Abstract
Stroke is the most debilitating cardiovascular event. It has a variety of causes that may be present simultaneously. In young or otherwise healthy people, the search for a patent foramen ovale (PFO) has become standard. In stroke of the elderly, atherosclerosis and atrial fibrillation are in the foreground but the PFO should not be ignored. The risk of a PFO-related stroke over time is controversial and so is its prevention by device closure. The association of proximal aortic plaques in arteries subtending the brain and stroke is considered strong, ignoring that it is as putative as that of the PFO. Statins can prevent progression of such plaques. Antiplatelet agents in asymptomatic and surgical endarterectomy in symptomatic patients or highly ulcerated lesions are the treatment of choice. Stenting with protection devices was shown competitive in selected patients.
Keywords: Secondary stroke prevention, Patent foramen ovale, Closure of PFO, Aortic plaque, Carotid stenosis, Endarterectomy, Carotid stenting
Introduction
Stroke is a devastating event and remains as the third leading cause of mortality and the most important cause of serious, long-term disability.1 Most strokes are of ischaemic origin. Atherosclerosis plays a causative role as do other factors that vary among countries, genders, lifestyles, and a number of well-documented risk factors.2 In the USA, it is estimated that almost 90% of the roughly 800 000 strokes per year are ischaemic.3 Cardioembolic reasons account for 19% and carotid disease for 15% of those. A patent foramen ovale (PFO) is per se not yet considered a primary cause for stroke. It is subsumed under the ∼40% labelled cryptogenic. This paper deals with the stroke causes that are easiest to document and treat in the sense of primary and secondary prevention.
Patent foramen ovale
Autopsy studies revealed that the foramen ovale remains dynamically patent in approximately one-fourth of the general population.4 The PFO thus represents the most common cardiac congenital abnormality (Figure 1). It permits intracardiac shunting while right atrial pressure exceeds left atrial pressure (Figure 2). The PFO accounts for up to 95% of right-to-left shunts. Pulmonary shunts account for about 4%, and atrial septal defects for less than 1%.
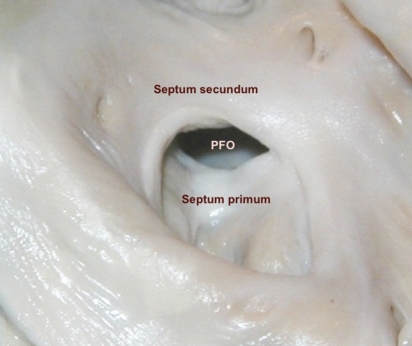
Figure 1.
Patent foramen ovale (PFO) seen from the right atrium. The caudal membranous septum primum approaches the cranial muscular septum secundum, thereby closing the PFO.
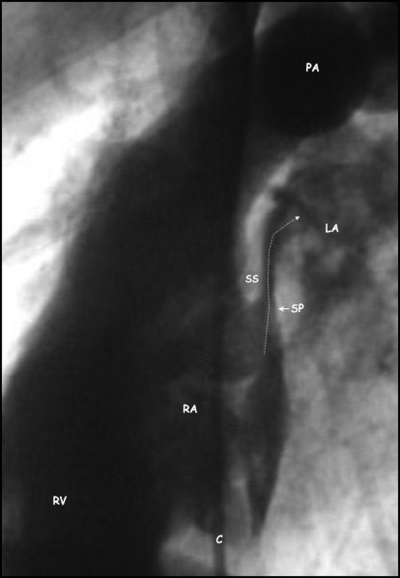
Figure 2.
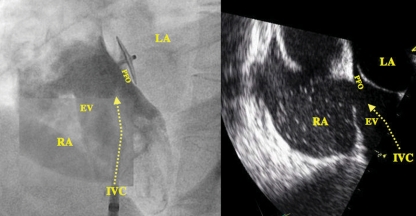
Angiographic lateral view of a patent foramen ovale showing contrast medium injected into the right atrium (RA) passing (arrow) through the patent foramen ovale between the thick septum secundum (SS) and the thin mobile septum primum (SP) into the left atrium (LA). C, catheter; PA, pulmonary artery; RV, right ventricle.
The prevalence of PFO declines from 34% during the first three decades to 25% for the fourth to eighth decade, and to 20% beyond that.4 Spontaneous closure even late in life or selective mortality (reduced life expectancy of PFO carriers) have to be accountable. In most individuals, a PFO will remain asymptomatic for life. However, since the initial link of a fatal stroke in a young woman to a PFO by Cohnheim in 1877, PFO and atrial septal aneurysm (ASA) have been increasingly recognized as potential mediators of systemic embolism.
ASA is a congenital abnormality of the interatrial septum characterized by a redundant, central part of the septum primum (Figure 3). The prevalence of ASA in the general population was about 1% in autopsy series5–7 and 2.2% in a population-based transoesophageal echocardiographic (TOE) study.8 ASA is associated with a PFO in 50–85% of cases7–9 and likely co-responsible for it. The constant motion of the ASA renders postnatal fusion difficult and thus begets PFOs. The criteria for distinction between a floppy interatrial septum and ASA vary between autopsy, transthoracic echocardiography (TTE), and TOE. ASA is generally diagnosed if the diameter of the base of the flimsy portion of the interatrial septum exceeds 15 mm and the excursion of the aneurysmal membrane is ≥10 mm in either left or right atrium, or if the sum of the total excursion is ≥10 mm.7
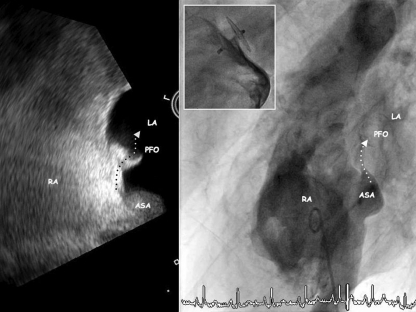
Figure 3.
Patent foramen ovale (PFO) associated with an atrial septal aneurysm (ASA) depicted by transoesophageal echocardiography (left panel) and angiography (right panel). The atrial septal aneurysm bulges into the left atrium (LA) after a sustained Valsalva manoeuvre allowing for a shunt into the left atrium (LA). The inset shows the situation after PFO closure with a 25 mm Amplatzer PFO occluder.
ASA has been associated with cerebral ischaemic events in numerous case–control studies.7–13 The combination of PFO and ASA must account for this and constitutes a particularly high-risk situation with a relative risk of 16 (95% CI 3–86) comparing ischaemic stroke with non-stroke control subjects, and a relative risk of 17 (95% CI 2–134) comparing cryptogenic stroke with known stroke cause control subjects (age <55 years).14 ASA may facilitate paradoxical embolism by leading to a more frequent and wider opening of the PFO channel15 or by promoting a right-to-left shunt by redirecting flow from the inferior vena cava towards the PFO.16
Patent foramen ovale and stroke
In younger patients, a classical aetiology is not found in up to 40% of ischaemic strokes despite an extensive diagnostic evaluation.17,18 Such strokes are then referred to as cryptogenic, a misnomer in the presence of a PFO. Despite the prevalence of around 25% of a PFO in the general population,4 paradoxical embolism is rare and typically assumed rather than proved.19 However, this holds true equally for strokes attributed to atrial fibrillation, prior myocardial infarction, or proximal arterial plaques.
The association of PFO with cryptogenic stroke, reported in 1988,20,21 as well as numerous case reports depicting a thrombus straddling the PFO establish paradoxical embolism as underlying mechanism. This is corroborated by an observational study of 139 patients suffering from major pulmonary embolism.22 Patients with PFO were more likely to die (44 vs. 13%, P = 0.02) and to suffer from stroke (13 vs. 2%, P = 0.02) or peripheral embolism (15 vs. 0%, P = 0.01) with the presence of a PFO emerging as an independent predictor of mortality. The higher frequency of pelvic vein thrombosis at magnetic resonance (MR) venograms within 2 days of the onset of symptoms in stroke patients with PFO (20%) than with conventional stroke causes (4%) again points to paradoxical embolism via PFO.23 So do an observational study of 202 patients with transvenous pacing leads, in whom the presence of intracardiac shunts was associated with a >2-fold increased risk of systemic embolism during long-term follow-up,24 and a large Danish population-based study on patients with deep venous thrombosis (n = 25 199) or pulmonary embolism (n = 16 925). Their relative risks of stroke during the first year after the thrombotic event were 2.2 (1.9–2.6) and 2.9 (2.3–3.7) fold increased compared with controls (N = 163 566).25
Patent foramen ovale and first ischaemic stroke
In the European population, the annual incidence of a first ischaemic stroke is 139 per 100 000 inhabitants.26 Since around 60% of these events can be attributed to conventional causes,17,18 the annual risk attributed to paradoxical embolism has been estimated at 28 per 100 000 persons with PFO per year.27 The association of PFO with cryptogenic stroke has been repeatedly confirmed.14,28 More recently, this observation has been extended to adults >55 years, with a significantly higher prevalence of PFO alone (28.3 vs. 11.9%; OR 2.9; 95% CI 1.7–5.0; P < 0.001) as well as of PFO associated with ASA (15.2 vs. 4.4%; OR 3.9; 95% CI 1.8–8.5; P < 0.001) among patients with cryptogenic stroke compared with those with conventional stroke causes.29 A recent meta-analysis of 23 case–control studies30 suggested that the odds of PFO were 2.9 times higher in patients with cryptogenic stroke when compared with controls (95% CI 2.1–4.0).
