Abstract
Alterations of B cell subpopulations have been described up to date as characterizing advanced stage of HIV-1 infection. However, whether such defects are relevant in subjects with a preserved number of CD4+ T cells (>350 cells/μL) is unclear. In a cross-sectional study, we investigated if signs of B cells exhaustion and impaired viral immune surveillance are present in a cohort of 43 asymptomatic HIV-1-infected patients with preserved CD4+ T cell counts (>350 cells/μL) and highly active antiretroviral therapy (HAART) untreated. A dramatic expansion of exhausted tissue-like memory B cells (CD10−CD21lowCD27−) was observed. B cells alteration was related to an increase in Torque teno virus (TTV) load, used as surrogate marker of immune function. Successfully HAART-treated patients showed normalization of B cell subpopulations frequency and TTV load. These results provide new insights on B cell in HIV-1 infection and show that development of B cell abnormalities precedes CD4+ T cell decline.
1. Introduction
Through direct or indirect effects, HIV-1 infection leads to several perturbations on most cells of the immune system. The persistent viral replication induces loss of CD4+ T cells and a general chronic cellular activation that affect viability, subsets distribution, phenotype, and function of all the major immune cell populations [1]. B cells exhibit numerous effects of HIV-1-induced immune activation. Increased expression of activation and proliferation markers, polyclonal B cell hyperactivation, and hypergammaglobulinemia are some of the B cell defects detectable in HIV-1-infected patients [2, 3]. Moreover, B cells derived from HIV-1-infected patients show several alterations in vitro, as high spontaneous antibodies secretion, defective responses to B cell stimuli, and impaired costimulatory functions [4–8]. These functional defects have been recently linked to perturbation in the frequency of several B cell subpopulations. Naïve B cells (CD21highCD27−) constitute the largest B cell population in the blood of healthy individuals, followed by memory B cells (CD21highCD27+). The frequency of memory B cells is strongly decreased in HIV-1-infected individuals, and this occurrence is accompanied by an increased levels of naïve B cells, activated mature B cells (CD20+CD21lowCD27+), and plasma cells (CD20−CD21lowCD27+) [9–11]. Moreover, a relevant expansion of B cell subpopulations characterized by a CD21lowCD27− phenotype has been recently described in chronically infected patients in advanced stage of HIV-1 infection with a pronounced CD4+ T cell lymphopenia and ongoing HIV-1 replication [12]. This B cell population, which is normally present at low frequency in the peripheral blood of healthy individuals, can be further divided, depending on the expression of CD10, into immature/transitional B cells (CD10+) or exhausted tissue-like memory B cells (CD10−) [13–15].
Torque teno virus (TTV) is a single-strand DNA virus harmlessly carried by the majority of healthy individuals [16]. TTV is considered a surrogate marker of the immunological status of the host [17]. In fact, TTV viral load has been found significantly higher in HIV-1-infected patients presenting AIDS and decreased survival than healthy individuals [18].
Here we demonstrate that asymptomatic patients naïve for HAART with preserved CD4+ T cell counts (>350 cells/μL) show an aberrant B cell subsets distribution and a concurrent increase in TTV viral load compared to HIV-1-uninfected individuals as well as HAART successfully treated patients.
2. Materials and Methods
2.1. Study Population
Blood samples were obtained from a cohort of 43 chronic HIV-1-infected patients naïve for HAART and without any history of opportunistic infections or malignancies. Clinical features of these patients are described in Table 1. Blood samples were also obtained from a cohort of 20 HAART-treated patients, aviremic for at least 12 months (Table 2), and from 34 healthy laboratory workers. The HIV-1-infected patients enrolled in this study were recruited at the Institute of Infectious and Tropical Diseases of the University of Brescia, provided their written informed consent. The study was approved by the Ethical Committee of the University Hospital of Brescia according to the declaration of Helsinki.
Table 1.
Profiles of asymptomatic HIV-1-infected patients.
| Patients | Sex | Age (years) | HIV-1 risk group | CDC stage | HIV-1 viral load (RNA copies/mL) | CD4 T cell count (cell/μL) |
|---|---|---|---|---|---|---|
| 1 | M | 49 | Heterosex | A1 | 6342 | 500 |
| 2 | F | 33 | Heterosex | A2 | 760 | 471 |
| 3 | M | 30 | Heterosex | A1 | <50* | 650 |
| 4 | F | 40 | Heterosex | A1 | 9893 | 470 |
| 5 | F | 21 | Heterosex | A1 | 160116 | 786 |
| 6 | F | 72 | Heterosex | A1 | 2940 | 536 |
| 7 | M | 55 | Homosex/bisex | A1 | 76871 | 477 |
| 8 | M | 27 | Heterosex | A1 | 39880 | 540 |
| 9 | F | 44 | Heterosex | A1 | 9337 | 487 |
| 10 | F | 42 | Heterosex | A1 | 74 | 857 |
| 11 | F | 48 | Heterosex | B1 | 62078 | 505 |
| 12 | M | 48 | Unknown | A1 | 6778 | 384 |
| 13 | M | 28 | Unknown | A1 | 58322 | 622 |
| 14 | M | 26 | Homosex/bisex | A1 | 274979 | 550 |
| 15 | F | 36 | Drugs user | B1 | 1332 | 454 |
| 16 | M | 43 | Homosex/bisex | A2 | 27684 | 356 |
| 17 | F | 43 | Heterosex | A1 | 1201 | 417 |
| 18 | M | 30 | Drugs user | A1 | 15756 | 523 |
| 19 | M | 33 | Heterosex | A1 | 789 | 742 |
| 20 | M | 42 | Hematic product contact | A1 | 107 | 570 |
| 21 | M | 47 | Drugs user | A1 | 323 | 552 |
| 22 | M | 47 | Drugs user | A1 | <50 | 684 |
| 23 | M | 50 | Heterosex | A1 | 55681 | 510 |
| 24 | M | 55 | Heterosex | A2 | 67431 | 481 |
| 25 | M | 45 | Heterosex | A1 | 14781 | 386 |
| 26 | F | 46 | Heterosex | A1 | 143 | 494 |
| 27 | F | 54 | Heterosex | A1 | 276 | 417 |
| 28 | M | 32 | Homosex/bisex | A1 | 11091 | 685 |
| 29 | M | 28 | Heterosex | A1 | 362 | 620 |
| 30 | M | 34 | Heterosex | A1 | 16841 | 668 |
| 31 | F | 48 | Drugs user | A1 | <50 | 640 |
| 32 | M | 44 | Drugs user | A1 | <50 | 2049 |
| 33 | F | 24 | Drugs user | A2 | 49716 | 469 |
| 34 | M | 49 | Homosex/bisex | A2 | 3569 | 489 |
| 35 | F | 28 | Heterosex | A1 | 6390 | 555 |
| 36 | M | 49 | Heterosex | A1 | 487 | 834 |
| 37 | M | 39 | Heterosex | A1 | 32750 | 523 |
| 38 | M | 35 | Homosex/bisex | A2 | 1121 | 408 |
| 39 | M | 49 | Homosex/bisex | A1 | 13057 | 496 |
| 40 | M | 39 | Homosex/bisex | A2 | 40811 | 478 |
| 41 | M | 40 | Homosex/bisex | A2 | 1713 | 474 |
| 42 | M | 43 | Homosex/bisex | A2 | 29763 | 355 |
| 43 | M | 43 | Homosex/bisex | A1 | 62035 | 574 |
*<50 HIV-1 RNA copies/mL: undetectable levels.
