEN) instrument and Sensimix Probe (Bioline) qPCR mix according to the manufacturers instructions, using previously published oligonucleotide sequences [20].
2.7. Plaque Index Amount
The plaque score was in GOT calculated using the Turesky modification of the Quigley-Hein index (TQHPI-index) [23]. The toothsurface coverage with plaque was for each tooth scored on six surfaces (mesiobuccal, buccal, distobuccal, distolingual, lingual, and mesiolingual) on a scale of 0–5. At ACTA, a modification of the Silness and Löe plaque index was used [24]. All buccal and lingual areas in the lower jaw were assessed for each tooth at six sites (mesiobuccal, buccal, distobuccal, distolingual, lingual, and mesiolingual) on a scale of 0–3.
2.8. Questionnaire
At the end of each test period, the volunteers were requested to complete a questionnaire with a Visual Analogue Scale (VAS) with a total of nine questions related to their experience of using the assigned mouth rinse solution. They marked their answer on a 100 mm line with the negative extreme on the left and the positive extreme on the right.
2.9. Statistical Analyses
The mean ± SD of all clinical parameters and individuals as calculated. For plaque pH, the mean of the values for the left and right side was collected. From each pH curve, the area under the curve (AUC5.7 and AUC6.2), minimum-pH, and maximum-pH decrease was calculated. For the plaque score, the mean score for each tooth was first calculated, after which the mean score for the whole dentition was calculated. Protein content was expressed in μg and the amount of organic acids as μmol/mg protein. For ACTA, the total number of the different microorganisms was calculated. For GOT, all microbiological data were transformed to logarithmic values. The distribution of mutans streptococci and total streptococci in comparison to the total streptococcal flora and total oral flora (%), respectively, was also calculated. For ACTA, the Log10 CFU was calculated. For the answers on the VAS, the distance (in mm) from the left side was measured for each question and a mean score was calculated.
In GOT, two-way analysis of variance, ANOVA, was used to test the significance of differences between the seven test occasions (after each test period and the washout periods). When ANOVA rejected the multisample hypothesis of equal means, multiple comparison testing was performed using Fisher's PLSD. P < 0.05 was regarded as statistically significant.
At ACTA, a paired t-test was used to compare the amounts of different organic acids in resting and fermented plaque from the same visit. The General Linear Model Repeated Measures Test and the Bonferroni post-hoc test were used to compare the output parameters (amount of each acid, relative abundance of oral microorganisms, protein amount) after each of the three treatment periods and separately from the test periods, that is, between pre-experimental baseline and each consecutive washout period. The difference between the mean plaque score at the start of each test period and upon completion of each test period was calculated and used as an input variable in GLM-RM test.
3. Results
3.1. Volunteers
All 30 and 35 individuals, respectively, completed the study, apart from the final washout period for one subject in GOT. The mean age of the volunteers was 31 ± 13 years (mean ± SD) at GOT, including 19 females/11 males, and 23 ± 3 years (mean ± SD) with 32 females/3 males at ACTA.
3.2. Plaque Acidogenicity
The most pronounced metabolic activity for the sucrose rinse at the end of the three test periods was found after rinsing with the placebo, and the least attenuated pH fall was found for the positive control (AmF-SnF2), while the active compound (shiitake) resulted in an intermediate position (Figure 1). A statistically significant difference when comparing the pH values at the different time points was found at 2 min between shiitake and placebo (P < 0.05). In the case of minimum pH, there was also a numerical difference between the three products, with a difference of 0.2 pH units between shiitake and placebo and the positive control, respectively (ns). Minor differences in plaque acidogenicity were found when evaluating the maximum pH decrease, as well as AUC5.7 and AUC6.2. Only minor numerical differences in plaque acidogenicity were found when comparing the results for the four washout periods (baseline and posttreatment; ns).
Figure 1.