In contrast, two prospective population-based studies failed to confirm PFO as an independent risk factor for cryptogenic stroke,31,32 with only an insignificant trend towards a higher incidence of stroke in persons with PFO. The Olmsted County Study enrolled 588 randomly selected subjects.31 PFO was identified using TTE in 24% and an ASA in 2%. During a mean follow-up of 5.1 years, cerebrovascular events (cerebrovascular death, ischaemic stroke, and TIA) occurred in 41 subjects (7%). After adjustment for age and co-morbidities, PFO was not an independent predictor of stroke (hazard ratio, HR 1.46; 95% CI 0.74–2.88; P = 0.28). The risk of stroke among subjects with ASA was almost four times greater than that in those without, but proportional hazard regression analysis did not establish statistical significance (HR 3.72; 95% CI 0.88–15.71; P = 0.074). The relatively small sample size and the advanced age (mean 67 years) of the study participants were criticised, in addition to the inadequate screening sensitivity resulting in a significant percentage of undetected PFOs.
Among the 1100 participants of the Northern Manhattan Study,32 TTE detected a PFO in only 15% and an ASA in 3%. During 6.6 years of follow-up, 68 subjects suffered an ischaemic stroke (6%). After adjustment for demographic and risk factors, PFO was not significantly associated with stroke (HR 1.64; 95% CI 0.87–3.09). Isolated ASA was associated with an elevated stroke incidence (HR 3.66; 95% CI 0.88–15.30), but ASA associated with PFO was not (HR 1.25; 95% CI 0.17–9.24). The low prevalence of PFO when compared with autopsy studies again unmasks TTE as not sensitive enough to screen for PFO.
Patent foramen ovale and recurrent cerebrovascular events
The natural history after cerebrovascular events in patients with PFO remains insufficiently defined, which is problematic since the risk of recurrence determines the therapeutic value of interventions aimed at secondary prevention. Traditionally, most patients with presumed paradoxical embolism were treated with antithrombotic medications. Data are scarce concerning the efficacy of oral anticoagulation as opposed to antiplatelet agents and the duration of treatment required. Observational studies on medical treatment in patients with PFO with either antiplatelet agents or coumarin reported a risk of recurrent stroke or TIA ranging from 3 to 12% during the first year.9,12,16,28,33,34 Both larger PFO size15,18,35,36 and a greater degree of right-to-left shunt15,16,35,37 signify a higher risk for paradoxical embolism. However, there are major differences in the baseline characteristics of the patient populations studied, which may account for the disparate recurrence rates reported. According to a recent meta-analysis of 15 studies of medical treatment in 2548 patients with cryptogenic cerebrovascular events, the pooled rate of recurrent ischaemic stroke or TIA was 4.0 events per 100 patient-years (95% CI 3.0–5.1) while the rate of recurrent ischaemic stroke was 1.6 events per 100 patient-years (95% CI 1.1–2.1).38 Of note, in trials with antiplatelet agents or oral anticoagulation, the risk of recurrence appeared lower with the latter. Current medical treatment recommendations are mainly based on one randomized clinical trial with blinded outcome assessment, the Patent foramen ovale In Cryptogenic Stroke Study (PICSS),28 and three prospective studies.9,16,33
Although medical treatment lacks the risk of interventional procedures, it is associated with other adverse effects, most notably an increased risk of bleeding. Thus, major bleeding amounted to 1.5–2.2 per 100 patient-years in the prospective PICSS28 and its subanalysis, the Warfarin-Aspirin Recurrent Stroke Study (WARSS),39 with no significant differences between acetylsalicylic acid and oral anticoagulation. Treatment with acetylsalicylic acid has been found insufficient in patients with PFO and associated ASA.9,12 Another important limitation of medical treatment is lack of compliance.
Percutaneous patent foramen ovale closure
Percutaneous (device) closure of the PFO has supplanted surgical PFO closure and constitutes an alternative treatment. It eliminates the pathway for paradoxical embolism and may thus circumvent the need for long-term blood thinners. However, it is associated with a small periprocedural risk and significant cost.
Bridges et al.40 introduced percutaneous PFO closure in 1992 to reduce the incidence of recurrent strokes. Since then, percutaneous PFO closure has been shown safe and feasible in numerous studies, using a variety of devices (Figure 4).41–52 The reported success rates varied between 90 and 100%, with complication rates between 0 and 10%. Complete PFO closure was reported in 51–100% of patients, and yearly recurrence rates of ischaemic strokes and TIAs varied between 0 and 3.4%.
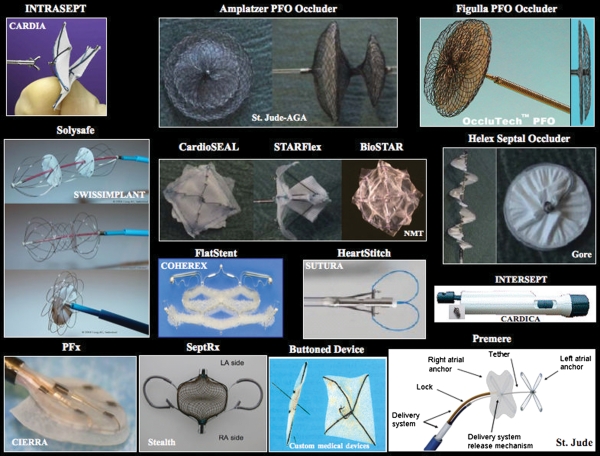
Figure 4.
Selection of clinically used closure devices for the patent foramen ovale. The device name is on top and the company name is inside the respective pictures.
All patients should undergo TOE prior to the intervention for initial diagnosis of PFO and detailed delineation of anatomy (i.e. associated ASA, Eustachian valve; Figures 3 and 5) including assessment of right-to-left shunt. The procedure can be performed on an outpatient basis under local anaesthesia and may take less than 30 min.52 Patients can be released to unrestricted physical activity as early as a few hours after the intervention. Antibiotics during the intervention are commonplace and prevention against endocarditis is recommended for a few months until the device is completely covered by tissue. Failed implantation due to inability to canulate the PFO is extremely rare (<1%). Periprocedural complications have fallen below 1% in experienced centres, and complete closure rates of >90% can be expected.52 Follow-up treatment includes acetylsalicylic acid (80–300 mg daily) for 1–6 months, with the addition of clopidogrel (75 mg qd) for 1–6 months at some centres. At 3–6 months after percutaneous PFO closure, a contrast TOE should be repeated, to assess for residual shunt following endothelial overgrowth and exclude thrombosis of the device. Transcranial Doppler constitutes an alternative. However, it cannot rule out a thrombus on the device. If the PFO proves completely closed, all medication can be discontinued, unless required for another indication, e.g. associated coronary artery disease.53 In case of persistence of a moderate or large residual shunt, implantation of a second device is recommended, which results in complete closure in ∼90% of cases.52
Figure 5.
Angiographic (left panel) and transoesophageal view (right panel) of a patent foramen ovale (PFO) associated with an Eustachian valve (EV) channelling the inflow from the inferior vena cava (IVC) directly onto the PFO. The left panel shows a catheter in the IVC and a 25 mm Amplatzer PFO occluder in the PFO. LA, left atrium; RA, right atrium.
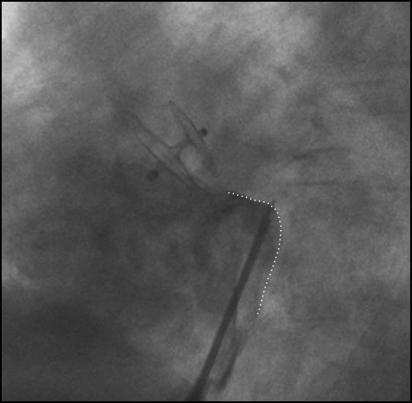
Complications consist mostly of arteriovenous fistulae at the groin and are device- and technique-related.41,51,54 The same holds true for residual shunts and thrombus formation.55 Erosion of the free atrial wall, device endocarditis, or need for surgical explantation are exceedingly rare. Long-term safety is of utmost importance for a preventive procedure against a low risk in natural history. Rarely, supraventricular arrhythmias can be induced or triggered by the device leading to need for anticoagulation or left atrial ablation. Transseptal puncture (for later left atrial interventions) is rather optically guided and not impeded after device implantation (Figure 6).
Figure 6.
Ideal projection and area (dotted line) of transseptal puncture after patent foramen ovale closure.
Comparison of medical treatment with patent foramen ovale closure
Available evidence of studies assessing medical treatment and percutaneous PFO closure encompass multiple observational single-arm studies, two comparative registries,56,57 and a systematic review of case series.58 None of the prospective, randomized clinical trials has been published to date. Oral presentation of the CLOSURE I trial (Prospective, Multicenter, Randomized Controlled Trial to Evaluate the Safety and Efficacy of the STARFlex Septal Closure System Versus Best Medical Therapy in Patients with a Stroke of Transient Ischemic Attack due to Presumed Paradoxical Embolism through a Patent Foramen Ovale) showed no significant advantage of PFO closure with the obsolete STARFlex device at 2 years of follow-up. Wöhrle58 recently summarized clinical outcomes from 8 studies comprising 998 medically treated patients and 12 studies with 2016 patients who underwent percutaneous PFO closure. The annual rate of stroke or TIA was significantly lower after percutaneous PFO closure (1.3%, 95% CI 1.0–1.8) compared with medical treatment (5.2%, 95% CI 4.4–6.2) and was comparable to event rates of patients without PFO.