Table 2.
Profiles of HAART-treated HIV-1-infected patients.
| Donors | Sex | Age (years) |
HIV-1 risk group | HIV-1 viral load | CD4 T cell count (cell/μL) |
CDC before therapy |
Nadir | Therapy |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 44 | Drug user | <50 | 481 | A3 | 93 | EFV+FTC+TDF |
| 2 | M | 43 | Drug user | <50 | 662 | A2 | 201 | NVP+3TC+TDF |
| 3 | F | 34 | Heterosex | <50 | 1124 | A2 | 308 | NVP+AZT+3TC |
| 4 | M | 36 | Homosex/bisex | <50 | 1031 | C3 | 9 | NVP+FTC+TDF |
| 5 | M | 46 | Drug user | <50 | 740 | C3 | 43 | AZT/r+FTC+TDF |
| 6 | M | 60 | Heterosex | <50 | 512 | C3 | 39 | AZT+3TC+ABC |
| 7 | M | 48 | Heterosex | <50 | 731 | A2 | 208 | NVP+3TC+ABC |
| 8 | M | 53 | Heterosex | <50 | 566 | A3 | 94 | EFV+FTC+TDF |
| 9 | M | 44 | Drug user | <50 | 527 | C2 | 274 | AZT/r+3TC+TDF |
| 10 | F | 41 | Heterosex | <50 | 636 | A2 | 311 | EFV+3TC+ABC |
| 11 | M | 56 | Homosex/bisex | <50 | 638 | C3 | 84 | AZT/r+3TC+ABC |
| 12 | M | 40 | Drug user | <50 | 823 | A2 | 365 | AZT+3TC+ABC |
| 13 | M | 49 | Heterosex | <50 | 754 | A3 | 23 | AZT+3TC+ABC |
| 14 | F | 31 | Drug user | <50 | 633 | A2 | 280 | FSP/r+3TC+ABC |
| 15 | F | 49 | Drug user | <50 | 846 | B3 | 57 | NVP+FTC+TDF |
| 16 | M | 39 | Homosex/bisex | <50 | 701 | A2 | 332 | AZT/r+FTC+TDF |
| 17 | F | 43 | Heterosex | <50 | 1067 | A2 | 339 | EFV+FTC+TDF |
| 18 | F | 40 | Drug user | <50 | 524 | B3 | 166 | NVP+3TC+ABC |
| 19 | M | 44 | Homosex/bisex | <50 | 701 | A2 | 239 | NVP+FTC+TDF |
| 20 | M | 44 | Heterosex | <50 | 564 | C3 | 23 | LPV/r+FTC+TDF |
<50 HIV-1 RNA copies/mL: undetectable table.
2.2. FACS Analysis
Blood samples were analyzed for 3- or 4-colors surface staining after lysis of red blood cells (FACS Lysing Solution, BD Bioscences, Milan, Italy). Monoclonal antibodies directly conjugated either to fluorescein-isothiocyanate (FITC), or phycoerythrin (PE), or peridinin-chlorophyll-protein (PerCP), or AlloPhycocyAnin (APC) specific for CD10, CD19, CD21, CD27, CD3, CD4, CD8, and CD127 were used (BD Bioscences). Flow cytometric analysis was performed using FACSCalibur flow cytometer (BD Biosciences), and 50.000 events (gated on lymphocytes) were acquired. Data were analyzed by CellQuest software.
2.3. IL-7 Serum Levels Detection
Detection of IL-7 serum concentration was performed through Quantikine HS IL-7 Immunoassay, according to the protocol provided by the supplier (R&D Systems, Minneapolis, USA).
2.4. Virus Detection and Quantification
HIV-1 plasma viral load was detected using Roche Amplicor PCR kit (RocheDiagnostic, Milan, Italy). DNA extracted from 200 μL of plasma samples was examined for TTV genome using a single step universal TaqMan real-time PCR assay as previously described [17]. The sensitivity of the assay was of 3.0 log10 DNA copies/mL of plasma. All samples were tested simultaneously in triplicate.
2.5. Statistical Analysis
Statistical analysis were performed using GraphPad Prism v4.0c for Mac OS X. The distributions of each immune parameter between healthy donors and HIV-1-infected patient cohorts were compared using the nonparametric Mann-Whitney U test. All P values were 2 sided and unadjusted. Correlation between variables was tested using Spearman's rank correlation coefficient test. For all statistical analysis, the 0.05 level of significance was used (P < 0.05). Data in the text were expressed as median.
3. Results
3.1. Several B Cell Subset Alterations in Asymptomatic and HAART-Naïve HIV-1-Infected Patients
In the present study we examined the frequency and the function of B cell populations in 43 HIV-1-infected patients characterized by being clinically asymptomatic, with CD4+ T cell count >350 cell/μL (median: 510 cell/μL), median viral load of 6779 RNA copies/mL and HAART-naïve. As control, B cell populations were examined in 34 healthy individuals.