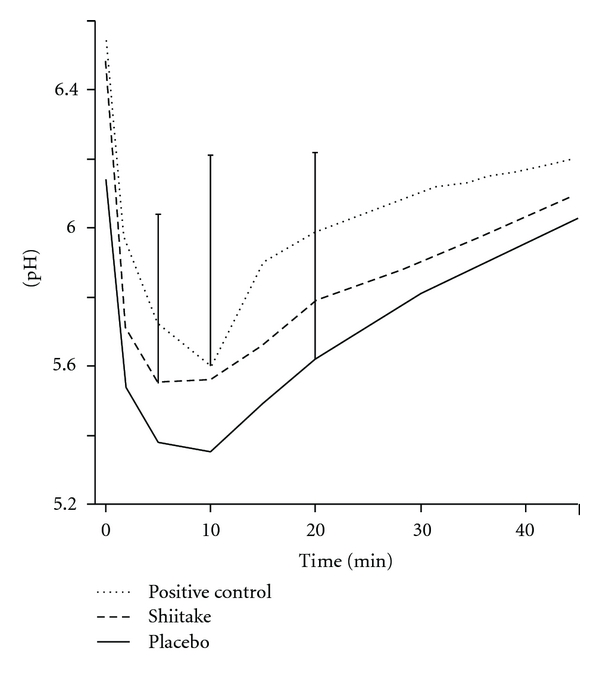
The changes in dental plaque pH up to 45 min after a mouth rinse with 10% sucrose for 1 min. The rinse was carried out after two weeks use of a mouth rinse with a shiitake mushroom extract, a placebo, or positive control (AmF-SnF2). Mean values for 30 subjects. The standard deviation for some of the time points is shown.
3.3. Protein and Organic Acids in Plaque
There was no difference between shiitake and placebo in plaque protein mass, while the positive control (AmF-SnF2) resulted in significantly less plaque protein than the test or placebo rinse (P < 0.001). No significant differences in plaque protein were found between the four washout periods. In the case of ACTA, there were no differences in the amount of protein from resting versus fermented plaque, except for the positive control washout period, where less protein was found in the resting plaque (P < 0.01). For GOT, there was a tendency towards a generally somewhat lower protein content for the resting plaque (ns). The protein plaque content varied for the seven test periods and two conditions (resting and fermented) for GOT between 14.7 ± 0.1 and 40.3 ± 31.4 μg and for ACTA between 30.3 ± 23.5 and 81.2 ± 65.3 μg. Significantly less protein was found for the positive control compared with the other two test products (P < 0.001) (ACTA).
The profiles of acetate, lactate, and minor acids (propionate, formate, succinate, and butyrate) for the resting and fermented plaque gave similar values for GOT and ACTA (Figure 2). The highest values for all the test sessions were found for lactate for fermented plaque, with a larger variation for GOT compared with ACTA. The rinse period of the positive control (AmF-SnF2) resulted in significantly less lactate and acetate for fermented plaque compared with shiitake and placebo for GOT (P < 0.01). The corresponding data for ACTA showed that the positive control period resulted in significantly less lactate and minor acids in fermented plaque compared with shiitake and placebo (P < 0.001). A higher amount of minor acids was found in resting plaque after the shiitake rinse compared with the positive control for ACTA (P < 0.01), while no such effect was seen for GOT.
Figure 2.
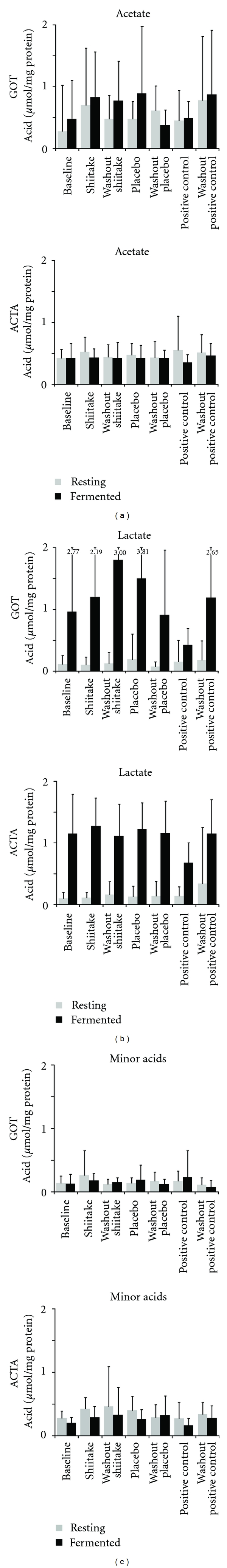
Amount of acetate, lactate, and minor acids (propionate, formate, succinate, and butyrate) in resting (presucrose) and fermented (postsucrose) dental plaque. After baseline, after the three legs of crossover (shiitake, placebo, positive control [AmF-SnF2]) and after three washout periods (washout shiitake, washout placebo, and washout positive control) are all shown. Data are shown separately for volunteers in Gothenburg (GOT) and Amsterdam (ACTA). Mean values ± SD for 30 (GOT) and 35 (ACTA) subjects, respectively. Due to the high standard deviation when analysing lactate in fermented plaque for five of the test sessions in GOT, the y-axis does not correspond to the actual figure. The total amount (mean ± SD) is given above each individual column.