Percutaneous PFO closure may be particularly valuable in patients with PFO and associated ASA. One study compared clinical outcomes following device closure between patients with both PFO and ASA (n = 141) and those with PFO alone (n = 220).41 Device implantation success and the rate of residual shunt assessed by contrast TOE 6 months after the procedure were similar for both groups. At 4 years of follow-up, the rate of recurrent stroke, TIA, or peripheral embolism amounted to 5.1 and 6.0% in patients with and without ASA, respectively (HR = 0.8, 95% CI 0.3–2.3, P = 0.70). Accordingly, device closure in patients with both PFO and ASA lowered the rate of recurrence to that of patients with PFO alone, which was considerably lower than the 19.2% rate reported in the French PFO/ASA study.9
However, the results of the above-mentioned studies have to be interpreted in light of limitations of non-randomized registries with unmeasured characteristics, lack of data monitoring, and independent event adjudication, and variations in definitions, outcome measures, and their assessment.
Current state of the subject
Despite growing recognition of the PFO, particularly when associated with an ASA, as risk factor for several disease manifestations, the optimal treatment strategy for patients with documented or suspected paradoxical embolism remains controversial. Percutaneous PFO closure is a minimally invasive procedure which can be performed with high success and low morbidity. With respect to secondary prevention of recurrent embolic events, percutaneous PFO closure appears to be clinically at least as effective as medical treatment.56,58 However, it has to be emphasized that the true therapeutic efficacy of percutaneous PFO closure as adjunct or alternative to medical treatment can only be ascertained with long-term randomized studies. The first such trial (CLOSURE I) showed no benefit but was limited to 2 years of follow-up and used a device since withdrawn. The Patent foramen ovale for Cryptogenic stroke (PC), the Randomised Evaluation of recurrent Stroke comparing PFO closure to Established Current standard of care Treatment (RESPECT), the Gore Helex Septal Occluder and Antiplatelet Medical Management for Reduction of Recurrent Stroke or Imaging-Confirmed TIA in Patients with Patent Foramen Ovale (REDUCE), and the Patent Foramen Ovale Closure or Anticoagulants Versus Antiplatelet Therapy to Prevent Stroke Recurrence (CLOSE) trials are ongoing. Enrollment is slow and skewed as most patients believed to be at high risk undergo percutaneous PFO closure. Due to lower-than expected event rates, the included patient numbers and follow-up duration risk to be insufficient to achieve satisfactory power. Besides, stroke pathophysiology is multifactorial, and the diagnosis of PFO-mediated paradoxical embolism is presumptive. Both a PFO and a cryptogenic stroke may coexist without causal relation in a given patient. In this case, PFO closure will not reduce the risk of recurrence.30 Notwithstanding, non-randomized studies show a consistent pattern in favour of percutaneous PFO closure in all-comers. At least patients with multiple embolic events warrant percutaneous PFO closure,56 especially if they already failed medical treatment.
Aortic plaques
It is controversial whether aortic plaques are a risk factor for stroke, a marker for generalized atherosclerosis, or a harmless incidental finding. Due to this uncertainty and the lack of evidence-based data to support specific drug therapy in patients with stroke and aortic plaques, clinicians face a dilemma in choosing proper secondary prophylaxis in patients with TIA or ischaemic stroke and aortic plaques on echocardiography.
Many retrospective case–control studies have reported a strong association between aortic atheromas and stroke.59–61 Independent of other traditional atherosclerotic risk factors, the incidence of atheromatous plaques involving the thoracic aorta was consistently higher in patients with cerebral embolism compared with control groups. As studies were of retrospective nature, true cause-and-effect relationship could not be established. Subsequently, several prospective follow-up studies have shown an association between aortic plaques and stroke.62,63 The risk of embolic stroke from aortic atheromatous plaque varies with different plaque morphology. Complex plaques, which are defined by the presence of protruding atheroma of more than 4 mm thickness, mobile debris, or plaque ulceration, are more likely to embolize than simple plaques. Lack of calcification independently increases the embolic risk.64 The suspected reason might be that non-calcified plaques are lipid-laden and prone to rupture and thrombosis.
Recent community-based studies found no association between aortic atheroma and future stroke. In a prospective study of Meissner et al.,65 581 participants of the Stroke Prevention Assessment of Risk in a Community (SPARC) study were followed for vascular events. In their observation, both simple and complex atheromatous plaques were not an independent predictor of either cardiac events or strokes. In a case–control analysis of Petty et al.,66 the prevalence of ascending and thoracic aortic atheroma among the SPARC participants (random controls) compared with three other groups—patients with cryptogenic stroke, patients with stroke attributed to another aetiology, and patients undergoing TOE for non-neurological indications (referred controls)—was not significantly associated with group status. Based on these results, the authors concluded that aortic atheroma was not a risk factor for cryptogenic stroke.
Russo et al.67 prospectively evaluated prevalence of aortic plaques and risk of vascular events and ischaemic stroke associated with plaques in the aortic arch or in the proximal portion of the descending aorta in 209 stroke-free subjects from the Aortic Plaques and Risk of Ischemic Stroke (APRIS) study. Large plaques in the aortic arch were not associated with an increased risk of vascular events over a 6-year follow-up, whereas plaques in the proximal descending aorta were associated with an increased risk of combined events in unadjusted analyses, but not after adjustment for other risk factors. The authors concluded that this observation may suggest that plaques in the descending aorta represent rather a marker of atherosclerosis than a direct cause for the events.
In contrast, Harloff et al.68,69 postulated that complex plaques of the aorta displayed an embolic high-risk source for stroke. By multidirectional-dimensional (3D) velocity mapping, they could prove the connection of complex plaques of the descending aorta with supra-aortic vessels that supplied the territory of visible acute and embolic retinal or cerebral infarction by retrograde flow. In a prospective study including 94 stroke patients, time-resolved 3D MR could explain potential embolization from the descending aorta in 19 of 57 patients (33.3%) with determined stroke aetiology and in 9 of 37 patients (24.3%) with cryptogenic stroke. However, the true incidence and clinical relevance of this mechanism remains unclear.
Apart from the fact that the real impact of aortic plaques on the aetiology of ischaemic stroke is still unsolved, optimal treatment strategies for patients with aortic atheromatous plaques are not well defined.
Statins
Statins seem to be a sensible concept in patients with aortic plaques because of their pleiotropic effects. Beyond lipid lowering, statins attenuate plaque inflammation, reduce endothelial dysfunction, and have antithrombotic properties.70 Aortic plaque regression during statin therapy has been reported through the use of MR monitoring.71 Therapy for 6 months led to significant aortic plaque regression and reverse remodelling which was strongly associated with low-density lipoprotein cholesterol reduction. In a randomized trial with either simvastatin or diet alone, Tahara et al.72 observed patients who underwent fluorodeoxy glucose (FDG) positron emission tomography scan for cancer screening and were found to have increased FDG uptake in the thoracic aorta or carotid artery. After 3 months, simvastatin reduced plaque inflammation significantly when compared with diet alone. However, these two studies did not monitor cerebrovascular endpoints. Independent from the use of statins in patients with aortic plaque, pravastatin has been found to reduce the incidence of stroke in patients with coronary artery disease.73,74 In an observational study of 519 patients followed for 3 years with severe aortic arch plaque, multivariate analysis showed that statin therapy was independently protective against recurrent events (number needed to treat = 6).75 No protective effect was found for the use of warfarin or antiplatelet drugs.
Anticoagulation and antiplatelet drugs
As unstable aortic plaques are associated with intravascular thrombus embolization, anticoagulation with warfarin has been used for primary or secondary prophylaxis in patients with aortic plaque. Although three non-randomized observational trials showed benefits of anticoagulation with warfarin,76–78 others did not.75,79 In a retrospective observational trial with 129 patients by Ferrari et al.,77 patients with complex plaques showed a decreased incidence of vascular events and death under treatment with oral anticoagulation when compared with antiplatelet therapy. In contrast, Tunick et al.75 found no significant benefit of warfarin or antiplatelet drugs on the incidence of stroke and other embolic events in 519 patients with severe thoracic aortic plaque. The French study of aortic plaques in stroke reported similar results.80 In WARSS, a recent double-blind randomized trial, therapy with warfarin or acetylsalicylic acid was compared in 516 patients with ischaemic stroke.79 Endpoints were recurrent ischaemic stroke or death over a 2-year follow-up period. Especially large plaques were associated with a significantly increased risk of events, but no interaction was observed between warfarin treatment and large plaques on the risk of events. In all these studies, the effect of statin therapy, which could improve aortic plaque stability, was not measured. As outlined above, this could act as a confounding factor and the actual effects of either anticoagulation or antiplatelet drugs could be underestimated or exaggerated. Besides, the use of dual antiplatelet therapy is not reported. The ongoing Aortic arch Related Cerebral Hazard (ARCH) trial, comparing warfarin and clopidogrel plus acetylsalicylic acid, may be the next step towards evidence-based data to secondary prevention in patients with aortic plaque. Future studies should also evaluate the value of newer, safer agents like direct thrombin inhibitors in this indication.
Surgery
Aortic arch endarterectomy during coronary artery bypass grafting has been suggested for aortic atheromatous disease.81 As intraoperative stroke rate is very high and long-term anticoagulation with warfarin and regular surveillance with TOE or MR are necessary because of increased risk of recurrence, surgery should only be considered in desperate cases of young patients with recurrent emboli, despite maximal medical treatment.
Current state of the subject
Although a retrospective study indicated a likely benefit from statins, the best medical treatment for patients with aortic plaque has not yet been determined. Statins are recommended in any patient with atherosclerosis, as these drugs have been shown to reduce the risk of stroke and myocardial infarction and have plaque stabilization properties. As unstable aortic plaques may develop superimposed thrombi, seen as mobile elements on TOE, anticoagulation with warfarin may be a reasonable therapy. Antiplatelet drugs have not been proved to be beneficial in patients with aortic plaques and ischaemic stroke. However, until a proper randomized control trial is done, oral anticoagulation cannot be definitely recommended.