We observed that the median of CD19+ B cell counts of the asymptomatic HIV-1-infected patients was superimposable to the median of healthy individuals (129.5 versus 143 cell/μL, resp.) (Figure 1(a)). Moreover, the frequency of B cell subsets, such as mature/activated, immature/transitional or tissue-like memory, resting/memory, and naïve B cells was analyzed in all tested individuals. Although the normal number of total B cells, several perturbations were detected in the B cell subpopulations of asymptomatic HIV-1-infected patients. As shown in Figures 1(b) and 1(c) among the CD21high B cells we observed that the frequencies of both resting/memory (CD27+), and naïve (CD27−) were significantly lower in the peripheral blood of the asymptomatic HIV-1-infected patients compared to healthy donors (median percentage: 23.5 versus 29.4, P = 0.026, and 54.2 versus 64.4, P < 0,005, resp.). However, we observed that activated/mature CD21lowCD27+ and immature/transitional or tissue-like memory CD21lowCD27− B cells were significantly higher in the asymptomatic HIV-1-infected patients compared to uninfected donors (median percentage: 9.6 versus 2.4, P < 0.0001, and 10.4 versus 2.1, P < 0.0001, resp.). A previous study from Malaspina et al. [13] showed that the frequency of CD21lowCD27− B cells was increased in HIV-1-infected patients in advanced stage of disease (CD4+T cell count <200 cell/μL). This cell subset included both CD10+elements, accounting for an immature/transitional B cell phenotype, and CD10− cells defining exhausted tissue-like memory B cells. In our cohort of asymptomatic HIV-1-infected patients the percentage of CD10+ B cells was particularly low and similar to those of healthy donors. The CD21lowCD27− B cells subtype was essentially negative for CD10, attesting for an exhausted tissue-like memory B cells phenotype (Figure 1(b)). The percentage of CD21lowCD27− B cells positively correlated to the HIV-1 viral load (r = 0.39, P = 0.012). A higher percentage of CD21lowCD27− B cells was detected in patients with high plasma viremia (>10000 RNA copies/mL), and, vice versa, a lower percentage of circulating CD21lowCD27− B cells was found in patients with low plasma viremia (<10000 RNA copies/mL) (P < 0.01) (Figure 2). No correlation was observed between the percentage of CD21lowCD27− B cells and CD4+ T cell count (r = −0.27, P = 0.085, not shown).
Figure 1.
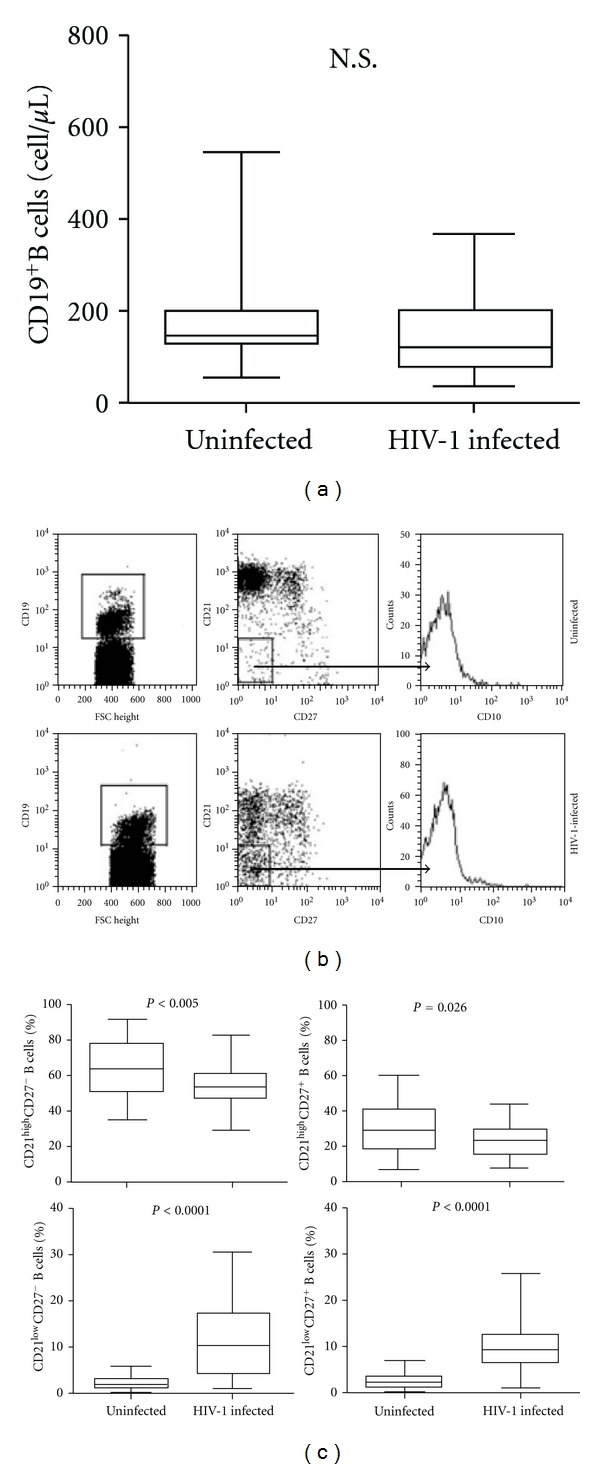
Peripheral blood B cell subsets alterations in asymptomatic HIV-1-infected patients. (a) Comparative analysis of CD19+ B cell counts (cell number/μL) in HIV-1-uninfected individuals and asymptomatic HIV-1-infected patients. (b) Analysis of CD21 and CD27 expression on B cells from HIV-1-uninfected individuals and asymptomatic HIV-1-infected patients, and analysis of CD10 expression on CD21low/CD27− B cells. Percentages of B cell subsets were determined by four-color flow cytometry analysis of CD19, CD21, CD27, and CD10 surface expression molecules. Profiles of expression of CD21 and CD27 are shown for one representative of each group. Cells were gated according to the lymphocytes forward and side scatter pattern and the CD19+ cells. (c) Comparative analysis of B cell subpopulations in HIV-1-uninfected individuals and asymptomatic HIV-1-infected patients. Frequency of CD21highCD27− naïve B cells (upper left panel), CD21highCD27+ resting memory B cells (upper right panel), CD10−CD21lowCD27− exhausted tissue-like memory B cells (lower left panel), and CD21lowCD27+ mature/activated B cells (lower right panel) are shown for the total of donors. Differences between groups were evaluated using the two-tailed Mann-Whitney U test, and were considered significant at P < 0.05. Into the box plots, horizontal lines represent median values for each group, boxes show the 25th and 75th percentiles, and bars show SD. N.S. = not significant.
Figure 2.