3.4. Microbiological Analyses
All the microbial data are presented in Table 1. For salivary microorganisms in GOT, no statistically significant differences were found for lactobacilli or mutans streptococci in saliva between the three test periods. Rinsing with the positive control (AmF-SnF2) resulted in a significantly lower number of oral streptococci (P < 0.05) and total number of microorganisms (P < 0.001) when compared with the shiitake rinse. The lowest proportion of mutans streptococci in comparison to the total number of streptococci was found for shiitake (ns). No significant differences were found for any of the groups of oral microorganisms or the proportion of bacteria when comparing baseline and posttreatment washout periods.
Table 1.
Number of salivary and plaque microorganisms and proportions of microorganisms after baseline, the three test periods (shiitake, placebo, positive control (AmF-SnF2; Pos Ctrl)) and three washout periods (washout shiitake, washout placebo, and washout positive control) for GOT (n = 30) and ACTA (n = 35). Mean ± SD.
| City/microorganisms | Test session | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | Shiitake | Washout | Placebo | Washout | Pos. Ctrl. | Washout | |
| shiitake | placebo | Pos. Ctrl. | |||||
| GOT | |||||||
| Mutans streptococci (log CFU/mL) | 4.8 ± 1.2 | 5.0 ± 1.0 | 4.8 ± 1.0 | 4.9 ± 1.1 | 4.9 ± 1.2 | 4.5 ± 1.2 | 4.8 ± 1.0 |
| Lactobacilli (log CFU/mL) | 3.6 ± 1.4 | 3.8 ± 1.3 | 3.8 ± 1.2 | 3.8 ± 1.3 | 3.7 ± 1.4 | 3.8 ± 1.4 | 3.5 ± 1.3 |
| Total streptococci (log CFU/mL) | 7.4 ± 0.5 | 7.6 ± 0.4 | 7.5 ± 0.4 | 7.5 ± 0.4 | 7.6 ± 0.3 | 7.2 ± 0.81 | 7.5 ± 0.4 |
| Total oral flora (log CFU/mL) | 8.0 ± 0.3 | 8.1 ± 0.3 | 8.1 ± 0.3 | 8.0 ± 0.3 | 8.1 ± 0.3 | 7.8 ± 0.63 | 8.0 ± 0.3 |
| Total streptococci/total flora (%) | 35.2 ± 19.4 | 41.0 ± 19.8 | 30.1 ± 16.0 | 32.5 ± 12.6 | 35.0 ± 19.0 | 31.9 ± 30.2 | 40.4 ± 23.7 |
| Mutans streptococci/total streptococci (%) | 1.1 ± 1.7 | 0.7 ± 0.9 | 1.1 ± 2.5 | 0.8 ± 1.1 | 1.1 ± 1.6 | 0.8 ± 1.5 | 0.7 ± 1.4 |
|
| |||||||
| ACTA | |||||||
| Universal probe counts (log10 CFU) | 7.8 ± 0.3 | 7.9 ± 0.5 | 7.8 ± 0.5 | 7.8 ± 0.5 | 7.8 ± 0.6 | 7.1 ± 0.83 | 7.9 ± 0.4 |
| L. casei (log10 CFU) | 2.1 ± 1.0 | 1.6 ± 1.2 | 1.3 ± 1.2 | 1.9 ± 1.0 | 1.5 ± 1.1 | 1.5 ± 1.13 | 1.4 ± 1.2 |
| V. dispar (log10 CFU) | 6.5 ± 0.6 | 6.6 ± 0.9 | 6.5 ± 0.7 | 6.5 ± 0.8 | 6.6 ± 0.8 | 5.4 ± 1.13 | 6.5 ± 0.6 |
| N. subflava (log10 CFU) | 6.7 ± 0.9 | 6.4 ± 1.12 | 6.7 ± 0.9 | 6.7 ± 0.9 | 6.7 ± 0.9 | 5.1 ± 1.01, 3 | 6.9 ± 0.7 |
| A. naeslundii (log10 CFU) | 5.1 ± 1.2 | 5.1 ± 1.0 | 5.0 ± 1.1 | 5.0 ± 1.2 | 5.0 ± 1.3 | 4.0 ± 1.63 | 4.9 ± 1.0 |
| P. intermedia (log10 CFU) | 0.7 ± 1.1 | 0.7 ± 1.2 | 0.8 ± 1.3 | 0.6 ± 1.0 | 0.8 ± 1.2 | 0.1 ± 0.63 | 0.7 ± 1.3 |
| S. sanguinis (log10 CFU) | 5.9 ± 0.5 | 5.9 ± 0.6 | 5.7 ± 0.6 | 5.8 ± 0.6 | 5.9 ± 0.7 | 4.7 ± 1.01, 3 | 5.9 ± 0.5 |
| S. mutans (log10 CFU) | 1.3 ± 2.2 | 1.4 ± 2.4 | 1.6 ± 2.4 | 1.2 ± 2.2 | 1.6 ± 2.5 | 0.7 ± 1.8 | 1.3 ± 2.4 |
1Statistically significantly different from shiitake group (GOT P < 0.05 (ANOVA), ACTA P < 0.01 (GLM-RM test)).