Carotid stenosis
Surgery vs. stenting
Two large randomized trials, the North American Symptomatic Carotid Endarterectomy Trial (NASCET) and the European Carotid Surgery Trial (ESC), found a clear benefit for carotid endarterectomy compared with medical treatment in patients with high-degree symptomatic stenosis of the internal carotid artery (ICA).82,83 Taken together, the trials found an absolute risk reduction of 13.5% over 5 years for the combined endpoint of stroke and death in favour of carotid endarterectomy.84 The risk reduction was even higher in patients with an ICA stenosis >90%. In patients with an ICA stenosis of 50–69%, the 5-year absolute risk reduction for the endpoint ipsilateral stroke was 4.6%. Patients with an ICA stenosis <50% did not benefit from carotid endarterectomy. The short-term complication rates (stroke and death) were 6.2% with an ICA stenosis >70 and 8.4% with an ICA stenosis of 50–69%. One should, however, keep in mind that these studies were performed at times when optimal treatment of vascular risk factors was not available.
Soon after balloon dilatation and stenting had been introduced in cardiology, these methods were applied in patients with significant carotid stenosis without scientific proof of efficacy or superiority over carotid endarterectomy.85,86 The following section reports the results of the recently published randomized trials comparing the two approaches, both in patients with symptomatic and asymptomatic carotid stenosis (Table 1).87–92
Table 1.
Risk of stroke or death from large-scale randomized trials comparing endovascular carotid artery stenting (CAS) and surgical carotid endarterectomy (CEA) treatment in patients with severe carotid-artery stenosis
| Patients | Any stroke or death at 30 days |
Disabling stroke or death at 30 days |
Ipsilateral stroke after 30 days |
||||
|---|---|---|---|---|---|---|---|
| CAS (n, %) | CEA (n, %) | CAS (n, %) | CEA (n, %) | CAS (n, %) | CEA (n, %) | ||
| CAVATAS87 | 504 | 25 (10.0) | 25 (9.9) | 16 (6.4) | 15 (5.9) | 6a | 10a |
| SAPPHIRE88 | 334 | 8 (4.8) | 9 (5.4) | n.a. | n.a. | n.a. | n.a. |
| SPACE89 | 1214 | 46 (7.7) | 38 (6.5) | 29 (4.8) | 23 (3.9) | 4 (0.7)b | 1 (0.2)b |
| EVA 3S90 | 527 | 25 (9.6) | 10 (3.9) | 9 (3.4) | 4 (1.5) | 2 (0.6)b | 1 (0.3)b |
| ICSSc91 | 1713 | 72 (8.5) | 40 (4.7) | 34 (4.0) | 27 (3.0) | 58 (6,8) | 30 (3.5) |
| CREST92 | 2502 | 55 (6.8) | 29 (3.6) | n.a. | n.a. | n.a. | n.a. |
n.a. = not available).
aFollow-up duration 1.95 years in mean.
bFollow-up duration up to 6 months.
cOutcome at 90 days (intention-to-treat data).
SPACE (Stent-protected Percutaneous Angioplasty of the Carotid vs. Endarterectomy) randomized 1200 symptomatic patients with a >50% stenosis (according to the NASCET criteria) or >70% (according to ESC criteria) to carotid endarterectomy or stenting within 6 months after a TIA or minor stroke.89 The primary endpoint, ipsilateral stroke, or death within 30 days occurred in 6.84% of patients undergoing stenting and 6.34% of patients undergoing carotid endarterectomy. A post-hoc subgroup analysis identified age <68 years as a factor being associated with a lower complication rate in patients treated with stenting. The complication rate of surgery was not age-dependent.93 In this study, the use of a protection system did not influence the complication rate. In the SAPPHIRE study, enrolling high-risk patients, complication rates were even slightly lower with carotid stenting than with carotid endarterectomy (Table 1).88 In contrast, the EVA-3S (Endarterectomy Versus Angioplasty in Patients with Severe Symptomatic Carotid Stenosis) was terminated prematurely after 527 patients were randomized due to a significant difference in the 30-day complication rate favouring carotid surgery (9.6 vs. 3.9%; OR 2.5; 95% CI 1.25–4.93).90 Of note, however, EVA-3S involved a considerable number of centres with very limited experience in carotid stenting which makes the interpretation of this study difficult. In addition, the complication rate of surgery was much lower than that observed in the SPACE study.
The International Carotid Stenting Study (ICSS) randomized 1713 patients (stenting group, n = 855; endarterectomy group, n = 858).91 Between randomization and 120 days, there were 34 (4.0%) events of disabling stroke or death in the stenting group compared with 27 (3.2%) events in the endarterectomy group (HR 1.28). The incidence of stroke, death, or procedural myocardial infarction was 8.5% (72) in the stenting group compared with 5.2% (44) in the endarterectomy group (HR 1.69, P = 0.006). Any stroke (65 vs. 35 events; HR 1.92) and all-cause death (19 vs. 7 events; HR 2.76) were more frequent in the stenting group than in the endarterectomy group.
CREST randomly assigned patients with symptomatic or asymptomatic carotid stenosis to carotid-artery stenting or carotid endarterectomy.92 The primary composite endpoint was stroke, myocardial infarction, or death from any cause during the periprocedural period (30 days) or any ipsilateral stroke within 4 years after randomization of 2502 patients observed over 2.5 years. There was no significant difference in the estimated 4-year rates of the primary endpoint between the stenting group and the endarterectomy group (7.2 and 6.8%, respectively; HR 1.11, P = 0.51). The 4-year rate of stroke or death was 6.4% with stenting and 4.7% with endarterectomy (HR 1.50, P = 0.03). The rates among symptomatic patients were higher than among asymptomatic patients, but in both groups there was no difference in outcomes between surgery and stenting. Periprocedural complications differed between the stenting group and the endarterectomy group (death: 0.7 vs. 0.3%, P = 0.18; stroke: 4.1 vs. 2.3%, P = 0.01 and myocardial infarction: 1.1 vs. 2.3%, P = 0.03).
A meta-analysis included 11 randomized trials (but not CREST) and reported outcomes in 4796 patients.84 The periprocedural risk of stroke or death was lower for carotid surgery (OR 0.67, P = 0.025) than for carotid stenting. The risk of myocardial infarction (OR 2.69, P = 0.036) and cranial nerve injury (OR 10.2, P < 0.001) was higher in patients undergoing carotid endarterectomy.
Taken together, the results of the so far published studies show a lower and in some cases a similar complication rate for endarterectomy compared with carotid stenting. The reported medium-time outcomes in a 2–4-year follow-up were comparable but the restenosis rate was higher after carotid stenting.95,96 It is likely that the success of carotid stenting (and for that matter also of carotid endarterectomy) depends heavily on the experience of a given centre.
There remain many unanswered research questions despite the 12 studies published so far. Most of the studies were underpowered to show non-inferiority or equivalence of carotid stenting compared with endarterectomy. In addition, the trials had different primary and secondary endpoints and different definitions for complications. Some studies included only patients with symptomatic carotid stenosis, whereas others had a mixture of symptomatic and asymptomatic patients. There seems to be an age effect on outcomes. Patients aged over 70–75 years had a higher early complication rate with carotid stenting while the rate in carotid endarterectomy was not age-dependent. None of the trials considered plaque morphology. One would expect that carotid stenosis with a heavy calcification load would benefit more from endarterectomy than from stenting.
Current state of the subject
Symptomatic patients with significant stenosis of the ICA should preferably undergo carotid endarterectomy. In experienced centres, carotid stenting can be equivalent in terms of benefit and complications. The benefit of surgery is no longer present when the complication rate exceeds 6%.96 Most of the randomized studies, however, achieved complication rates below that. Therefore, patient selection is of major importance. In addition, one has to consider the countries in which the trials were performed. For patients enrolled in the USA (CREST) or Germany, Austria, and Switzerland (SPACE), carotid surgery and carotid stenting seem to be similar. Therefore, experience and complication rate of a particular vascular surgeon, interventionalist, or team may decide which method is preferred.
Funding
The Department of Neurology at the University Duisburg-Essen received research grants from the German Research Council (DFG), German Ministry of Education and Research (BMBF), European Union, NIH, Bertelsmann Foundation, and Heinz-Nixdorf Foundation.
Conflict of interest: B.M. received research grants and speaker fees from St. Jude-AGA Medical.