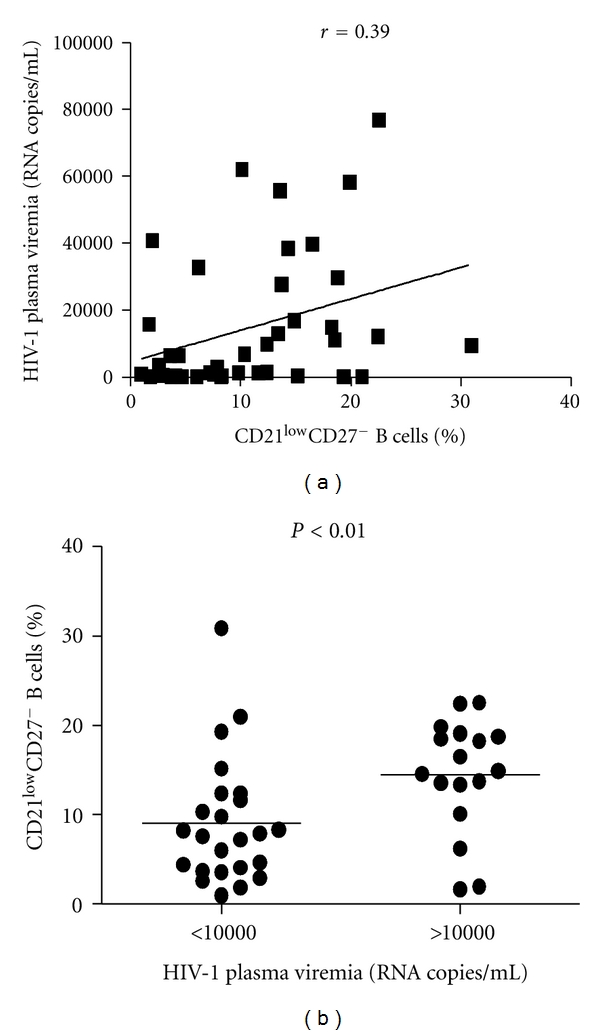
Association between exhausted tissue-like memory B cells and HIV plasma viremia. Correlation between CD10−CD21lowCD27− B cell percentages and HIV-1 plasma viremia (RNA copies/mL) in asymptomatic HIV-1-infected patients (a). Comparative analysis of CD10−CD21lowCD27− B cell percentages in asymptomatic patients with HIV-1 plasma viremia ≤ to 10000 RNA copies/mL and asymptomatic patients with HIV-1 plasma viremia > to 10000 RNA copies/mL (b). Association of 2 different data groups were analyzed by Spearman's rank test; P and rho values are indicated. Each symbol represents one individual, and regression line is shown. Differences between groups were evaluated using the two-tailed Mann-Whitney U test, and were considered significant at P < 0.05. Into the box plots, horizontal lines represent median values for each group, boxes show the 25th and 75th percentiles, and bars show SD.
3.2. Increased Levels of IL-7 in Sera of Asymptomatic HIV-1-Infected Patients
IL-7 is a pleiotropic cytokine controlling T lymphopoiesis and T cell peripheral expansion through interaction with its surface receptor (IL-7Rα or CD127) [19]. Several investigators observed increased serum levels of IL-7 in HIV-1-infected patients characterized by severe CD4+ lymphopenia, and showed direct correlation between IL-7 serum concentration and viral load, loss of CD4+ T cells, and alteration of B cell subsets [13, 20]. Moreover, the frequency of immature/transitional CD10+CD21lowCD27− B cells is positively correlated to the IL-7 concentration detected in lymphopenic HIV-1-infected patients [13]. For these reasons, high levels of IL-7 are considered a hallmark of advanced HIV-1 disease. However, we observed a dramatic increase of IL-7 serum levels also in asymptomatic HIV-1-infected patients compared to healthy individuals (median concentration: 12.9 pg/mL versus 1.7 pg/mL, P < 0.01) despite a relatively preserved number of CD4+ T cells (median number count: 510 versus 831 cell/μL) (Figure 3(a)). No correlation between IL-7 serum levels and CD4+ T cell counts or exhausted tissue-like memory B cell subset frequency were detected (Figure 3(b)).
Figure 3.
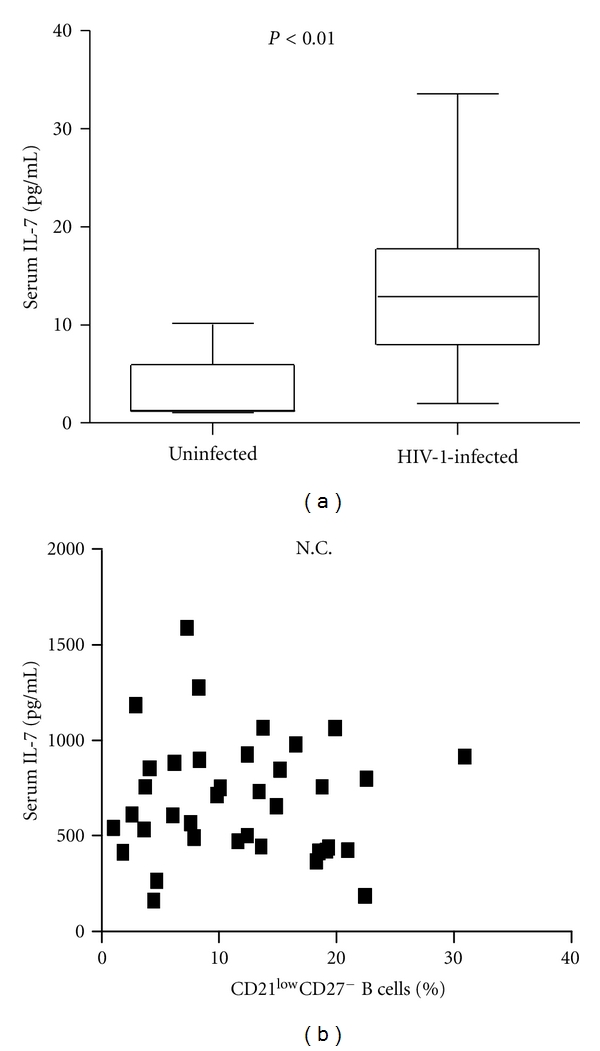
IL-7 serum levels in asymptomatic HIV-1-infected patients and correlation with CD10−CD21lowCD27−B cells frequency. (a) Comparative analysis of IL-7 serum levels (pg/mL) in HIV-1-uninfected donors and asymptomatic HIV-1-infected patients. ELISA was performed to measure the IL-7 concentration. (b) Correlation between IL-7 serum levels and percentage of CD10−CD21lowCD27− B cells in asymptomatic HIV-1-infected patients. Differences between groups were evaluated using the two tailed Mann-Whitney U test, and were considered significant at P < 0.05. Into the box plots, horizontal lines represent median values for each group, boxes show the 25th and 75th percentiles, and bars show SD. Association of 2 different data groups were analyzed by Spearman's rank test. Each symbol represents one individual, and regression line is shown. N.C.= no correlation.