2Statistically significantly different from placebo group (ACTA P < 0.05 (GLM-RM test)).
3Statistically significantly different from shiitake and placebo groups (GOT P < 0.001 respective P < 0.01 (ANOVA), ACTA P < 0.001 (GLM-RM test)).
For plaque samples from ACTA analysed by PCR assays, Neisseria subflava was the most predominant microorganism of all the tested organisms, followed by Veillonella dispar and Fusobacterium nucleatum. There were significantly fewer microbial cells and individual organisms in the panel, apart from S. mutans counts in plaque samples collected after positive control than after shiitake or placebo periods (P < 0.001). The shiitake mouth rinse reduced the proportion of N. subflava significantly compared with the placebo rinse (P < 0.05), while N. subflava and S. sanguinis were significantly reduced by the positive control compared with shiitake (P < 0.01).
3.5. Plaque Index Score
The plaque scores are shown in Tables 2 and 3. For GOT, the three test periods resulted in the numerically lowest plaque scores (ns), while the positive control resulted in significantly less plaque than shiitake and placebo (P < 0.001) for ACTA. For the washout periods, significantly less plaque was found for GOT after the shiitake washout period compared with placebo (P < 0.05). For ACTA, significantly less plaque was found at the overall preexperimental baseline compared with the other three washout periods (P < 0.001).
Table 2.
Quigley-Hein plaque index score (Turesky modification 1970) (mean ± SD) after baseline, the three legs of the crossover (shiitake, placebo, positive control (AmF-SnF2; Pos Ctrl)) and three washout periods (washout shiitake, washout plaque, and washout positive control) for GOT (n = 30). Mean ± SD.
| City | Test session | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | Shiitake | Washout | Placebo | Washout | Pos. Ctrl. | Washout | |
| shiitake | placebo | Pos. Ctrl. | |||||
| GOT | 2.0 ± 0.9 | 1.7 ± 0.8 | 1.8 ± 0.8 | 1.7 ± 0.8 | 1.9 ± 0.8 | 1.6 ± 0.6 | 1.8 ± 0.81 |
1Statistically significantly different from the placebo test period (P < 0.001, paired samples t-test).
Table 3.
Silness & Löe plaque index score (as modified by Danser et al. [24]) before and after each of the three legs of the crossover (shiitake, placebo, positive control (AmF-SnF2)) and three washout periods (washout shiitake, washout plaque, and washout positive control) for ACTA (n = 35). Mean ± SD.
| Shiitake | Placebo | Positive control | |
|---|---|---|---|
| Plaque score before test period | 1.5 ± 0.4 | 1.5 ± 0.4 | 1.5 ± 0.3 |
| Plaque score after test period | 1.6 ± 0.3 | 1.6 ± 0.3 | 1.2 ± 0.41 |
| Plaque score after washout | 1.6 ± 0.3 | 1.7 ± 0.2 | 1.6 ± 0.3 |
| The mean difference in plaque score before and after test period | −0.1 ± 0.4 | −0.1 ± 0.3 | 0.3 ± 0.52 |
1Statistically significantly different from the respective plaque score obtained before the test period (P < 0.001, paired samples t-test).
2Statistically significant plaque score reduction compared to other test periods (P < 0.001, GLM-RM; Bonferroni post-hoc test).