H.C.D. received honoraria for participation in clinical trials, contribution to advisory boards, or oral presentations from: Abbott, AstraZeneca, Bayer Vital, BMS, Boehringer Ingelheim, CoAxia, D-Pharm, Fresenius, GlaxoSmithKline, Janssen-Cilag, Knoll, MSD, Medtronic, MindFrame, Neurobiological Technologies, Novartis, Novo-Nordisk, Paion, Parke-Davis, Pfizer, Sanofi-Aventis, Sankyo, Schering-Plough, Servier, Solvay, Thrombogenics, Wyeth and Yamaguchi. H.C.D. has no ownership interest and does not own stocks of any pharmaceutical company.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. doi:10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Borglykke A, Andreasen AH, Kuulasmaa K, Sans S, Ducimetiere P, Vanuzzo D, Ferrario MM, Palmieri L, Karvanen J, Tunstall-Pedoe H, Jorgensen T. Stroke risk estimation across nine European countries in the MORGAM project. Heart. 2010;96:1997–2004. doi: 10.1136/hrt.2010.207555. doi:10.1136/hrt.2010.207555. [DOI] [PubMed] [Google Scholar]
- 3.Marsh JD, Keyrouz SG. Stroke prevention and treatment. J Am Coll Cardiol. 2010;56:683–691. doi: 10.1016/j.jacc.2009.12.072. doi:10.1016/j.jacc.2009.12.072. [DOI] [PubMed] [Google Scholar]
- 4.Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc. 1984;59:17–20. doi: 10.1016/s0025-6196(12)60336-x. [DOI] [PubMed] [Google Scholar]
- 5.Silver MD, Dorsey JS. Aneurysms of the septum primum in adults. Arch Pathol Lab Med. 1978;102:62–65. [PubMed] [Google Scholar]
- 6.Hanley PC, Tajik AJ, Hynes JK, Edwards WD, Reeder GS, Hagler DJ, Seward JB. Diagnosis and classification of atrial septal aneurysm by two-dimensional echocardiography: report of 80 consecutive cases. J Am Coll Cardiol. 1985;6:1370–1382. doi: 10.1016/s0735-1097(85)80228-x. doi:10.1016/S0735-1097(85)80228-X. [DOI] [PubMed] [Google Scholar]
- 7.Pearson AC, Nagelhout D, Castello R, Gomez CR, Labovitz AJ. Atrial septal aneurysm and stroke: a transesophageal echocardiographic study. J Am Coll Cardiol. 1991;18:1223–1229. doi: 10.1016/0735-1097(91)90539-l. doi:10.1016/0735-1097(91)90539-L. [DOI] [PubMed] [Google Scholar]
- 8.Agmon Y, Khandheria BK, Meissner I, Gentile F, Whisnant JP, Sicks JD, O'Fallon WM, Covalt JL, Wiebers DO, Seward JB. Frequency of atrial septal aneurysms in patients with cerebral ischemic events. Circulation. 1999;99:1942–1944. doi: 10.1161/01.cir.99.15.1942. [DOI] [PubMed] [Google Scholar]
- 9.Mas JL, Arquizan C, Lamy C, Zuber M, Cabanes L, Derumeaux G, Coste J. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med. 2001;345:1740–1746. doi: 10.1056/NEJMoa011503. doi:10.1056/NEJMoa011503. [DOI] [PubMed] [Google Scholar]
- 10.Cabanes L, Mas JL, Cohen A, Amarenco P, Cabanes PA, Oubary P, Chedru F, Guerin F, Bousser MG, de Recondo J. Atrial septal aneurysm and patent foramen ovale as risk factors for cryptogenic stroke in patients less than 55 years of age: a study using transesophageal echocardiography. Stroke. 1993;24:1865–1873. doi: 10.1161/01.str.24.12.1865. doi:10.1161/01.STR.24.12.1865. [DOI] [PubMed] [Google Scholar]
- 11.Mugge A, Daniel WG, Angermann C, Spes C, Khandheria BK, Kronzon I, Freedberg RS, Keren A, Denning K, Engberding R, et al. Atrial septal aneurysm in adult patients: a multicenter study using transthoracic and transesophageal echocardiography. Circulation. 1995;91:2785–2792. doi: 10.1161/01.cir.91.11.2785. [DOI] [PubMed] [Google Scholar]
- 12.Mas JL, Zuber M. Recurrent cerebrovascular events in patients with patent foramen ovale, atrial septal aneurysm, or both and cryptogenic stroke or transient ischemic attack. French Study Group on Patent Foramen Ovale and Atrial Septal Aneurysm. Am Heart J. 1995;130:1083–1088. doi: 10.1016/0002-8703(95)90212-0. doi:10.1016/0002-8703(95)90212-0. [DOI] [PubMed] [Google Scholar]
- 13.Mattioli AV, Aquilina M, Oldani A, Longhini C, Mattioli G. Atrial septal aneurysm as a cardioembolic source in adult patients with stroke and normal carotid arteries: a multicentre study. Eur Heart J. 2001;22:261–268. doi: 10.1053/euhj.2001.2293. doi:10.1053/euhj.2001.2293. [DOI] [PubMed] [Google Scholar]
- 14.Overell JR, Bone I, Lees KR. Interatrial septal abnormalities and stroke: a meta-analysis of case–control studies. Neurology. 2000;55:1172–1179. doi: 10.1212/wnl.55.8.1172. [DOI] [PubMed] [Google Scholar]
- 15.Homma S, Di Tullio MR, Sacco RL, Mihalatos D, Li Mandri G, Mohr JP. Characteristics of patent foramen ovale associated with cryptogenic stroke: a biplane transesophageal echocardiographic study. Stroke. 1994;25:582–586. doi: 10.1161/01.str.25.3.582. doi:10.1161/01.STR.25.3.582. [DOI] [PubMed] [Google Scholar]
- 16.De Castro S, Cartoni D, Fiorelli M, Rasura M, Anzini A, Zanette EM, Beccia M, Colonnese C, Fedele F, Fieschi C, Pandian NG. Morphological and functional characteristics of patent foramen ovale and their embolic implications. Stroke. 2000;31:2407–2413. doi: 10.1161/01.str.31.10.2407. doi:10.1161/01.STR.31.10.2407. [DOI] [PubMed] [Google Scholar]
- 17.Sacco RL, Ellenberg JH, Mohr JP, Tatemichi TK, Hier DB, Price TR, Wolf PA. Infarcts of undetermined cause: the NINCDS Stroke Data Bank. Ann Neurol. 1989;25:382–390. doi: 10.1002/ana.410250410. doi:10.1002/ana.410250410. [DOI] [PubMed] [Google Scholar]
- 18.Steiner MM, Di Tullio MR, Rundek T, Gan R, Chen X, Liguori C, Brainin M, Homma S, Sacco RL. Patent foramen ovale size and embolic brain imaging findings among patients with ischemic stroke. Stroke. 1998;29:944–948. doi: 10.1161/01.str.29.5.944. doi:10.1161/01.STR.29.5.944. [DOI] [PubMed] [Google Scholar]
- 19.Meier B, Lock JE. Contemporary management of patent foramen ovale. Circulation. 2003;107:5–9. doi: 10.1161/01.cir.0000046073.34261.c1. doi:10.1161/01.CIR.0000046073.34261.C1. [DOI] [PubMed] [Google Scholar]
- 20.Lechat P, Mas JL, Lascault G, Loron P, Theard M, Klimczac M, Drobinski G, Thomas D, Grosgogeat Y. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med. 1988;318:1148–1152. doi: 10.1056/NEJM198805053181802. doi:10.1056/NEJM198805053181802. [DOI] [PubMed] [Google Scholar]
- 21.Webster MW, Chancellor AM, Smith HJ, Swift DL, Sharpe DN, Bass NM, Glasgow GL. Patent foramen ovale in young stroke patients. Lancet. 1988;2:11–12. doi: 10.1016/s0140-6736(88)92944-3. doi:10.1016/S0140-6736(88)92944-3. [DOI] [PubMed] [Google Scholar]
- 22.Konstantinides S, Geibel A, Kasper W, Olschewski M, Blumel L, Just H. Patent foramen ovale is an important predictor of adverse outcome in patients with major pulmonary embolism. Circulation. 1998;97:1946–1951. doi: 10.1161/01.cir.97.19.1946. [DOI] [PubMed] [Google Scholar]
- 23.Cramer SC, Rordorf G, Maki JH, Kramer LA, Grotta JC, Burgin WS, Hinchey JA, Benesch C, Furie KL, Lutsep HL, Kelly E, Longstreth WT., Jr Increased pelvic vein thrombi in cryptogenic stroke: results of the Paradoxical Emboli from Large Veins in Ischemic Stroke (PELVIS) study. Stroke. 2004;35:46–50. doi: 10.1161/01.STR.0000106137.42649.AB. doi:10.1161/01.STR.0000106137.42649.AB. [DOI] [PubMed] [Google Scholar]
- 24.Khairy P, Landzberg MJ, Gatzoulis MA, Mercier LA, Fernandes SM, Cote JM, Lavoie JP, Fournier A, Guerra PG, Frogoudaki A, Walsh EP, Dore A. Transvenous pacing leads and systemic thromboemboli in patients with intracardiac shunts: a multicenter study. Circulation. 2006;113:2391–2397. doi: 10.1161/CIRCULATIONAHA.106.622076. doi:10.1161/CIRCULATIONAHA.106.622076. [DOI] [PubMed] [Google Scholar]
- 25.Sorensen HT, Horvath-Puho E, Pedersen L, Baron JA, Prandoni P. Venous thromboembolism and subsequent hospitalisation due to acute arterial cardiovascular events: a 20-year cohort study. Lancet. 2007;370:1773–1779. doi: 10.1016/S0140-6736(07)61745-0. doi:10.1016/S0140-6736(07)61745-0. [DOI] [PubMed] [Google Scholar]