3.3. Increase of TTV Load in Asymptomatic HIV-1-Infected Patients and Correlation with the CD21lowCD27− B Cells Frequency
So far, our findings showed profound phenotypic alterations in the B cell compartment of patients in the asymptomatic stage of HIV-1 infection. Previous studies showed that B cell subsets, abnormally expressed in the peripheral blood of HIV-1-infected patients in advanced stages of disease, and scarcely detectable in healthy individuals, displayed several functional defects [13, 21]. The aim of our study was trying to understand the impact of these uncommon B cell subsets on immunological surveillance in vivo.
TTV is an ubiquitous virus which infects up to 80% of the healthy population [22], and that is present at high titer in the blood of patients in advanced stages of HIV-1 infection compared to healthy subjects. In HIV-1-infected patients TTV load inversely correlated with the CD4+ T cell count [18]. TTV plasma viremia was measured in all individuals enrolled in our study. Asymptomatic HIV-1-infected patients showed a significantly higher amount of circulating TTV compared to healthy individuals (titer median: 5.7 versus 4.6 log DNA copies/mL, P < 0.005, Table 3). A direct correlation was observed between TTV load and both HIV-1 viremia and percentage of CD21lowCD27− B lymphocytes (r = 0.49 P < 0.001, and r = 0.40, P < 0.001 resp.) (Figure 4). These data suggest that in asymptomatic HIV-1-infected patients the increased percentage of CD21lowCD27− B cells may be related to the lack of in vivo immunological control of TTV replication.
Table 3.
Effects of HAART on B cells frequency, IL-7 serum levels, and TTV viral load.
| Healthy | Asymptomatic HIV-1 infected | HAART treated | P (HAART treated versus healthy) | P (HAART treated versus asymptomatic HIV-1 infected) | |
|---|---|---|---|---|---|
| CD19+ B cell count (cell/μL) | 143.0 | 129.5 | 232.5 | <0.01 | <0.005 |
| CD21−CD27− B cell frequency (%) | 2.1 | 10.4 | 2.7 | NS | <0.0001 |
| CD21−CD27+ B cell frequency (%) | 2.4 | 9.6 | 1.7 | NS | <0.0001 |
| CD21+CD27+ B cell frequency (%) | 29.4 | 23.5 | 22.4 | <0.05 | NS |
| CD21+CD27− B cell frequency (%) | 64.4 | 54.2 | 73.0 | NS | <0.0005 |
| IL-7 serum level (pg/mL) | 1.7 | 12.9 | 2.0 | NS | <0.01 |
| TTV plasma viremia (log DNA copies/mL) | 4.6 | 5.7 | 4.1 | NS | <0.001 |
NS: not significant.
Results are expressed as median.
Figure 4.
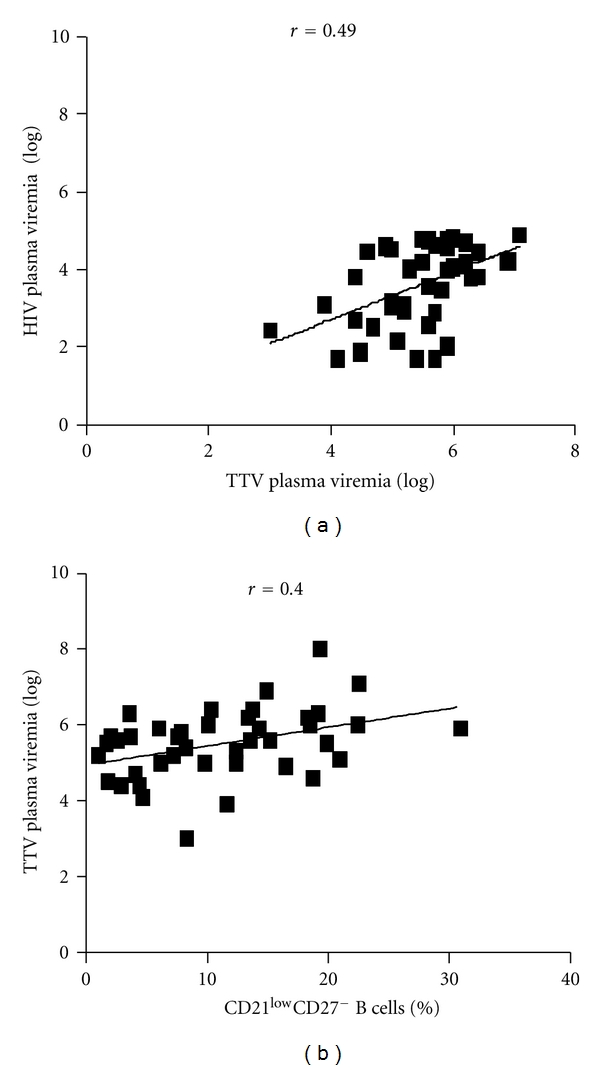
Perturbation in TTV immune surveillance in asymptomatic HIV-1-infected patients. (a) Correlation between TTV and HIV-1 viral loads (log DNA copies/mL, and log RNA copies/mL, resp.) in plasma derived from asymptomatic HIV-1-infected patients. TTV and HIV viral loads were measured by PCR and RT-PCR, respectively. (b) Correlation between plasma TTV titer and percentage of CD10−CD21lowCD27− B cells in asymptomatic HIV-1-infected patients. Association of 2 different data groups were analyzed by Spearman's rank test; P and rho values are indicated. Each symbol represents one individual, and regression line is shown.