3.6. Questionnaire
Taste experience was, when marked from very poor to very good, described as significantly worse by the volunteers after the shiitake test period (GOT 47.0 ± 32.7, ACTA 3.0 ± 4.6; mean ± SD) compared to both the placebo (GOT 62.4 ± 22.4, ACTA 59.2 ± 21.5) and positive control (GOT 70.6 ± 18.9, ACTA 54.9 ± 25.6); rinses for both GOT and ACTA (P < 0.01 or P < 0.001). Similar significant differences were found for duration of the taste, taste perception, and experienced rinsing time (data not shown). A significantly higher perception of sensitivity of the teeth after shiitake (36.2 ± 27.2) compared to the positive control (19.6 ± 27.7) and higher perception of staining after shiitake (24.8 ± 27.2) in comparison to the placebo (12.1 ± 18.8) rinse (P < 0.05) was reported for ACTA. The burning sensation of the mouth was also significantly higher after the shiitake (GOT 27.4 ± 28.6, ACTA, 43.2 ± 30.8) and placebo (GOT 29.1 ± 31.5, ACTA 38.2 ± 29.1) rinses compared to the positive control (GOT 12.6 ± 18.8, ACTA 15.0 ± 23.4) for both test series (GOT P < 0.01, ACTA P < 0.002).
4. Discussion
The scientific approach and study design of this paper are based on the results of previous studies performed within the Nutrident project. The study was planned as a consequence of the initial chemical characterization of the shiitake mushroom [15], evaluation of the fractions and subfractions of the shiitake mushroom, and further evaluation in different in vitro settings [16, 25]. Due to different technical limitations at each of the two centres, the study did not follow the design of a multicentre approach. However, the fact that the conclusions are based on the results from 30 and 35 volunteers from two international centres strengthens its scientific value. No direct comparison of the subjects from the perspective of caries activity was made. However, the additional inclusion criteria in GOT, where the subjects are known to have reduced their plaque pH by at least one pH unit after a sugar rinse, indicates that these subjects may have higher caries activity. This hypothesis is also supported by their higher bacterial counts and plaque scores. The possibility that the numerical variation seen between the two substudies may be a consequence of the selection of subjects cannot, therefore, be ruled out. Due to the above-mentioned factors, the data have been handled separately for GOT and ACTA.
The main finding in this study is that rinsing twice daily with a natural food extract may reduce the metabolic activity of the dental biofilm. Although not evaluated in the present study, a reduction of this kind may result in the long term in a lower degree of demineralisation. This is supported by recent data where a subfraction of shiitake showed a strong inhibiting effect on dentine demineralisation when evaluated in an environment using saliva-derived microcosms [16]. There may be multiple explanations for the present findings of a change in the acidogenic potential of the biofilm. Previous work focusing on shiitake mushroom extract has demonstrated biological activity relevant to caries prevention [16, 25]. This includes mechanisms such as bactericidal activity against cariogenic microorganisms, the prevention of the coaggregation of cariogenic microorganisms, the induction of the detachment of cariogenic microorganisms from hydroxyapaite, and changes in cell surface hydrophobicity.
The antimicrobial, antiadhesive, and antiplaque properties of polyphenol-rich beverages have previously been demonstrated [26, 27]. Recent studies have focused on the oral health variables of tea in particular, both when consumed naturally or when evaluating tea and cranberry in an in vitro or in vivo design [28–31]. Similar findings relating to the plaque-lowering potential have been found both after using both different sweeteners [32] and essential oils [33].
The interpretation of the acid anion profiles of the resting and fermented plaque is complicated. Although a corresponding pattern when comparing the data with the results from the plaque-pH measurements would have seemed logical, it was difficult to obtain a clear and consistent picture from the current data. This may be related to the low “in vivo” activity of the food compound that was tested, a poor cooperation of the subjects, or a weak experimental design. When evaluating the same low-molecular-weight fraction of the shiitake mushroom in an in vitro caries model, a stronger inhibitory effect on acid production potential was observed by one of the subfractions (SF4) in comparison to the whole low-molecular-weight fraction [16].
Microbiological analyses included both the total cell count and bacteria related to periodontal diseases, dental caries, and oral health. Only minor differences in both the salivary levels (GOT) and the plaque levels (ACTA) of oral microorganisms were found between the different visits. The numerically lowest salivary number of mutans streptococci in comparison to the total number of streptococci was found for shiitake. For plaque microflora, significantly reduced proportions of microorganisms were only found for the Gram-negative organism N. subflava when comparing the shiitake mouth rinse with placebo. These findings are supported by a recent study in which 11 days of frequent mouth rinses with the same mushroom extract resulted in a reduced amount of plaque but a weaker effect on the decrease in total bacterial counts as well as some specific oral pathogens when compared with a placebo test period [34].