- 26.Alzamora MT, Sorribes M, Heras A, Vila N, Vicheto M, Fores R, Sanchez-Ojanguren J, Sancho A, Pera G. Ischemic stroke incidence in Santa Coloma de Gramenet (ISISCOG), Spain: a community-based study. BMC Neurol. 2008;8:5. doi: 10.1186/1471-2377-8-5. doi:10.1186/1471-2377-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kraywinkel K, Jauss M, Diener HC, Weimar C. Patent foramen ovale, atrial septum aneurysm, and stroke: an examination of the status of recent evidence. Nervenarzt. 2005;76:935–942. doi: 10.1007/s00115-004-1874-5. doi:10.1007/s00115-004-1874-5. [DOI] [PubMed] [Google Scholar]
- 28.Homma S, Sacco RL, Di Tullio MR, Sciacca RR, Mohr JP. Effect of medical treatment in stroke patients with patent foramen ovale: patent foramen ovale in cryptogenic stroke study. Circulation. 2002;105:2625–2631. doi: 10.1161/01.cir.0000017498.88393.44. doi:10.1161/01.CIR.0000017498.88393.44. [DOI] [PubMed] [Google Scholar]
- 29.Handke M, Harloff A, Olschewski M, Heztel A, Geibel A. Patent foramen ovale and cryptogenic stroke in older patients. N Engl J Med. 2007;357:2262–2268. doi: 10.1056/NEJMoa071422. doi:10.1056/NEJMoa071422. [DOI] [PubMed] [Google Scholar]
- 30.Alsheikh-Ali AA, Thaler DE, Kent DM. Patent foramen ovale in cryptogenic stroke: incidental or pathogenic? Stroke. 2009;40:2349–2355. doi: 10.1161/STROKEAHA.109.547828. doi:10.1161/STROKEAHA.109.547828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meissner I, Khandheria BK, Heit JA, Petty GW, Sheps SG, Schwartz GL, Whisnant JP, Wiebers DO, Covalt JL, Petterson TM, Christianson TJ, Agmon Y. Patent foramen ovale: innocent or guilty? Evidence from a prospective population-based study. J Am Coll Cardiol. 2006;47:440–445. doi: 10.1016/j.jacc.2005.10.044. doi:10.1016/j.jacc.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 32.Di Tullio MR, Sacco RL, Sciacca RR, Jin Z, Homma S. Patent foramen ovale and the risk of ischemic stroke in a multiethnic population. J Am Coll Cardiol. 2007;49:797–802. doi: 10.1016/j.jacc.2006.08.063. doi:10.1016/j.jacc.2006.08.063. [DOI] [PubMed] [Google Scholar]
- 33.Bogousslavsky J, Garazi S, Jeanrenaud X, Aebischer N, Van Melle G. Stroke recurrence in patients with patent foramen ovale: the Lausanne Study. Lausanne Stroke with Paradoxal Embolism Study Group. Neurology. 1996;46:1301–1305. doi: 10.1212/wnl.46.5.1301. [DOI] [PubMed] [Google Scholar]
- 34.Nedeltchev K, Arnold M, Wahl A, Sturzenegger M, Vella EE, Windecker S, Meier B, Mattle HP. Outcome of patients with cryptogenic stroke and patent foramen ovale. J Neurol Neurosurg Psychiatry. 2002;72:347–350. doi: 10.1136/jnnp.72.3.347. doi:10.1136/jnnp.72.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hausmann D, Mugge A, Daniel WG. Identification of patent foramen ovale permitting paradoxic embolism. J Am Coll Cardiol. 1995;26:1030–1038. doi: 10.1016/0735-1097(95)00288-9. doi:10.1016/0735-1097(95)00288-9. [DOI] [PubMed] [Google Scholar]
- 36.Schuchlenz HW, Weihs W, Horner S, Quehenberger F. The association between the diameter of a patent foramen ovale and the risk of embolic cerebrovascular events. Am J Med. 2000;109:456–462. doi: 10.1016/s0002-9343(00)00530-1. doi:10.1016/S0002-9343(00)00530-1. [DOI] [PubMed] [Google Scholar]
- 37.Stone DA, Godard J, Corretti MC, Kittner SJ, Sample C, Price TR, Plotnick GD. Patent foramen ovale: association between the degree of shunt by contrast transesophageal echocardiography and the risk of future ischemic neurologic events. Am Heart J. 1996;131:158–161. doi: 10.1016/s0002-8703(96)90065-4. doi:10.1016/S0002-8703(96)90065-4. [DOI] [PubMed] [Google Scholar]
- 38.Almekhlafi MA, Wilton SB, Rabi DM, Ghali WA, Lorenzetti DL, Hill MD. Recurrent cerebral ischemia in medically treated patent foramen ovale: a meta-analysis. Neurology. 2009;73:89–97. doi: 10.1212/WNL.0b013e3181aa2a19. doi:10.1212/WNL.0b013e3181aa2a19. [DOI] [PubMed] [Google Scholar]
- 39.Mohr JP, Thompson JLP, Lazar RM, Levin B, Sacco RL, Furie KL, Kistler JP, Albers GW, Pettigrew LC, Adams HP, Jackson CM, Pullicino P for the Warfarin-Aspirin Recurrent Stroke Study Group. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med. 2001;345:1444–1451. doi: 10.1056/NEJMoa011258. doi:10.1056/NEJMoa011258. [DOI] [PubMed] [Google Scholar]
- 40.Bridges ND, Hellenbrand W, Latson L, Filiano J, Newburger JW, Lock JE. Transcatheter closure of patent foramen ovale after presumed paradoxical embolism. Circulation. 1992;86:1902–1908. doi: 10.1161/01.cir.86.6.1902. [DOI] [PubMed] [Google Scholar]
- 41.Wahl A, Krumsdorf U, Meier B, Sievert H, Ostermayer S, Billinger K, Schwerzmann M, Becker U, Seiler C, Arnold M, Mattle HP, Windecker S. Transcatheter treatment of atrial septal aneurysm associated with patent foramen ovale for prevention of recurrent paradoxical embolism in high-risk patients. J Am Coll Cardiol. 2005;45:377–380. doi: 10.1016/j.jacc.2004.10.043. doi:10.1016/j.jacc.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 42.Windecker S, Wahl A, Chatterjee T, Garachemani A, Eberli FR, Seiler C, Meier B. Percutaneous closure of patent foramen ovale in patients with paradoxical embolism: long-term risk of recurrent thromboembolic events. Circulation. 2000;101:893–898. doi: 10.1161/01.cir.101.8.893. [DOI] [PubMed] [Google Scholar]
- 43.Hung J, Landzberg MJ, Jenkins KJ, King ME, Lock JE, Palacios IF, Lang P. Closure of patent foramen ovale for paradoxical emboli: intermediate-term risk of recurrent neurological events following transcatheter device placement. J Am Coll Cardiol. 2000;35:1311–1316. doi: 10.1016/s0735-1097(00)00514-3. doi:10.1016/S0735-1097(00)00514-3. [DOI] [PubMed] [Google Scholar]
- 44.Wahl A, Meier B, Haxel B, Nedeltchev K, Arnold M, Eicher E, Sturzenegger M, Seiler C, Mattle HP, Windecker S. Prognosis after percutaneous closure of patent foramen ovale for paradoxical embolism. Neurology. 2001;57:1330–1332. doi: 10.1212/wnl.57.7.1330. [DOI] [PubMed] [Google Scholar]
- 45.Martin F, Sanchez PL, Doherty E, Colon-Hernandez PJ, Delgado G, Inglessis I, Scott N, Hung J, King ME, Buonanno F, Demirjian Z, de Moor M, Palacios IF. Percutaneous transcatheter closure of patent foramen ovale in patients with paradoxical embolism. Circulation. 2002;106:1121–1126. doi: 10.1161/01.cir.0000027819.19722.ee. doi:10.1161/01.CIR.0000027819.19722.EE. [DOI] [PubMed] [Google Scholar]
- 46.Braun MU, Fassbender D, Schoen SP, Haass M, Schraeder R, Scholtz W, Strasser RH. Transcatheter closure of patent foramen ovale in patients with cerebral ischemia. J Am Coll Cardiol. 2002;39:2019–2025. doi: 10.1016/s0735-1097(02)01904-6. doi:10.1016/S0735-1097(02)01904-6. [DOI] [PubMed] [Google Scholar]
- 47.Onorato E, Melzi G, Casilli F, Pedon L, Rigatelli G, Carrozza A, Maiolino P, Zanchetta M, Morandi E, Angeli S, Anzola GP. Patent foramen ovale with paradoxical embolism: mid-term results of transcatheter closure in 256 patients. J Interv Cardiol. 2003;16:43–50. doi: 10.1046/j.1540-8183.2003.08002.x. doi:10.1046/j.1540-8183.2003.08002.x. [DOI] [PubMed] [Google Scholar]
- 48.Braun M, Gliech V, Boscheri A, Schoen S, Gahn G, Reichmann H, Haass M, Schraeder R, Strasser RH. Transcatheter closure of patent foramen ovale (PFO) in patients with paradoxical embolism: periprocedural safety and mid-term follow-up results of three different device occluder systems. Eur Heart J. 2004;25:424–430. doi: 10.1016/j.ehj.2003.10.021. doi:10.1016/j.ehj.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 49.Spies C, Strasheim R, Timmermanns I, Schraeder R. Patent foramen ovale closure in patients with cryptogenic thrombo-embolic events using the Cardia PFO occluder. Eur Heart J. 2006;27:365–371. doi: 10.1093/eurheartj/ehi617. doi:10.1093/eurheartj/ehi617. [DOI] [PubMed] [Google Scholar]
- 50.Harms V, Reisman M, Fuller CJ, Spencer MP, Olsen JV, Krabill KA, Gray WA, Jesurum JT. Outcomes after transcatheter closure of patent foramen ovale in patients with paradoxical embolism. Am J Cardiol. 2007;99:1312–1315. doi: 10.1016/j.amjcard.2006.12.055. doi:10.1016/j.amjcard.2006.12.055. [DOI] [PubMed] [Google Scholar]