3.4. Normal B Cell Subsets Frequency and Normal TTV Plasma Levels in HAART-Treated Patients
We then investigated the effect of HAART on the B cell compartment of a cohort of HIV-1-infected individuals who experienced low levels of CD4+ T cell counts during the course of infection (median nadir: 183 cell/μL), and maintained undetectable HIV-1 viral load for the last 12 months of therapy. At the time of our study, all patients belonging to this cohort displayed CD4+ T cell counts higher than asymptomatic therapy-naïve HIV-1-infected patients (median 682 cells/μL versus 510 cell/μL, P < 0.0001). The absolute number of B cells was significantly higher in HAART-treated patients as compared to both asymptomatic HIV-1-infected HAART-naïve patients and healthy individuals (median: 233 cell/μL versus 129.5 cell/μL, P < 0.005, and versus 143 cell/μL, P < 0.01, resp.) (Table 3). In the cohort of HAART-treated patients we observed no presence of uncommon B cell populations with percentages of CD21lowCD27− and of CD21lowCD27+ B cells superimposable to those of healthy individuals (Figure 5). At the same time, the percentage of CD21highCD27− B cell subpopulation was higher in HAART-treated than asymptomatic HIV-1-infected cohort patients (P < 0.0005), and the IL-7 levels of treated-patients were superimposable to those of healthy donors. Finally, a successful immunological surveillance was observed in HAART-treated patients as they displayed a TTV plasma viremia within ranges commonly observed in healthy subjects (median: 4.1 versus 4.6 log DNA copies/mL).
Figure 5.
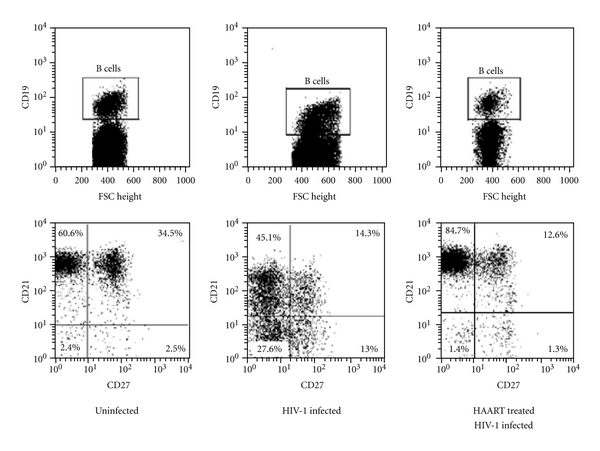
Effect of HAART in B cell subsets distribution in HIV-1-infected patients. Comparative analysis of CD21 and CD27 expression on B cells from HIV-1-uninfected individuals, asymptomatic HIV-1-infected patients, and HAART-treated patients. Profiles of expression of CD21 and CD27 are shown for one representative of each groups (lower panel). Cells were gated according to the lymphocytes forward and side scatter pattern and the CD19+ cells (upper panel).
4. Discussion
Disturbances in differentiation and function of B cells characterize HIV-1 infection, and mostly, these impairments are correlated to the loss of CD4+ T cells and the increase of HIV-1 load [12]. In the present study, we demonstrate that asymptomatic HIV-1-infected patients naïve for HAART are characterized by normal B cell numbers but impaired B cell subsets frequencies. In particular, we observed that CD21lowCD27− and CD21lowCD27+ B cells were overexpressed, whereas the frequencies of CD21highCD27− and CD21highCD27+ B cells, which respectively account for naïve and resting memory B cells, were lower compared to healthy donors. The increased uncommon CD21lowCD27− B cell population in the asymptomatic HIV-1-infected patients consisted exclusively of exhausted tissue-like memory B cells since no CD10 molecule surface was detected in this cellular subset. It is likely that the appearance of CD21lowCD27+ B cells, which have undergone HIV-1-induced activation and differentiation to plasma blasts, and are usually referred as activated mature elements, as well as the appearance of CD21lowCD27− B cells, which derive from exhausted B cell subpopulation, can be ascribed to the B cell hyperactivation driven by the chronic yet asymptomatic presence of HIV-1 in patients. The presence of exhausted B cells in asymptomatic patients is strongly correlated to the HIV-1 load but not to the CD4+ T cell counts. Immature/transitional CD10+CD21lowCD27− B cells, previously described in the peripheral blood of HIV-1 positive patients in advanced stages of disease, were not present in the cohort of asymptomatic patients probably because they appear consequently to a strong reduction of CD4+ T cell counts [13, 15]. In fact, immature/transitional B cells were also observed in patients with non-HIV-1-related idiopathic CD4+ lymphopenia suggesting that HIV-1-induced CD4+ lymphopenia (and not HIV-1 viremia itself) drives the expansion of these elements in HIV-1-infected patients [23]. Therefore, our data show that exhausted tissue-like memory B cells emerge independently from immature/transitional B cells in HIV-1-infected patients, and precede the appearance of the latter possibly arising from homeostatic pressures induced by lymphopenia, and/or from conditions that increase the expression levels of early B cell stimulatory factors such as IL-7 [13, 23].
Several studies report that CD4+ lymphopenia driven by HIV-1 infection or bone marrow transplantation and idiopathic CD4+ T lymphopenia are often associated with increased serum levels of IL-7 [21, 23–25]. In order to explain the increased levels of IL-7 in CD4+ T lymphopenic individuals, it has been suggested that IL-7 augmentation occurs as part of a compensatory effect to enhance differentiation, survival, and expansion of T cells in order to respond to the HIV-1-mediated T cell depletion [24]. Surprisingly, we detected increased levels of IL-7 in sera of asymptomatic HIV-1-infected patients, but no correlations with CD4+ T cells count or HIV-1 load were detected. This result may find a possible explanation in the recent discovery by Guimond et al. [26] that systemic IL-7 concentrations increase solely because of diminished use of the lymphokine by target cells.
So, it is possible to hypothesize that the presence of exhausted memory B cells in asymptomatic HIV-1-infected patients is likely to prejudice the proper functioning of immune cells, and, therefore, a suitable immune surveillance.
So far, no pathological effect can be ascribable to TTV [22, 27]. TTV viral load seems to depend on the immunological status of the host, being patients with tumours or HIV-1 infection characterized by a higher TTV load compared to healthy individuals [16, 28]. Moreover, recent findings obtained by studying TTV in bone marrow transplanted patients show that the increase of viral loads is correlated with an increase in the percentages of dysfunctional CD8+CD57+ T lymphocytes circulating in blood [17, 29]. In the study we show that asymptomatic HIV-1-infected individuals have TTV viral load higher than healthy controls and that its titre is directly correlated to the HIV-1 viral load but not to the CD4+ T cells count. Moreover, TTV viral load directly correlated to the percentage of CD21lowCD27− B cells. These correlations may attest for a scarce immune control of TTV replication by B cells and for an already compromised immune surveillance in untreated asymptomatic HIV-1-infected patients.