While GOT found a significant reduction in plaque score when comparing shiitake with placebo, no such difference was found for ACTA. However, a reduction in dental plaque deposition has also been found when evaluating the active compound against gingivitis- and periodontitis-related variables [34]. This finding is furthermore supported by previous studies in which inhibited plaque formation was found when using mouth rinses of oolong tea [35] and pomegranate [36]. Neither the mushroom extract nor the placebo was capable of reducing plaque formation to the same degree as chlorhexidine, an antimicrobial compound known to inhibit biofilm development and maturation [37].
The subjects reported a less favourable outcome for the different questions related to taste for the shiitake extract mouth rinse. All the subjects gave an assurance that they had followed the given instructions. However, following the reported negative reaction to the taste of the shiitake mouth rinse by a large number of the volunteers, one cannot exclude that this may have had a negative impact on compliance. As a consequence, some of the subjects may not have rinsed with the active compound according to instructions and that they may have rinsed their mouth with water shortly after using the active substance cannot be excluded. In order to secure the regular use of potential future products, it is important that this aspect is also considered seriously, as this factor alone may determine whether or not an oral health product is used. Aspects related to food safety also need to be taken into account.
Functional foods have not been introduced in order to replace traditional caries-prevention strategies but instead to add another tool to offer patients at higher risk. The positive finding of reduced plaque fermentation activity indicates that there is an opportunity to add one more strategy to the palette of preventive methods. It is not surprising that a stronger effect was found for this variable and that only a limited effect was found for several of the other variables. The metabolic activity of the dental biofilm is the end result of a large number of biological and biochemical caries-related factors. As shown by previous laboratory work [16, 25], the active compounds of shiitake mushroom may exert multiple actions on different caries-related variables. Even if the effect of each of them may appear weak, they may interfere in a positive way in the complex and diverse microbial community constituted by the biofilm.
The limited effect on several dental biofilm properties seen in the present study may indicate that frequent exposure for a longer period is needed. One important factor is believed to be the contact time between the active compound and the different oral properties. The repeated rinsing with 10 + 10 mL for 30 + 30 sec was used to diminish the dilution effect of saliva and to prolong the contact time of active compounds with the oral cavity. For this reason, both further laboratory and clinical studies are needed in order to evaluate not least the effect of a longer exposure period or variations in the concentration of these naturally derived biologically active compounds.
5. Conclusions
The main finding of this study is that frequent mouth rinses with a natural food extract (shiitake mushroom) may reduce the metabolic activity of the dental biofilm. Only a limited effect on other dental plaque properties related to the caries disease was found and not to the same extent as the positive control.
Acknowledgments
The research leading to these results has received funding from the European Union's Sixth Framework Programme (FP6) under the contract FOOD-CT-2006-036210 (project NUTRIDENT). Sincere thanks to Ann-Charlotte Börjesson and Ann-Britt Lundberg, Department of Cariology, University of Gothenburg, for technical support. The clinical and logistic support of Nienke Hennequin-Hoenderdos, Department of Periodontology, Academic Centre for Dentistry Amsterdam, is gratefully acknowledged.
References
- 1.Lingström P, van Ruyven FOJ, van Houte J, Kent R. The pH of dental plaque in its relation to early enamel caries and dental plaque flora in humans. Journal of Dental Research. 2000;79(2):770–777. doi: 10.1177/00220345000790021101. [DOI] [PubMed] [Google Scholar]