- 51.Wahl A, Kunz M, Moschovitis A, Nageh T, Schwerzmann M, Seiler C, Mattle HP, Windecker S, Meier B. Long-term results after fluoroscopy-guided closure of patent foramen ovale for secondary prevention of paradoxical embolism. Heart. 2008;94:336–341. doi: 10.1136/hrt.2007.118505. doi:10.1136/hrt.2007.118505. [DOI] [PubMed] [Google Scholar]
- 52.Wahl A, Tai T, Praz F, Schwerzmann M, Seiler C, Nedeltchev K, Windecker S, Mattle HP, Meier B. Late results after percutaneous closure of patent foramen ovale for secondary prevention of paradoxical embolism using the Amplatzer PFO occluder without intraprocedural echocardiography. J Am Coll Cardiol Interv. 2009;2:116–123. doi: 10.1016/j.jcin.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 53.Wahl A, Praz F, Seiler C, Windecker S, Meier B. Clinical relevance of coronary angiography at the time of percutaneous closure of a patent foramen ovale. Catheter Cardiovasc Interv. 2007;70:641–645. doi: 10.1002/ccd.21247. doi:10.1002/ccd.21247. [DOI] [PubMed] [Google Scholar]
- 54.Schwerzmann M, Windecker S, Wahl A, Mehta H, Nedeltchev K, Mattle HP, Seiler C, Meier B. Percutaneous closure of patent foramen ovale: impact of device design on safety and efficacy. Heart. 2004;90:186–190. doi: 10.1136/hrt.2002.003111. doi:10.1136/hrt.2002.003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krumsdorf U, Ostermayer S, Billinger K, Trepels T, Zadan E, Horvath K, Sievert H. Incidence and clinical course of thrombus formation on atrial septal defect and patient foramen ovale closure devices in 1000 consecutive patients. J Am Coll Cardiol. 2004;43:302–309. doi: 10.1016/j.jacc.2003.10.030. doi:10.1016/j.jacc.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 56.Schuchlenz HW, Weihs W, Berghold A, Lechner A, Schmidt R. Secondary prevention after cryptogenic cerebrovascular events in patients with patent foramen ovale. Int J Cardiol. 2005;101:77–82. doi: 10.1016/j.ijcard.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 57.Wahl A, Jüni P, Mono M, Kalesan B, Praz F, Geister L, Räber L, Nedeltchev K, Mattle H, Windecker S, Meier B. Long-term propensity score-matched comparison of percutaneous closure of patent foramen ovale with medical treatment after paradoxical embolism. Circulation. 2012;125:803–812. doi: 10.1161/CIRCULATIONAHA.111.030494. [DOI] [PubMed] [Google Scholar]
- 58.Wöhrle J. Closure of patent foramen ovale after cryptogenic stroke. Lancet. 2006;368:350–352. doi: 10.1016/S0140-6736(06)69087-9. doi:10.1016/S0140-6736(06)69087-9. [DOI] [PubMed] [Google Scholar]
- 59.Amarenco P, Duyckaerts C, Tzourio C, Henin D, Bousser MG, Hauw JJ. The prevalence of ulcerated plaques in the aortic arch in patients with stroke. N Engl J Med. 1992;326:221–225. doi: 10.1056/NEJM199201233260402. doi:10.1056/NEJM199201233260402. [DOI] [PubMed] [Google Scholar]
- 60.Stone DA, Hawke MW, LaMonte M, Kittner SJ, Acosta J, Corretti M, Sample C, Price TR, Plotnick GD. Ulcerated atherosclerotic plaques in the thoracic aorta are associated with cryptogenic stroke: a multiplane transesophageal echocardiographic study. Am Heart J. 1995;130:105–108. doi: 10.1016/0002-8703(95)90243-0. doi:10.1016/0002-8703(95)90243-0. [DOI] [PubMed] [Google Scholar]
- 61.Di Tullio MR, Sacco RL, Savoia MT, Sciacca RR, Homma S. Aortic atheroma morphology and the risk of ischemic stroke in a multiethnic population. Am Heart J. 2000;139:329–336. doi: 10.1067/mhj.2000.101225. [DOI] [PubMed] [Google Scholar]
- 62.Mitusch R, Doherty C, Wucherpfennig H, Memmesheimer C, Tepe C, Stierle U, Kessler C, Sheikhzadeh A. Vascular events during follow-up in patients with aortic arch atherosclerosis. Stroke. 1997;28:36–39. doi: 10.1161/01.str.28.1.36. doi:10.1161/01.STR.28.1.36. [DOI] [PubMed] [Google Scholar]
- 63.Fujimoto S, Yasaka M, Otsubo R, Oe H, Nagatsuka K, Minematsu K. Aortic arch atherosclerotic lesions and the recurrence of ischemic stroke. Stroke. 2004;35:1426–1429. doi: 10.1161/01.STR.0000127788.32550.d4. doi:10.1161/01.STR.0000127788.32550.d4. [DOI] [PubMed] [Google Scholar]
- 64.Cohen A, Tzourio C, Bertrand B, Chauvel C, Bousser MG, Amarenco P. Aortic plaque morphology and vascular events: a follow-up study in patients with ischemic stroke. FAPS Investigators. French Study of Aortic Plaques in Stroke. Circulation. 1997;96:3838–3841. doi: 10.1161/01.cir.96.11.3838. [DOI] [PubMed] [Google Scholar]
- 65.Meissner I, Khandheria BK, Sheps SG, Schwartz GL, Wiebers DO, Whisnant JP, Covalt JL, Petterson TM, Christianson TJ, Agmon Y. Atherosclerosis of the aorta: risk factor, risk marker, or innocent bystander? A prospective population-based transesophageal echocardiography study. J Am Coll Cardiol. 2004;44:1018–1024. doi: 10.1016/j.jacc.2004.05.075. doi:10.1016/j.jacc.2004.05.075. [DOI] [PubMed] [Google Scholar]
- 66.Petty GW, Khandheria BK, Meissner I, Whisnant JP, Rocca WA, Sicks JD, Christianson TJ, O'Fallon WM, McClelland RL, Wiebers DO. Population-based study of the relationship between atherosclerotic aortic debris and cerebrovascular ischemic events. Mayo Clin Proc. 2006;81:609–614. doi: 10.4065/81.5.609. doi:10.4065/81.5.609. [DOI] [PubMed] [Google Scholar]
- 67.Russo C, Jin Z, Rundek T, Homma S, Sacco RL, Di Tullio MR. Atherosclerotic disease of the proximal aorta and the risk of vascular events in a population-based cohort: the Aortic Plaques and Risk of Ischemic Stroke (APRIS) study. Stroke. 2009;40:2313–2318. doi: 10.1161/STROKEAHA.109.548313. doi:10.1161/STROKEAHA.109.548313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harloff A, Strecker C, Dudler P, Nussbaumer A, Frydrychowicz A, Olschewski M, Bock J, Stalder AF, Stroh AL, Weiller C, Hennig J, Markl M. Retrograde embolism from the descending aorta: visualization by multidirectional 3D velocity mapping in cryptogenic stroke. Stroke. 2009;40:1505–1508. doi: 10.1161/STROKEAHA.108.530030. doi:10.1161/STROKEAHA.108.530030. [DOI] [PubMed] [Google Scholar]
- 69.Harloff A, Simon J, Brendecke S, Assefa D, Helbing T, Frydrychowicz A, Weber J, Olschewski M, Strecker C, Hennig J, Weiller C, Markl M. Complex plaques in the proximal descending aorta: an underestimated embolic source of stroke. Stroke. 2010;41:1145–1150. doi: 10.1161/STROKEAHA.109.577775. doi:10.1161/STROKEAHA.109.577775. [DOI] [PubMed] [Google Scholar]
- 70.Rauch U, Osende JI, Chesebro JH, Fuster V, Vorchheimer DA, Harris K, Harris P, Sandler DA, Fallon JT, Jayaraman S, Badimon JJ. Statins and cardiovascular diseases: the multiple effects of lipid-lowering therapy by statins. Atherosclerosis. 2000;153:181–189. doi: 10.1016/s0021-9150(00)00397-x. doi:10.1016/S0021-9150(00)00397-X. [DOI] [PubMed] [Google Scholar]
- 71.Lima JA, Desai MY, Steen H, Warren WP, Gautam S, Lai S. Statin-induced cholesterol lowering and plaque regression after 6 months of magnetic resonance imaging-monitored therapy. Circulation. 2004;110:2336–2341. doi: 10.1161/01.CIR.0000145170.22652.51. doi:10.1161/01.CIR.0000145170.22652.51. [DOI] [PubMed] [Google Scholar]
- 72.Tahara N, Kai H, Ishibashi M, Nakaura H, Kaida H, Baba K, Hayabuchi N, Imaizumi T. Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol. 2006;48:1825–1831. doi: 10.1016/j.jacc.2006.03.069. doi:10.1016/j.jacc.2006.03.069. [DOI] [PubMed] [Google Scholar]
- 73.Plehn JF, Davis BR, Sacks FM, Rouleau JL, Pfeffer MA, Bernstein V, Cuddy TE, Moye LA, Piller LB, Rutherford J, Simpson LM, Braunwald E. Reduction of stroke incidence after myocardial infarction with pravastatin: the Cholesterol and Recurrent Events (CARE) study. The Care Investigators. Circulation. 1999;99:216–223. doi: 10.1161/01.cir.99.2.216. [DOI] [PubMed] [Google Scholar]
- 74.White HD, Simes RJ, Anderson NE, Hankey GJ, Watson JD, Hunt D, Colquhoun DM, Glasziou P, MacMahon S, Kirby AC, West MJ, Tonkin AM. Pravastatin therapy and the risk of stroke. N Engl J Med. 2000;343:317–326. doi: 10.1056/NEJM200008033430502. doi:10.1056/NEJM200008033430502. [DOI] [PubMed] [Google Scholar]