Several studies have shown that HAART-mediated suppression of HIV-1 plasma viremia is followed by normalization of B cell counts and hyperactivation. In addition, reduction of HIV-1 viremia by HAART is associated with decreased frequency of exhausted tissue-like memory B cells [30]. Here we have described that patients who experienced HAART, leading to an undetectable HIV-1 viremia for the last 12 months of therapy, display a normal B cell subsets distribution. The role of HAART in the normalization of IL-7 serum levels is controversial: some investigators showed only a partial decrease in the IL-7 levels, never declining toward normal values whereas others documented a complete normalization of this immunological parameter [19, 31]. In our study, successfully HAART-treated patients showed IL-7 values superimposable to those of healthy HIV-1-uninfected individuals.
Interestingly, we reported that all HAART-treated patients experienced TTV load to levels usually detected in healthy individuals. This finding shows that antiretroviral therapies aimed at controlling HIV-1 load and normalizing B cell subpopulations can help to recover immune functions to levels capable of controlling the replication of this endogenous virus. Normalization of B cell counts and subpopulations by HAART is followed by improved B cell responses to both T cell-independent and T cell-dependent immunogens [29, 32–34]. Of particular interest is the recent observation that an early control of viral replication through HAART preserves the longevity of B cell responses in vaccinated HIV-1-infected children thus underscoring the direct role of HIV-1 viremia in B cell terminal differentiation [35]. These observations have important implications not only for preserving immune responses against secondary pathogens but also for maintaining or boosting HIV-1 specific immune responses, including antibody responses.
5. Conclusions
We have provided evidences that asymptomatic HIV-1-infected patients with a preserved CD4+ T cell number, and naïve for HAART, display alterations in B cell subset phenotype and impaired immune responses, as manifested by their inability to control TTV replication. On the other hand, normal B cell phenotypes and counts were found in patients who initiated HAART during chronic disease and displayed a negative HIV-1 viral load in the last 12 months of therapy. Normal B cell subpopulations in HAART-treated patients were mirrored by a normal TTV viral load, attesting for a recovery of adequate immunological surveillance. From this point of view, HAART as well as therapies aimed at decreasing immune cell deregulation can help to better control the loss of immune function if administered earlier during HIV-1 infection.
Acknowledgments
The authors wish to thank the persons who participated in this study and the team which enrolled them. They also acknowledge the skillful technical assistance of Chiara Minini. This paper was supported by Istituto Superiore di Sanità (National Program AIDS Research, Grants: 45G.5 and 40G.16).
References
- 1.Lawn SD, Butera ST, Folks TM. Contribution of immune activation to the pathogenesis and transmission of human immunodeficiency virus type 1 infection. Clinical Microbiology Reviews. 2001;14(4):753–777. doi: 10.1128/CMR.14.4.753-777.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lane HC, Masur H, Edgar LC, Whalen G, Rook AH, Fauci AS. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. New England Journal of Medicine. 1983;309(8):453–458. doi: 10.1056/NEJM198308253090803. [DOI] [PubMed] [Google Scholar]
- 3.Shirai A, Cosentino M, Leitman-Klinman SF, Klinman DM. Human immunodeficiency virus infection induces both polyclonal and virus-specific B cell activation. Journal of Clinical Investigation. 1992;89(2):561–566. doi: 10.1172/JCI115621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amadori A, De Rossi A, Faulkner-Valle GP, Chieco-Bianchi L. Spontaneous in vitro production of virus-specific antibody by lymphocytes from HIV-infected subjects. Clinical Immunology and Immunopathology. 1988;46(3):342–351. doi: 10.1016/0090-1229(88)90053-0. [DOI] [PubMed] [Google Scholar]
- 5.Fournier AM, Fondere JM, Alix-Panabieres C, et al. Spontaneous secretion of immunoglobulins and anti-HIV-1 antibodies by in vivo activated B lymphocytes from HIV-1-infected subjects: monocyte and natural killer cell requirement for in vitro terminal differentiation into plasma cells. Clinical Immunology. 2002;103(1):98–109. doi: 10.1006/clim.2001.5195. [DOI] [PubMed] [Google Scholar]
- 6.De Milito A, Nilsson A, Titanji K, et al. Mechanisms of hypergammaglobulinemia and impaired antigen-specific humoral immunity in HIV-1 infection. Blood. 2004;103(6):2180–2186. doi: 10.1182/blood-2003-07-2375. [DOI] [PubMed] [Google Scholar]
- 7.De Milito A. B lymphocyte dysfunctions in HIV infection. Current HIV Research. 2004;2(1):11–21. doi: 10.2174/1570162043485068. [DOI] [PubMed] [Google Scholar]
- 8.Malaspina A, Moir S, Kottilil S, et al. Deleterious effect of HIV-1 plasma viremia on B cell costimulatory function. Journal of Immunology. 2003;170(12):5965–5972. doi: 10.4049/jimmunol.170.12.5965. [DOI] [PubMed] [Google Scholar]
- 9.Chong Y, Ikematsu H, Yamamoto M, et al. Increased frequency of CD27− (Naive) B cells and their phenotypic alteration in HIV type 1-infected patients. AIDS Research and Human Retroviruses. 2004;20(6):621–629. doi: 10.1089/0889222041217455. [DOI] [PubMed] [Google Scholar]
- 10.De Milito A, Mörch C, Anders Sönnerborg A, Chiodi F. Loss of memory (CD27) B lymphocytes in HIV-1 infection. AIDS. 2001;15(8):957–964. doi: 10.1097/00002030-200105250-00003. [DOI] [PubMed] [Google Scholar]
- 11.Moir S, Malaspina A, Pickeral OK, et al. Decreased survival of B cells of HIV-viremic patients mediated by altered expression of receptors of the TNF superfamily. Journal of Experimental Medicine. 2004;200(5):587–599. doi: 10.1084/jem.20032236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moir S, Fauci AS. B cells in HIV infection and disease. Nature Reviews Immunology. 2009;9(4):235–245. doi: 10.1038/nri2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malaspina A, Moir S, Ho J, et al. Appearance of immature/transitional B cells in HIV-infected individuals with advanced disease: correlation with increased IL-7. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(7):2262–2267. doi: 10.1073/pnas.0511094103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuss AK, Avery DT, Cannons JL, et al. Expansion of functionally immature transitional B cells is associated with human-immunodeficient states characterized by impaired humoral immunity. Journal of Immunology. 2006;176(3):1506–1516. doi: 10.4049/jimmunol.176.3.1506. [DOI] [PubMed] [Google Scholar]