- 2.Kleinberg I. Controversy: a mixed-bacteria ecological approach to understanding the role of the oral bacteria in dental caries causation: an alternative to Streptococcus mutans and the specific-plaque hypothesis. Critical Reviews in Oral Biology and Medicine. 2002;13(2):108–125. doi: 10.1177/154411130201300202. [DOI] [PubMed] [Google Scholar]
- 3.Selwitz RH, Ismail AI, Pitts NB. Dental caries. The Lancet. 2007;369(9555):51–59. doi: 10.1016/S0140-6736(07)60031-2. [DOI] [PubMed] [Google Scholar]
- 4.SBU. Prevention of dental caries: a systematic review. The Swedish Council on Technology Assessment in Health Care. 2002;(, Report 161)
- 5.Marsh PD. Dental plaque as a biofilm and a microbial community—implications for health and disease. BMC Oral Health. 2006;6, supplement 1, article S14 doi: 10.1186/1472-6831-6-S1-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Autio-Gold J. The role of chlorhexidine in caries prevention. Operative Dentistry. 2008;33(6):710–716. doi: 10.2341/08-3. [DOI] [PubMed] [Google Scholar]
- 7.van Loveren C. Sugar alcohols: what is the evidence for caries-preventive and caries-therapeutic effects? Caries Research. 2004;38(3):286–295. doi: 10.1159/000077768. [DOI] [PubMed] [Google Scholar]
- 8.Buzalaf MA, Pessan JP, Honòrio HM, ten Cate JM. Mechanisms of action of floride for caries control. Monographic Oral Sciences. 2011;22:97–114. doi: 10.1159/000325151. [DOI] [PubMed] [Google Scholar]
- 9.Newman DJ. Natural products as leads to potential drugs: an old process or the new hope for drug discovery? Journal of Medicinal Chemistry. 2008;51(9):2589–2599. doi: 10.1021/jm0704090. [DOI] [PubMed] [Google Scholar]
- 10.Ferrazzano GF, Amato I, Ingenito A, Zarrelli A, Pinto G, Pollio A. Plant polyphenols and their anti-cariogenic properties: a review. Molecules. 2011;16(2):1486–1507. doi: 10.3390/molecules16021486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Signoretto C, Canepari P, Pruzzo C, Gazzani G. Anticaries and antiadhesive properties of food constituents and plant extracts and implications for oral health. In: Wilson M, editor. Food Constituents and Oral Health: Current Status and Future Prospects. Cambridge, UK: Woodhead Publishing Limited; 2009. [Google Scholar]
- 12.Shouji N, Takada K, Fukushima K, Hirasawa M. Anticaries effect of a component from shiitake (an edible mushroom) Caries Research. 2000;34(1):94–98. doi: 10.1159/000016559. [DOI] [PubMed] [Google Scholar]
- 13.Venturini ME, Rivera CS, Gonzalez C, Blanco D. Antimicrobial activity of extracts of edible wild and cultivated mushrooms against foodborne bacterial strains. Journal of Food Protection. 2008;71(8):1701–1706. doi: 10.4315/0362-028x-71.8.1701. [DOI] [PubMed] [Google Scholar]
- 14.van Nevel CJ, Decuypere JA, Dierick N, Molly K. The influence of Lentinus edodes (Shiitake mushroom) preparations on bacteriological and morphological aspects of the small intestine in piglets. Archives of Animal Nutrition. 2003;57(6):399–412. doi: 10.1080/0003942032000161054. [DOI] [PubMed] [Google Scholar]
- 15.Daglia M, Papetti A, Mascherpa D, et al. Plant and fungal food components with potential activity on the development of microbial oral diseases. Journal of Biomedicine and Biotechnology. 2011;2011:9 pages. doi: 10.1155/2011/274578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaura E, Buijs MJ, Hoogenkamp MA, et al. The effects of fractions from shiitake mushroom on composition and cariogenicity of dental plaque microcosms in an in vitro caries model. doi: 10.1155/2011/135034. Journal of Biomedicine and Biotechnology. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerardu VAM, van Loveren C, Heijnsbroek M, Buijs MJ, van der Weijden GA, Ten Cate JM. Effects of various rinsing protocols after the use of amine fluoride/stannous fluoride toothpaste on the acid production of dental plaque and tongue flora. Caries Research. 2006;40(3):245–250. doi: 10.1159/000092233. [DOI] [PubMed] [Google Scholar]
- 18.Lingström P, Imfeld T, Birkhed D. Comparison of three different methods for measurement of plaque-pH in humans after consumption of soft bread and potato chips. Journal of Dental Research. 1993;72(5):865–870. doi: 10.1177/00220345930720050601. [DOI] [PubMed] [Google Scholar]
- 19.Scheie AA, Fejerskov O, Lingström P, Birkhed D, Manji F. Use of palladium touch microelectrodes under field conditions for in vivo assessment of dental plaque pH in children. Caries Research. 1992;26(1):44–51. doi: 10.1159/000261426. [DOI] [PubMed] [Google Scholar]