- 75.Tunick PA, Nayar AC, Goodkin GM, Mirchandani S, Francescone S, Rosenzweig BP, Freedberg RS, Katz ES, Applebaum RM, Kronzon I. Effect of treatment on the incidence of stroke and other emboli in 519 patients with severe thoracic aortic plaque. Am J Cardiol. 2002;90:1320–1325. doi: 10.1016/s0002-9149(02)02870-9. doi:10.1016/S0002-9149(02)02870-9. [DOI] [PubMed] [Google Scholar]
- 76.Dressler FA, Craig WR, Castello R, Labovitz AJ. Mobile aortic atheroma and systemic emboli: efficacy of anticoagulation and influence of plaque morphology on recurrent stroke. J Am Coll Cardiol. 1998;31:134–138. doi: 10.1016/s0735-1097(97)00449-x. doi:10.1016/S0735-1097(97)00449-X. [DOI] [PubMed] [Google Scholar]
- 77.Ferrari E, Vidal R, Chevallier T, Baudouy M. Atherosclerosis of the thoracic aorta and aortic debris as a marker of poor prognosis: benefit of oral anticoagulants. J Am Coll Cardiol. 1999;33:1317–1322. doi: 10.1016/s0735-1097(99)00003-0. doi:10.1016/S0735-1097(99)00003-0. [DOI] [PubMed] [Google Scholar]
- 78.Blackshear JL, Zabalgoitia M, Pennock G, Fenster P, Strauss R, Halperin J, Asinger R, Pearce LA. Warfarin safety and efficacy in patients with thoracic aortic plaque and atrial fibrillation. SPAF TEE Investigators. Stroke Prevention and Atrial Fibrillation. Transesophageal echocardiography. Am J Cardiol. 1999;83:453–455. doi: 10.1016/s0002-9149(98)00886-8. A459 doi:10.1016/S0002-9149(98)00886-8. [DOI] [PubMed] [Google Scholar]
- 79.Di Tullio MR, Russo C, Jin Z, Sacco RL, Mohr JP, Homma S. Aortic arch plaques and risk of recurrent stroke and death. Circulation. 2009;119:2376–2382. doi: 10.1161/CIRCULATIONAHA.108.811935. doi:10.1161/CIRCULATIONAHA.108.811935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.The French Study of Aortic Plaques in Stroke Group. Atherosclerotic disease of the aortic arch as a risk factor for recurrent ischemic stroke. N Engl J Med. 1996;334:1216–1221. doi: 10.1056/NEJM199605093341902. doi:10.1056/NEJM199605093341902. [DOI] [PubMed] [Google Scholar]
- 81.Choukroun EM, Labrousse LM, Madonna FP, Deville C. Mobile thrombus of the thoracic aorta: diagnosis and treatment in 9 cases. Ann Vasc Surg. 2002;16:714–722. doi: 10.1007/s10016-001-0314-2. doi:10.1007/s10016-001-0314-2. [DOI] [PubMed] [Google Scholar]
- 82.Barnett HJ, Taylor DW, Eliasziw M, Fox AJ, Ferguson GG, Haynes RB, Rankin RN, Clagett GP, Hachinski VC, Sackett DL, Thorpe KE, Meldrum HE, Spence JD. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998;339:1415–1425. doi: 10.1056/NEJM199811123392002. doi:10.1056/NEJM199811123392002. [DOI] [PubMed] [Google Scholar]
- 83.European Carotid Surgery Trialists’ Collaborative Group. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST) Lancet. 1998;351:1379–1387. doi:10.1016/S0140-6736(97)09292-1. [PubMed] [Google Scholar]
- 84.Rothwell PM, Eliasziw M, Gutnikov SA, Warlow CP, Barnett HJ. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet. 2004;363:915–924. doi: 10.1016/S0140-6736(04)15785-1. doi:10.1016/S0140-6736(04)15785-1. [DOI] [PubMed] [Google Scholar]
- 85.Mathias K, Bockenheimer S, von Reutern G, Heiss HW, Ostheim-Dzerowycz W. Catheter dilatation of arteries supplying the brain. Radiologe. 1983;23:208–214. [PubMed] [Google Scholar]
- 86.Yadav JS, Roubin GS, King P, Iyer S, Vitek J. Angioplasty and stenting for restenosis after carotid endarterectomy: initial experience. Stroke. 1996;27:2075–2079. doi: 10.1161/01.str.27.11.2075. doi:10.1161/01.STR.27.11.2075. [DOI] [PubMed] [Google Scholar]
- 87.CAVATAS I. Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trial. Lancet. 2001;357:1729–1737. doi:10.1016/S0140-6736(00)04893-5. [PubMed] [Google Scholar]
- 88.Yadav JS, Wholey MH, Kuntz RE, Fayad P, Katzen BT, Mishkel GJ, Bajwa TK, Whitlow P, Strickman NE, Jaff MR, Popma JJ, Snead DB, Cutlip DE, Firth BG, Ouriel K. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351:1493–1501. doi: 10.1056/NEJMoa040127. doi:10.1056/NEJMoa040127. [DOI] [PubMed] [Google Scholar]
- 89.Ringleb PA, Allenberg J, Bruckmann H, Eckstein HH, Fraedrich G, Hartmann M, Hennerici M, Jansen O, Klein G, Kunze A, Marx P, Niederkorn K, Schmiedt W, Solymosi L, Stingele R, Zeumer H, Hacke W. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet. 2006;368:1239–1247. doi: 10.1016/S0140-6736(06)69122-8. doi:10.1016/S0140-6736(06)69122-8. [DOI] [PubMed] [Google Scholar]
- 90.Mas JL, Chatellier G, Beyssen B, Branchereau A, Moulin T, Becquemin JP, Larrue V, Lievre M, Leys D, Bonneville JF, Watelet J, Pruvo JP, Albucher JF, Viguier A, Piquet P, Garnier P, Viader F, Touze E, Giroud M, Hosseini H, Pillet JC, Favrole P, Neau JP, Ducrocq X. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355:1660–1671. doi: 10.1056/NEJMoa061752. doi:10.1056/NEJMoa061752. [DOI] [PubMed] [Google Scholar]
- 91.Ederle J, Dobson J, Featherstone RL, Bonati LH, van der Worp HB, de Borst GJ, Lo TH, Gaines P, Dorman PJ, Macdonald S, Lyrer PA, Hendriks JM, McCollum C, Nederkoorn PJ, Brown MM. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trial. Lancet. 2010;375:985–997. doi: 10.1016/S0140-6736(10)60239-5. doi:10.1016/S0140-6736(10)60239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brott TG, Hobson RW, II, Howard G, Roubin GS, Clark WM, Brooks W, Mackey A, Hill MD, Leimgruber PP, Sheffet AJ, Howard VJ, Moore WS, Voeks JH, Hopkins LN, Cutlip DE, Cohen DJ, Popma JJ, Ferguson RD, Cohen SN, Blackshear JL, Silver FL, Mohr JP, Lal BK, Meschia JF. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363:11–23. doi: 10.1056/NEJMoa0912321. doi:10.1056/NEJMoa0912321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stingele R, Berger J, Alfke K, Eckstein HH, Fraedrich G, Allenberg J, Hartmann M, Ringleb PA, Fiehler J, Bruckmann H, Hennerici M, Jansen O, Klein G, Kunze A, Marx P, Niederkorn K, Schmiedt W, Solymosi L, Zeumer H, Hacke W. Clinical and angiographic risk factors for stroke and death within 30 days after carotid endarterectomy and stent-protected angioplasty: a subanalysis of the SPACE study. Lancet Neurol. 2008;7:216–222. doi: 10.1016/S1474-4422(08)70024-3. doi:10.1016/S1474-4422(08)70024-3. [DOI] [PubMed] [Google Scholar]
- 94.Meier P, Knapp G, Tamhane U, Chaturvedi S, Gurm HS. Short term and intermediate term comparison of endarterectomy versus stenting for carotid artery stenosis: systematic review and meta-analysis of randomised controlled clinical trials. Br Med J. 2010;340:c467. doi: 10.1136/bmj.c467. doi:10.1136/bmj.c467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mas JL, Trinquart L, Leys D, Albucher JF, Rousseau H, Viguier A, Bossavy JP, Denis B, Piquet P, Garnier P, Viader F, Touze E, Julia P, Giroud M, Krause D, Hosseini H, Becquemin JP, Hinzelin G, Houdart E, Henon H, Neau JP, Bracard S, Onnient Y, Padovani R, Chatellier G. Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial: results up to 4 years from a randomised, multicentre trial. Lancet Neurol. 2008;7:885–892. doi: 10.1016/S1474-4422(08)70195-9. doi:10.1016/S1474-4422(08)70195-9. [DOI] [PubMed] [Google Scholar]
- 96.Eckstein HH, Ringleb P, Allenberg JR, Berger J, Fraedrich G, Hacke W, Hennerici M, Stingele R, Fiehler J, Zeumer H, Jansen O. Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: a multinational, prospective, randomised trial. Lancet Neurol. 2008;7:893–902. doi: 10.1016/S1474-4422(08)70196-0. doi:10.1016/S1474-4422(08)70196-0. [DOI] [PubMed] [Google Scholar]
- 97.Rothwell PM, Warlow CP. Prediction of benefit from carotid endarterectomy in individual patients: a risk-modelling study. European Carotid Surgery Trialists’ Collaborative Group. Lancet. 1999;353:2105–2110. doi: 10.1016/s0140-6736(98)11415-0. doi:10.1016/S0140-6736(98)11415-0. [DOI] [PubMed] [Google Scholar]