- 15.Moir S, Ho J, Malaspina A, et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. Journal of Experimental Medicine. 2008;205(8):1797–1805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bendinelli M, Pistello M, Maggi F, Fornai C, Freer G, Vatteroni ML. Molecular properties, biology, and clinical implications of TT virus, a recently identified widespread infectious agent of humans. Clinical Microbiology Reviews. 2001;14(1):98–113. doi: 10.1128/CMR.14.1.98-113.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Focosi D, Maggi F, Albani M, et al. Torquetenovirus viremia kinetics after autologous stem cell transplantation are predictable and may serve as a surrogate marker of functional immune reconstitution. Journal of Clinical Virology. 2010;47(2):189–192. doi: 10.1016/j.jcv.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 18.Christensen JK, Eugen-Olsen J, SŁrensen M, et al. Prevalence and prognostic significance of infection with TT virus in patients infected with human immunodeficiency virus. Journal of Infectious Diseases. 2000;181(5):1796–1799. doi: 10.1086/315440. [DOI] [PubMed] [Google Scholar]
- 19.Beq S, Delfraissy JF, Theze J. Interleukin-7 (IL-7): immune function, involvement in the pathogenesis of HIV infection and therapeutic potential. European Cytokine Network. 2004;15(4):279–289. [PubMed] [Google Scholar]
- 20.Llano A, Barretina J, Gutiérrez A, et al. Interleukin-7 in plasma correlates with CD4 T-cell depletion and may be associated with emergence of syncytium-inducing variants in human immunodeficiency virus type 1-positive individuals. Journal of Virology. 2001;75(21):10319–10325. doi: 10.1128/JVI.75.21.10319-10325.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maggi F, Andreoli E, Riente L, et al. Torquetenovirus in patients with arthritis. Rheumatology. 2007;46(5):885–886. doi: 10.1093/rheumatology/kem032. [DOI] [PubMed] [Google Scholar]
- 22.Malaspina A, Moir S, Chaitt DG, et al. Idiopathic CD4+ T lymphocytopenia is associated with increases in immature/transitional B cells and serum levels of IL-7. Blood. 2007;109(5):2086–2088. doi: 10.1182/blood-2006-06-031385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ho J, Moir S, Malaspina A, et al. Two overrepresented B cell populations in HIV-infected individuals undergo apoptosis by different mechanisms. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(51):19436–19441. doi: 10.1073/pnas.0609515103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Napolitano LA, Grant RM, Deeks SG, et al. Increased production of IL-7 accompanies HIV-1-mediated T-cell depletion: implications for T-cell homeostasis. Nature Medicine. 2001;7(1):73–79. doi: 10.1038/83381. [DOI] [PubMed] [Google Scholar]
- 25.Bolotin E, Annett G, Parkman R, Weinberg K. Serum levels of IL-7 in bone marrow transplant recipients: relationship to clinical characteristics and lymphocyte count. Bone Marrow Transplantation. 1999;23(8):783–788. doi: 10.1038/sj.bmt.1701655. [DOI] [PubMed] [Google Scholar]
- 26.Guimond M, Veenstra RG, Grindler DJ, et al. Interleukin 7 signaling in dendritic cells regulates the homeostatic proliferation and niche size of CD4+ T cells. Nature Immunology. 2009;10(2):149–157. doi: 10.1038/ni.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hino S, Miyata H. Torque teno virus (TTV): current status. Reviews in Medical Virology. 2007;17(1):45–57. doi: 10.1002/rmv.524. [DOI] [PubMed] [Google Scholar]
- 28.Zhong S, Yeo W, Tang MW, et al. Gross elevation of TT virus genome load in the peripheral blood mononuclear cells of cancer patients. Annals of the New York Academy of Sciences. 2001;945:84–92. doi: 10.1111/j.1749-6632.2001.tb03868.x. [DOI] [PubMed] [Google Scholar]
- 29.Maggi F, Ricci V, Bendinelli M, et al. Changes in CD8+57+ T lymphocyte expansions after autologous hematopoietic stem cell transplantation correlate with changes in torquetenovirus viremia. Transplantation. 2008;85(12):1867–1868. doi: 10.1097/TP.0b013e31817615e6. [DOI] [PubMed] [Google Scholar]
- 30.Moir S, Malaspina A, Ho J, et al. Normalization of B cell counts and subpopulations after antiretroviral therapy in chronic HIV disease. Journal of Infectious Diseases. 2008;197(4):572–579. doi: 10.1086/526789. [DOI] [PubMed] [Google Scholar]
- 31.Mastroianni CM, Forcina G, d’Ettorre G, et al. Circulating levels of interleukin-7 in antiretroviral-naïve and highly active antiretroviral therapy-treated HIV-infected patients. HIV Clinical Trials. 2001;2(2):108–112. doi: 10.1310/6V29-4UU5-Y3FP-JERT. [DOI] [PubMed] [Google Scholar]
- 32.Feikin DR, Feldman C, Schuchat A, Janoff EN. Global strategies to prevent bacterial pneumonia in adults with HIV disease. Lancet Infectious Diseases. 2004;4(7):445–455. doi: 10.1016/S1473-3099(04)01060-6. [DOI] [PubMed] [Google Scholar]
- 33.Overton ET, Sungkanuparph S, Powderly WG, Seyfried W, Groger RK, Aberg JA. Undetectable plasma HIV RNA load predicts success after hepatitis B vaccination in HIV-infected persons. Clinical Infectious Diseases. 2005;41(7):1045–1048. doi: 10.1086/433180. [DOI] [PubMed] [Google Scholar]
- 34.Kroon FP, Rimmelzwaan GF, Roos MTL, et al. Restored humoral immune response to influenza vaccination in HIV-infected adults treated with highly active antiretroviral therapy. AIDS. 1998;12(17):F217–F223. doi: 10.1097/00002030-199817000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Pensieroso S, Cagigi A, Palma P, et al. Timing of HAART defines the integrity of memory B cells and the longevity of humoral responses in HIV-1 vertically-infected children. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(19):7939–7944. doi: 10.1073/pnas.0901702106. [DOI] [PMC free article] [PubMed] [Google Scholar]