- 20.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Analytical Biochemistry. 1976;72(7):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 21.Ciric L, Pratten J, Wilson M, Spratt D. Development of a novel multi-triplex qPCR method for the assessment of bacterial community structure in oral populations. Environmental Microbiology Reports. 2010;2(6):770–774. doi: 10.1111/j.1758-2229.2010.00183.x. [DOI] [PubMed] [Google Scholar]
- 22.Griffiths RI, Whiteley AS, O’Donnell AG, Bailey MJ. Rapid method for coextraction of DNA and RNA from natural environments for analysis of ribosomal DNA- and rRNA-based microbial community composition. Applied and Environmental Microbiology. 2000;66(12):5488–5491. doi: 10.1128/aem.66.12.5488-5491.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turesky S, Gilmore ND, Glickman I. Reduced plaque formation by the chloromethyl analogue of victamine C. Journal of Periodontology. 1970;41(1):41–43. doi: 10.1902/jop.1970.41.41.41. [DOI] [PubMed] [Google Scholar]
- 24.Danser MM, Timmerman MF, Jzerman Y, Piscaer MI, van der Velden U, van der Weijden GA. Plaque removal with a novel manual toothbrush (X-Active) and the Braun Oral-B 3D Plaque Remover. Journal of Clinical Periodontology. 2003;30(2):138–144. doi: 10.1034/j.1600-051x.2003.00114.x. [DOI] [PubMed] [Google Scholar]
- 25.Spratt D, O'Donnell D, Ciric L, et al. Evaluation of fungal extracts for their anti-gingivitis and anti-caries activity. doi: 10.1155/2012/510198. Submitted to Journal of Biomedicine and Biotechnology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu CD, Wei GX. Tea as a functional food for oral health. Nutrition. 2002;18(5):443–444. doi: 10.1016/s0899-9007(02)00763-3. [DOI] [PubMed] [Google Scholar]
- 27.Yoo S, Murata RM, Duarte S. Antimicrobial traits of tea- and cranberry-derived polyphenols against Streptococcus mutans. Caries Research. 2011;45(4):327–335. doi: 10.1159/000329181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamilton-Miller JMT. Anti-cariogenic properties of tea (Camellia sinensis) Journal of Medical Microbiology. 2001;50(4):299–302. doi: 10.1099/0022-1317-50-4-299. [DOI] [PubMed] [Google Scholar]
- 29.Elvin-Lewis M, Steelman R. The anticariogenic effects of tea drinking among Dallas school children. Journal of Dental Research. 1986;65(3):p. 198. [Google Scholar]
- 30.Weiss EI, Kozlovsky A, Steinberg D, et al. A high molecular mass cranberry constituent reduces mutans streptococci level in saliva and inhibits in vitro adhesion to hydroxyapatite. FEMS Microbiology Letters. 2004;232(1):89–92. doi: 10.1016/S0378-1097(04)00035-7. [DOI] [PubMed] [Google Scholar]
- 31.Koo H, Duarte S, Murata RM, et al. Influence of cranberry proanthocyanidins on formation of biofilms by Streptococcus mutans on saliva-coated apatitic surface and on dental caries development in vivo. Caries Research. 2010;44(2):116–126. doi: 10.1159/000296306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lif Holgerson P, Stecksén-Blicks C, Sjöström I, Twetman S. Effect of xylitol-containing chewing gums on interdental plaque-pH in habitual xylitol consumers. Acta Odontologica Scandinavica. 2005;63(4):233–238. doi: 10.1080/00016350510019883. [DOI] [PubMed] [Google Scholar]
- 33.Albertsson KW, Persson A, Lingström P, van Dijken JWV. Effects of mouthrinses containing essential oils and alcohol-free chlorhexidine on human plaque acidogenicity. Clinical Oral Investigations. 2010;14(1):107–112. doi: 10.1007/s00784-009-0273-5. [DOI] [PubMed] [Google Scholar]
- 34.Signoretto C, Burlacchini G, Marchi A, et al. Testing a low molecular mass fraction of a mushroom (Lentinus edodes) extract formulated as an oral rinse in a cohort of volunteers. doi: 10.1155/2011/857987. Journal of Biomedicine and Biotechnology. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ooshima T, Minami T, Aono W, Tamura Y, Hamada S. Reduction of dental plaque deposition in humans by oolong tea extract. Caries Research. 1994;28(3):146–149. doi: 10.1159/000261636. [DOI] [PubMed] [Google Scholar]
- 36.Bhadbhade SJ, Acharya AB, Rodrigues SV, Thakur SL. The antiplaque efficacy of pomegranate mouthrinse. Quintessence International. 2011;42(1):29–36. [PubMed] [Google Scholar]
- 37.Baehni PC, Takeuchi Y. Anti-plaque agents in the prevention of biofilm-associated oral diseases. Oral Diseases. 2003;9, supplement 1:23–29. doi: 10.1034/j.1601-0825.9.s1.5.x. [DOI] [PubMed] [Google Scholar]


